L2: Actin organisation in vivo
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
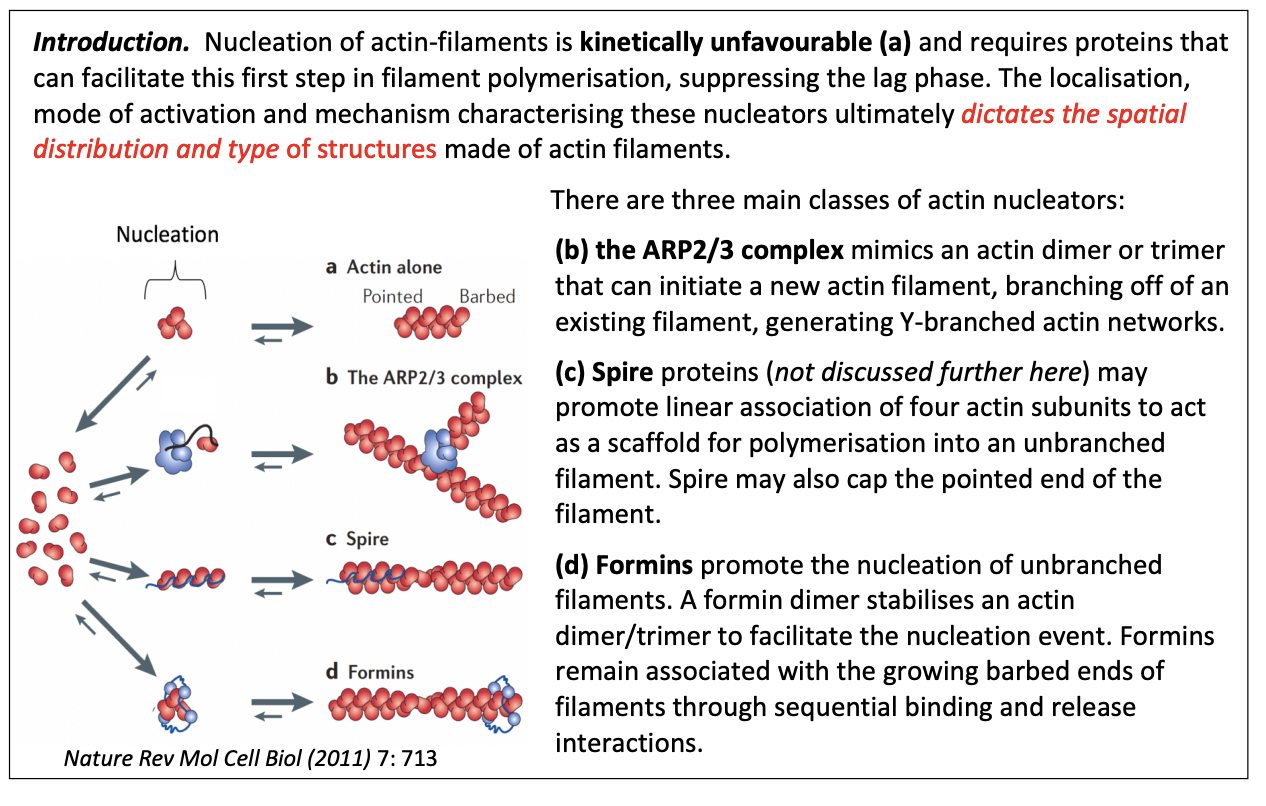
Why are nucleation factors (nucleators) required for nucleation of actin-filaments
kinetically unfavvourable
need to suppress the lag phase
What ultimately dictates the spatial distribution and type of strucutres made of actin filaments
localisation
mode of activation
mechanism
characterising these nnucleators
Three main classes of actin nucleators
ARP2/3 complex
Spire
Formins
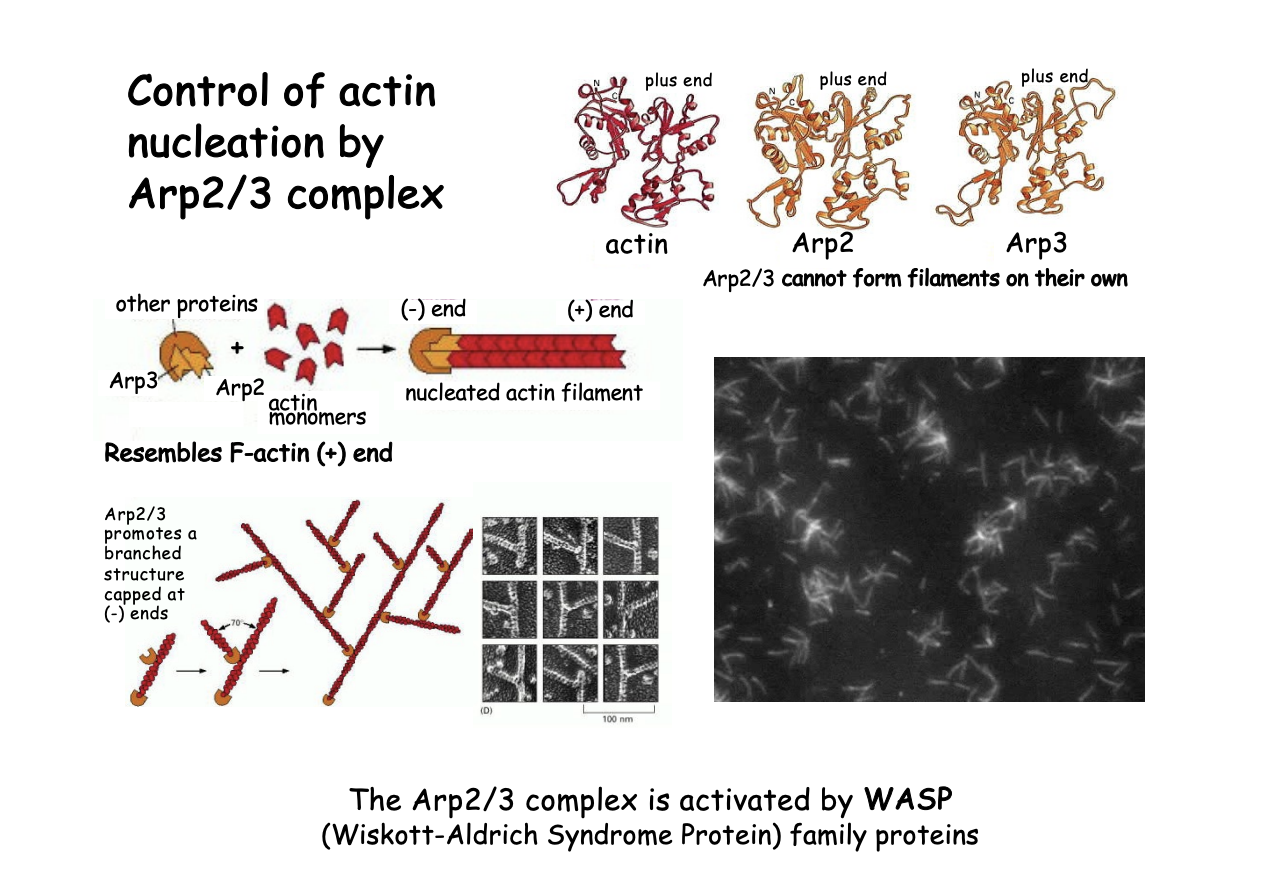
ARP2/3 complex
similar to actin monomer + end ‘actin related protein’
mimics and actin dimer or trimer
What they do
can initiate a new actin filament
can branch off of an existing filament→ generate Y branched actin networks
stays at the branch point
cannot form filaments on their own
