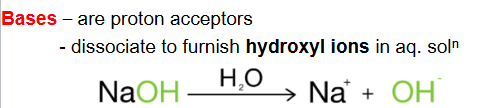MLT 118 Exam 2
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
All are relative expression except:
Concentrated water
Saturated Solution
0.9% Saline
Super saturated water
0.9% saline
V/V W/V and W/W are what kind of expressions?
Quantitative Expressions (physical)
What is a system used to ensure the validity of overall analytic performance of lab procedures?
Quality Assurance
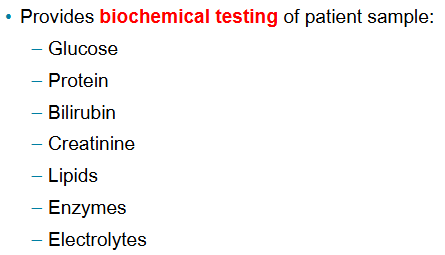
What is a substance or constituent in which the lab conducts tests?
Analyte
What is the procedural steps performed to determine the kind or amount of analyze in a specimen?
Analysis
What’s the name of a chemical that is used to convert the analyze from the sample into a measurable form? It can be a solution or powder.
Reagent
The action of assessing, setting of correcting a device by adjusting it to match or conform to a reliable, known measure is called…
calibration
What are the three types of analysis?
Qualitative
Semi-quantitative
Quantitative
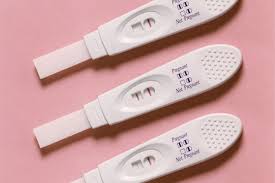
Pregnancy test came back positive (+), this is a ____ result of analysis.
qualitative
The patients blood glucose results came back, a total of 300mg/dL, this is a ______ result of analysis.
quantitative
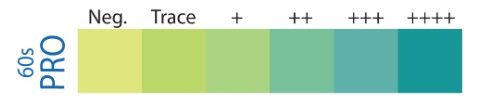
The protein and glucose on reagent strips are examples of what analysis? ( 1+, 2+. +/- )
semi- quantitative

The blood column on reagent test strips is an example of what analysis type?
qualitative
Pre-filtration, distillation, deionization, reverse osmosis, ultra filtration and nano-filtration are…
methods of water purification

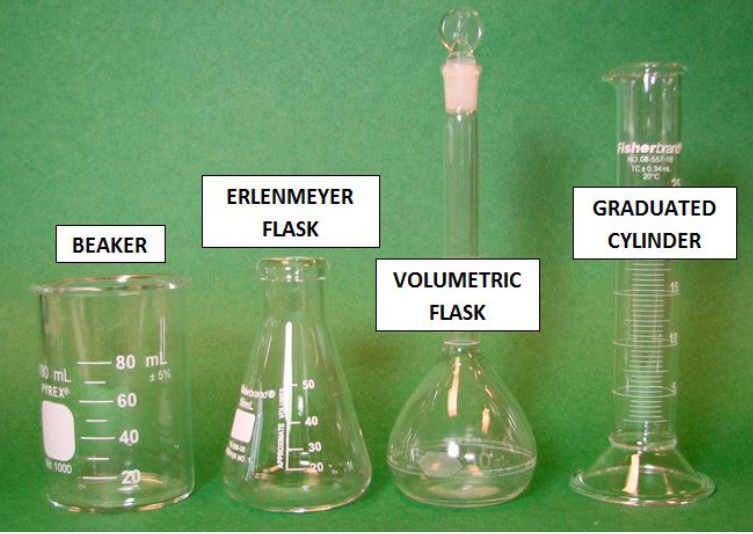
What is number 1?
BEAKER
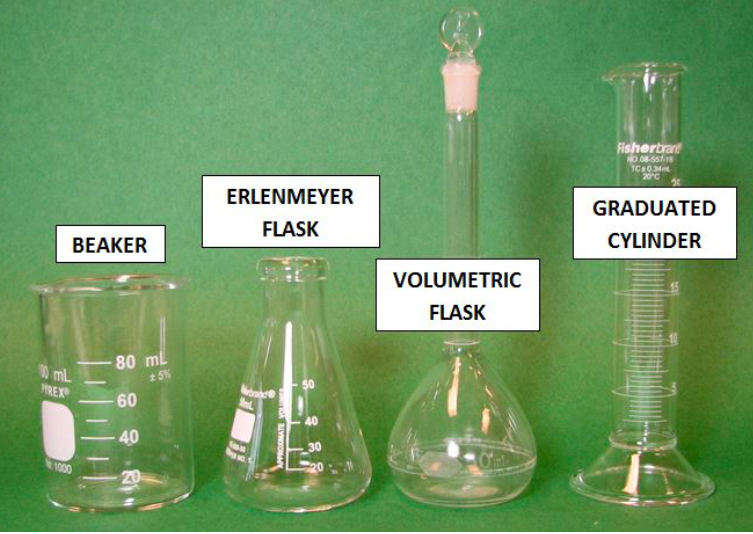