Filaments continue to grow at the + end, while the - end is capped by Arp2/3 complex
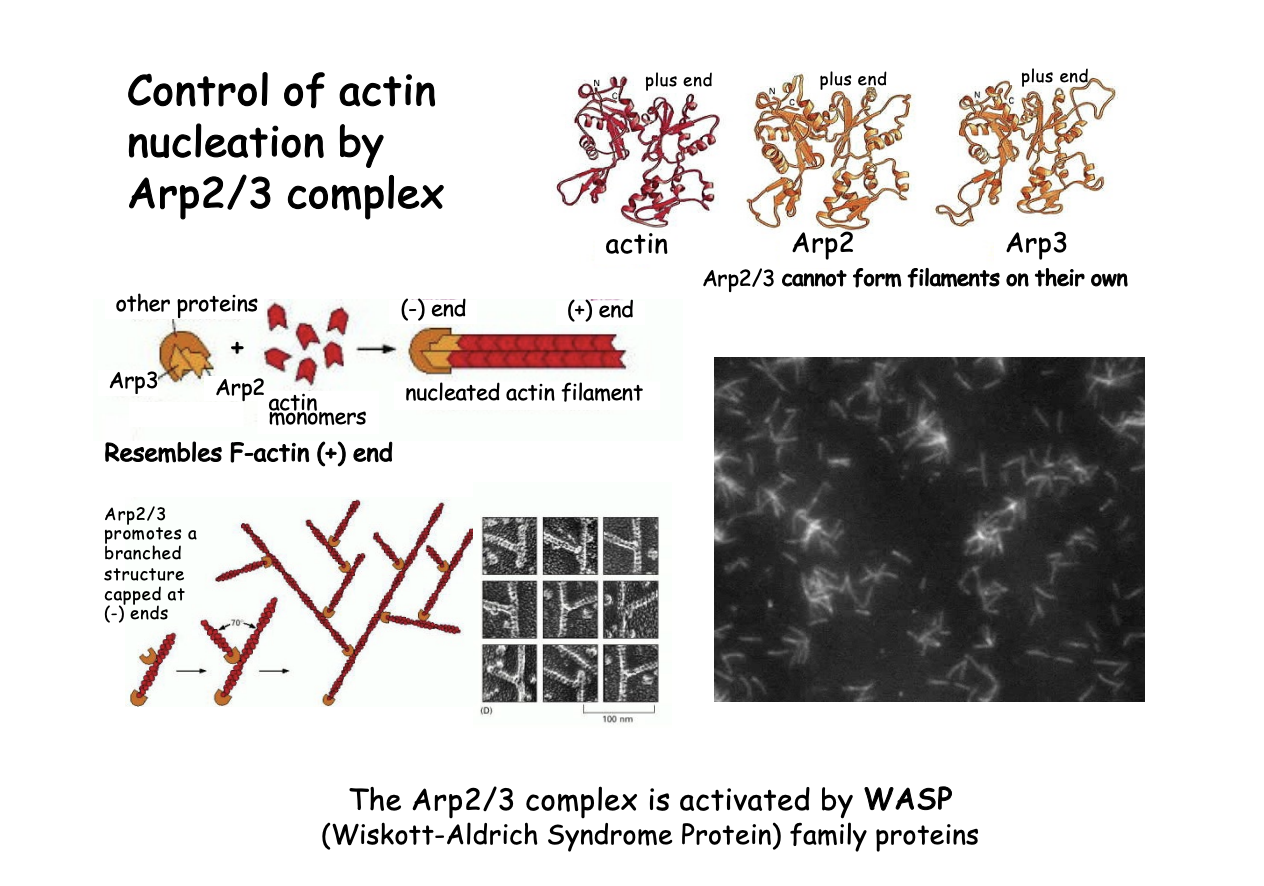
What activates the ARP2/3 complex in vivo
Associated with WASP (Wiskott-Aldrich Syndrome Protein)
family proteins
Nucleation-promoting factors
name→ genetic sex linked disorder
migration of actin function and tendency to malignancies
How it works
Signals
WASP released from an auto inhibitory conformation
Open conformation permits binding and activation to Arp2/3
Spire protein
may promote linear association of 4 actin subunits
to act as scaffold for polyermisation into an unbranched filament
may also cap the pointed end of the filament
Formins
Promote nucleation of unbranched filaments
dimer stabilises an actin dimer/trimer to facilitate the nucleation event
remain associated with growing barbed ends of filaments
through sequential binding and release interactions
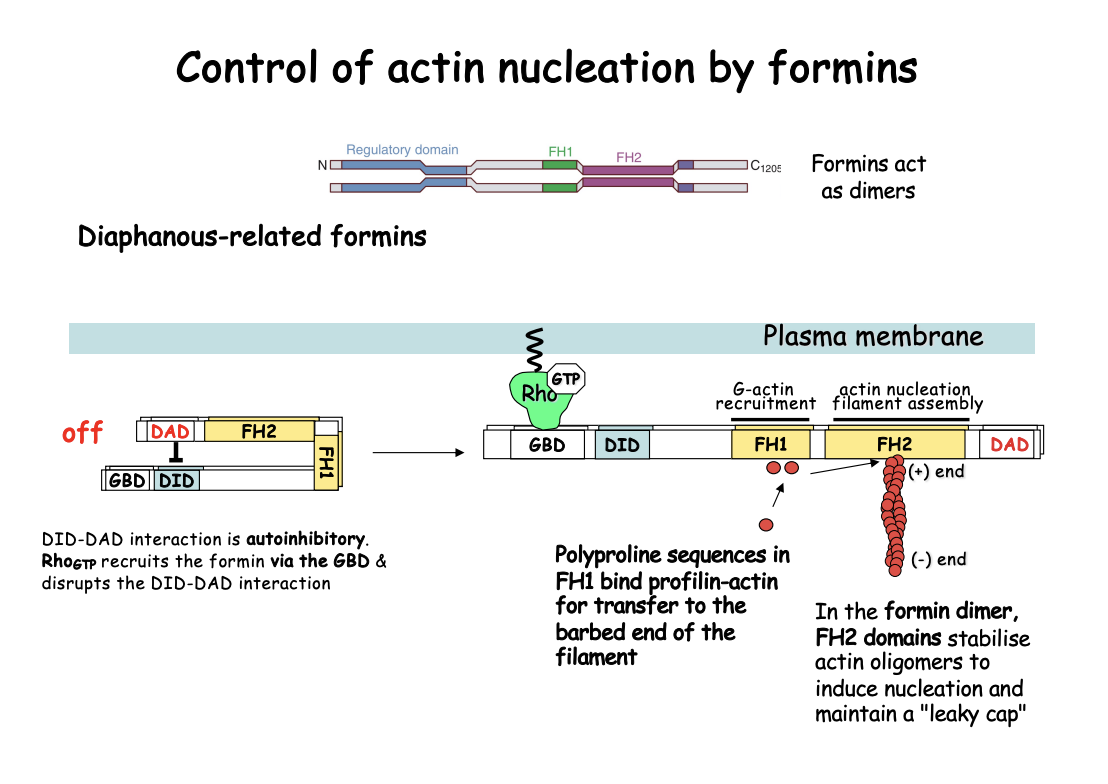
How do formins work→ e.g Diaphanous (Dia)-related formins
Act as dimers
Contain FH1 and FH2 signature domains
Adopt an open, active conformation upon recruitment by GTP-bound Rho-like GTPases
Act as dimers and nucleate from G-actin only→ producing linear filaments
How it works
FH1 domains recruit profilin-actin complexes (by polyproline sequences)
can increase the rate of elongation
i.e brings actin monomers to close proximity
FH2→ processively tracks the growing barbed end of actin filament
Protecting ti from capping proteins
i.e actively does the polymerisation
by causing a ring conformation that allows nucleation that be elongated
Stablise actin oliogmers to induce nucleation and maintain a leaky cap