What is number 2?
ERLENMEYER FLASK
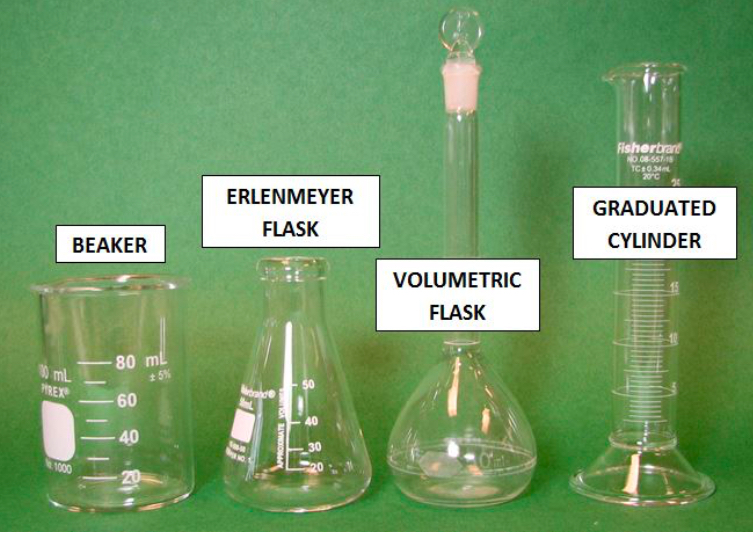

What is number 3?
VOLUMETRIC FLASK
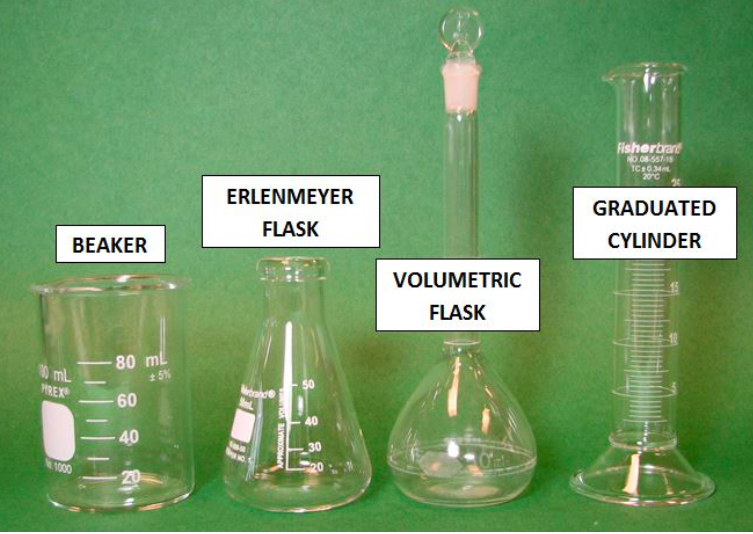

What is number 4?
GRADUATED CYLINDER

Whats the name of this container in the image?
Florence flask
What are the correct names for glassware?
borosilicate and low actinic
What type of glassware has high degree of thermal resistance, low alkali content and free of heavy chemicals?
borosilicate

Which glassware has high thermal resistance, protects light-sensitive substances and is amber/red in color?
low actinic