note: this also involves APC
What is meant by the leaky cap on the barbed end
Formins act as as leaky caps at the + end
cap the end but still permit polymer elongation by allowing monomers to add on
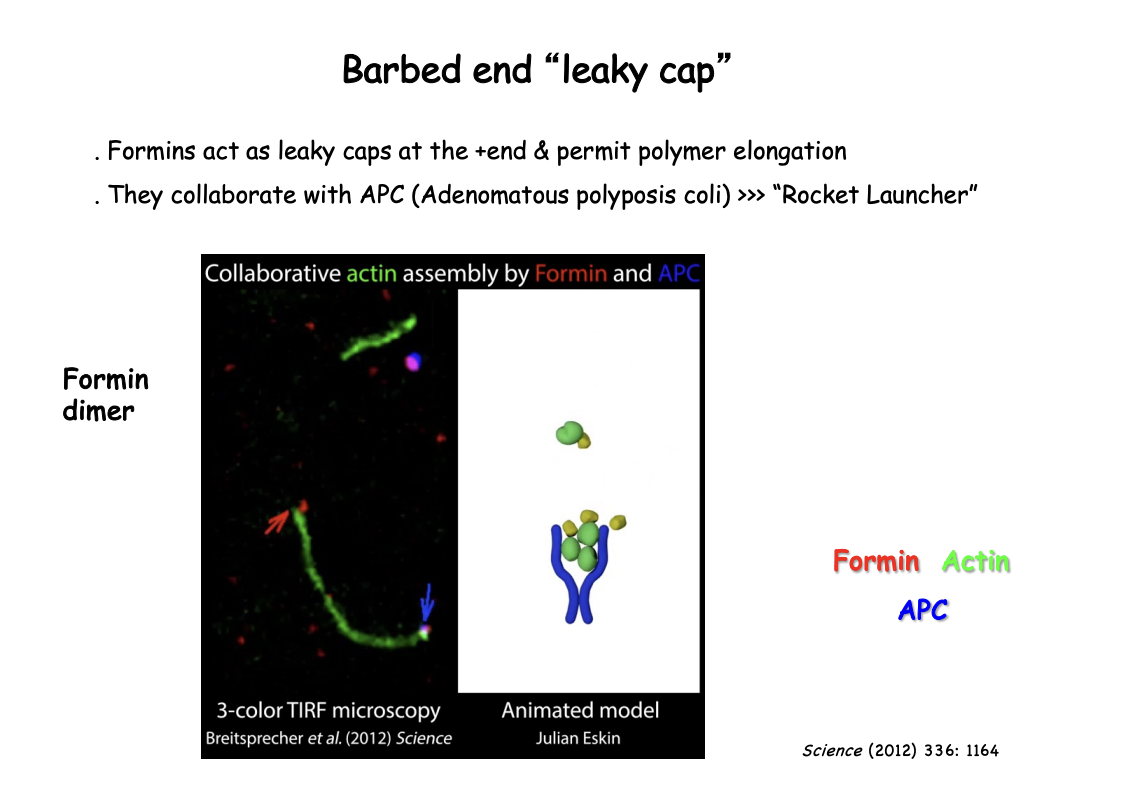
What is APC
Adenomatous polyposis coli
nucleation promoting factor
reamins at the base of where actin starts
collaborates with Formin
How APC and formin work together→ Rocket Launcher Model
Formin at the + end→ walks along to stay on the + end
APC→ remains at the base
helps aggregate the monomers and keep monomers together
Starts off the elongation
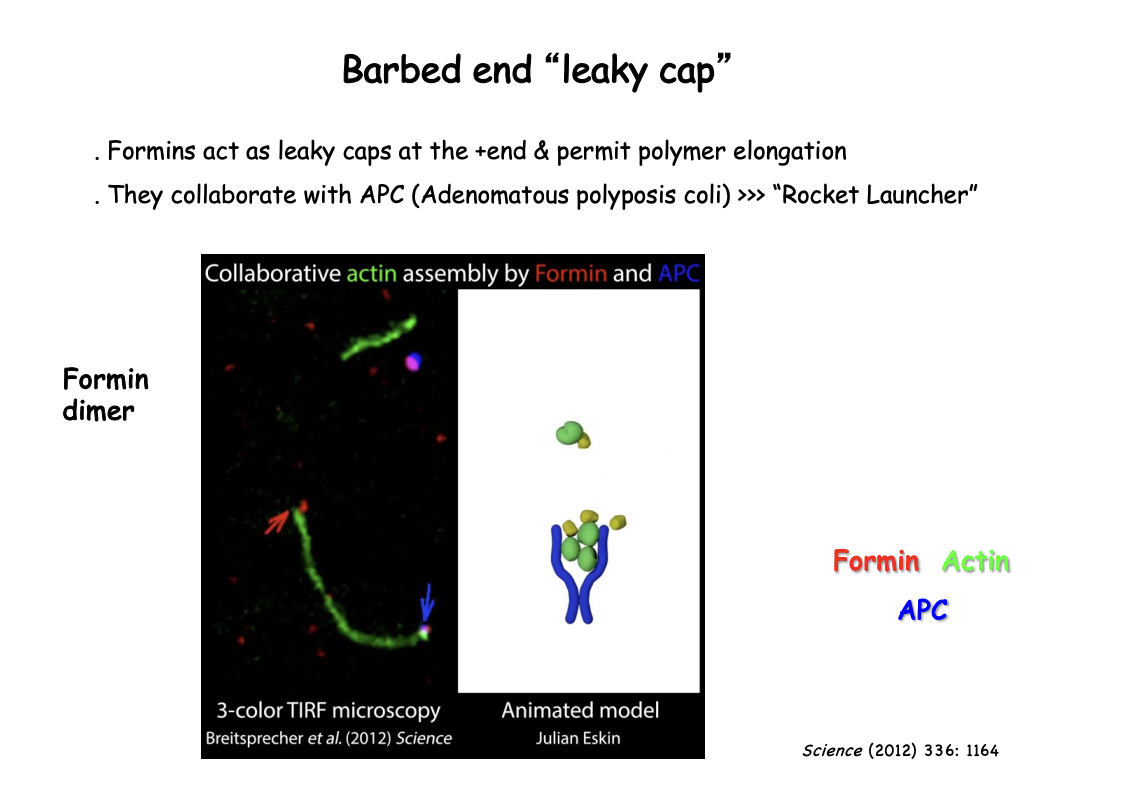
How was this visualised
Single-molecule formin association to the barbed end
followed in reconstitution experiments by total internal reflection fluoresence microscopy (TIRFM)