A salt sample is placed into some water and all of it dissolves without stirring or heating. When you add another pinch of salt it continue to dissolve. The resulting solution is:
unsaturated
You have water(solvent) & acid(solute) need for the solution. Which goes first?
water
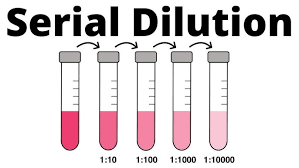
_________________is a type of dilution with multiple, progressive dilution processes that may be required for certain quantitative tests.
Serial dilution or sequential dilution
Blood samples are usually spun in a ____________________to get serum or plasma.
centrifuge
All of the following are true of pipets EXCEPT:
All micropepettes measure the same volume.
Micropipets measure and deliver in microliter (µL).
Pipette calibration is done by Gravimetric procedure.
Volumetric pipettes can be used to reconstitute lyophilized controls and calibrators
All micropepettes measure the same volume.
The speed of a centrifuge should be checked at least once every 3 months with a (n):
Potentiometer
Tachometer
Ergometer
Wiper
Tachometer
Which of the following is not %V/V (volume by volume) expression?
0.9% Saline
70% Ethanol
60% Isopropanol
10% Bleach
0.9% Saline
Which of the following does NOT require calibration in the clinical laboratory?
Erlenmeyer flask
Centrifuge
Electronic balance
Micropipette
Erlenmeyer flask
Solution = _________________ + ___________________
Solvent : Solute
There is water and salt in a container: define which is the solute and solvent.
water is solvent and salt is the solute
Solution properties that depend on the concentration of solute particles, not their identity are called…
Colligative properties
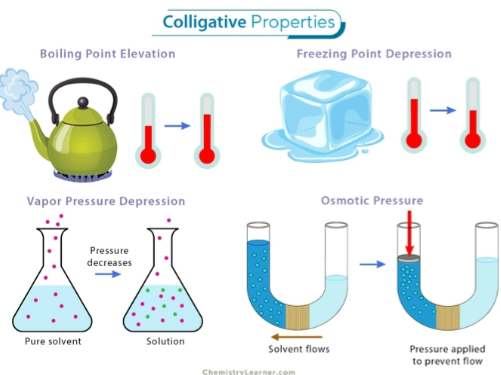
Glucose and creatinine are called what in clinical terms?
clinical analytes
Name 3-4 Clinical analytes.
glucose, creatinine, bilirubin, sodium, albumin protein and cholesterol
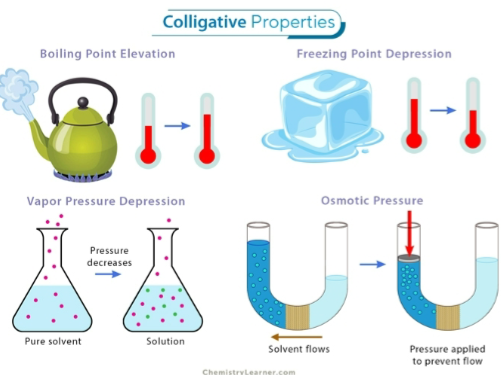
vapor pressure depression,
boiling point elevation,
freezing point depression
osmotic pressure
These are all called…
the four colligative properties
Which of the following statements is true about colligative properties?
The more particles dissolved in a solution will make the solution's freezing and boiling points higher in temperature.
Increasing the number of particles dissolved in a solution will make the solution's boiling point extremely higher.
The more particles dissolved in a solvent the lower the solution's boiling point.
The greater the number of particles dissolved in a solution the lower the solution's freezing point.
The greater the number of particles dissolved in a solution the lower the solution's freezing point.
Is a mixture of two or more solids…
solid solution
A homogenous mixture of two ore more gases..
Gaseous solution
The solvent is always liquid; the solute can be any state of matter..
liquid solution
Which of the following is not relative expression of solutions?
Diluted acetic acid
1M hydrogen peroxide
Concentrated urine
Saturated glucose
1M hydrogen peroxide
__________is a process by which the concentration of a given solution is decreased by the addition of solvents to make a weaker solution.
Dilution
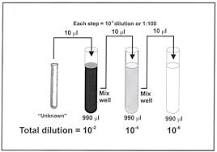
A type of dilution where one part of the original solution
simple dilution
A type of dilution using larger numbers; useful when the volume of concentrate or diluents is in short supply
serial dilution

what is this tool in the image and what’s its purpose?
rocker ; it is for mixing
Graduated cylinders:
are made only of plastic
are used by reading volume at meniscus
range in size from 5–50 mL
have a flared neck
are used by reading volume at meniscus
What form of temperature scale is used in clinical lab?
Celsius
A solution is made by mixing 70mL of alcohol and 30mL of water in a volumetric flask. The alcohol is referred to as the______________
solvent
solute
filtrate
solute
A noncritical measurement can be:
made using a volumetric flask
used when making a standard
made using an Erlenmeyer flask
made using a volumetric pipet
made using an Erlenmeyer flask
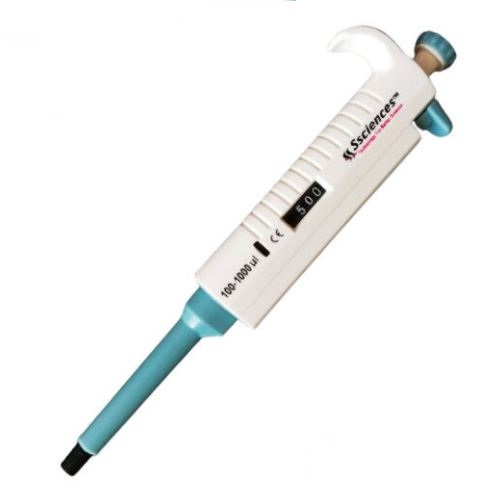
Look the below laboratory equipment and answer the following questions:
A) What is the range of volume measured by this equipment? ______________________
B) Can you use it to measure 50μl of sample? ________________________
A. 100-1000 microliter
B.NO
You have been instructed to create a working solution of HCl acid. You start to mix the working solution from a very concentrated stock solution of the acid. In what order should you complete this task?
__ Get a clean beaker
__ Carefully add acid slowly and in small amounts
__ Label the beaker clearly with the contents it will contain
__ Add the designated amount of water needed to make the working solution
1, 4, 2, 3
pH is defined as the negative logarithm of the ______________ ion concentration
Hydrogen, H
A proton donor which furnish hydrogen ions is called
an acid
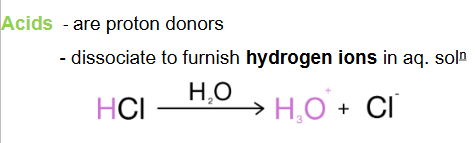
A proton acceptor which furnish hydroxyl ions is called
a base