high resolution cryo-EM showed actin filaments bound to formins
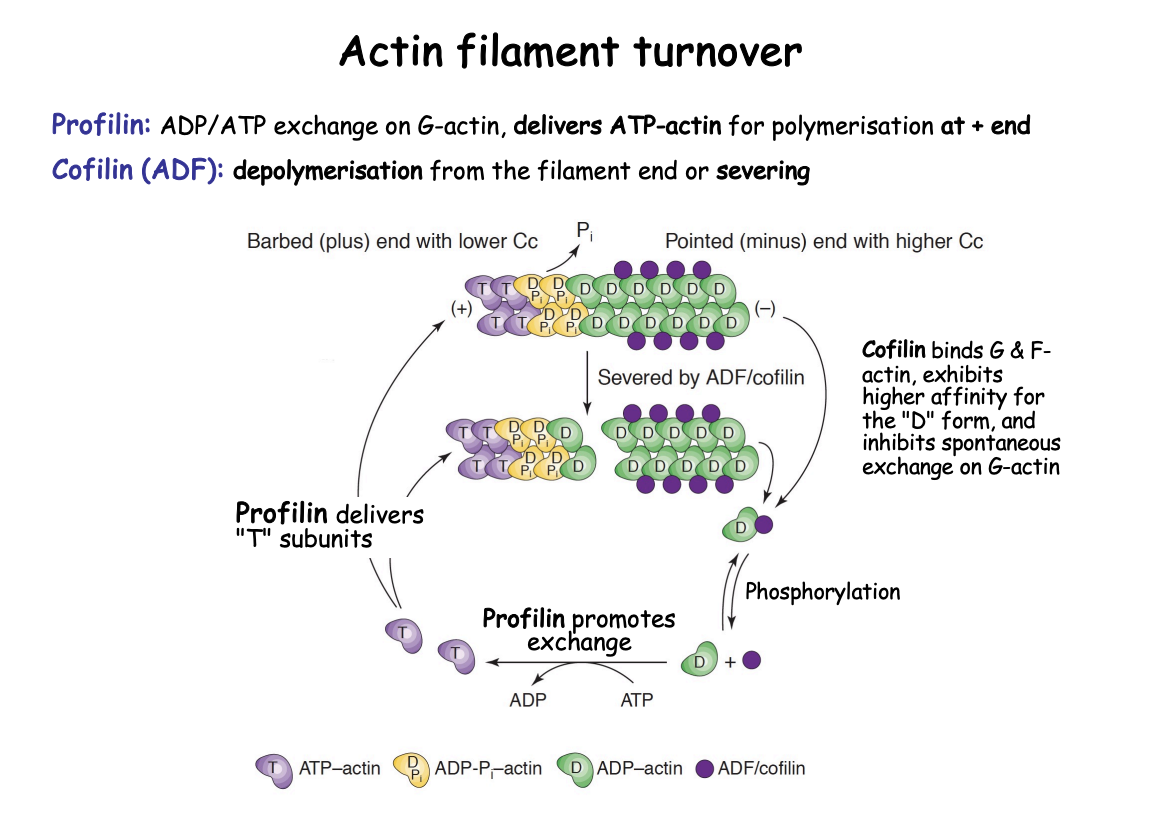
What is the actin filament turnover regulated by
Profilin
Cofilin (ADF→ Actin-Depolymerising factor)
How does the actin filament turnover work
Profilin (polymerisation)
Bind exclusively G actin
exchanges ADP→ ATP
allowing recycling of D subunits back into T subunits
T subunits delivered to + end as a profilin-actin complex
Bind to + end and flatten→ into D form?
Decrease Affinity for Profilin
Profilin released
Cofilin (De-polymerisation of older filaments)
Old filaments→ are in D form
Target D subunits (due to higher affinity) (in - end)
binds to the filament to drop D subunits and causes stress→ fragmentation (severing)
Accelerates turnover of filaments
Cofilin phosphorylated→ releases D subunits
once D monomers released→ can be re used again by the action of profilin
Cofilin also blocks polyermisation at the - end
by inhibiting spontaneous ADP>ATP exchange on G-actin
What does binding to PIP2 or to the formin FH1 domain help do
modulates locally profilin delivery of actin monomers
to sites of polymerisation near the plasma membrane
What are motor proteins
Mechanochemical enzymes
move uni-directionally along a cytoskeletal track
by coupling ATP hydrlysis with specific conformational changes
The direction of the movement is dictate by
the motor properties
polarity of the track
Features of motor proteins
Motor domain/ head region
has ATP and track binding sites
hydrolyses atp
cycles between sates in which binds and releases filament→ causes walking
The structure of the motor domain dictates the
Choice of cytoskeletal filament
Direction of movement
Tail Region
interacts with a ‘cargo’
determines the specific biological function of the motor protein
depending on what cargo it binds to
One type of motor protein→ actin based
Myosins
Superfamily→ 18 members
First found myosin 2 (in muscles) but found to be in all cells
What they do:
Use energy fromATP hydrlysis to move along actin filaments
Role they play:
carry organelles along actin tracks
cause adjacent actin filaments to slide pas each other in contractile bundles
In which direction do myosins move
Move towards the + end
(except type VI)
Example 1: Myosin II→ structure
elongated protein
two heavy chains
globular head at N-terminus→ force generating machinery
coiled coil tail→ mediates heavy chain dimerization
bundles itself with the tails of other myosin molecules
two essential light chains
bind close to the head domain
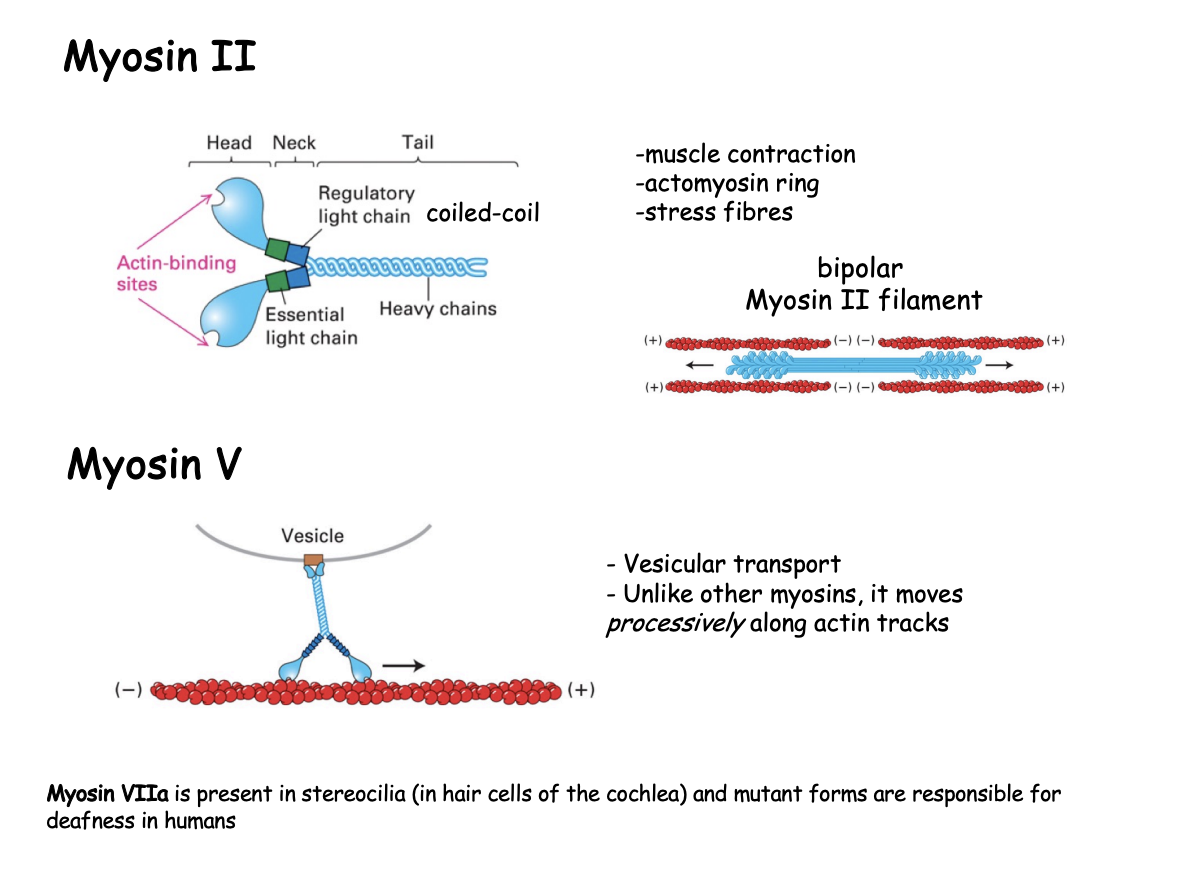
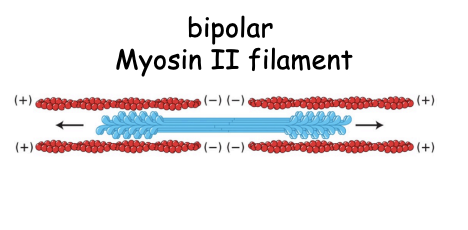
What does the bundling of the myosin tails do
generates bipolar ‘thick filaments’
with several hundreed myosin heads
orientated in opposite directions at the two ends of the thick filaments
allows contractile acitivty due to directionality
Role of myosin II
contractile activity in muscle and non-muscles cells
cytokinesis→ (as component of actomyosin ring)
cell migration→ Forward translocation of the cell body
How does the fibrous tail help with contraction
formation of filaments with heads pointing away from centre
a bipolar strucutre than can drive contraction by promoting the sliding of actin filaments past each other
as the heads both move towards polar ends
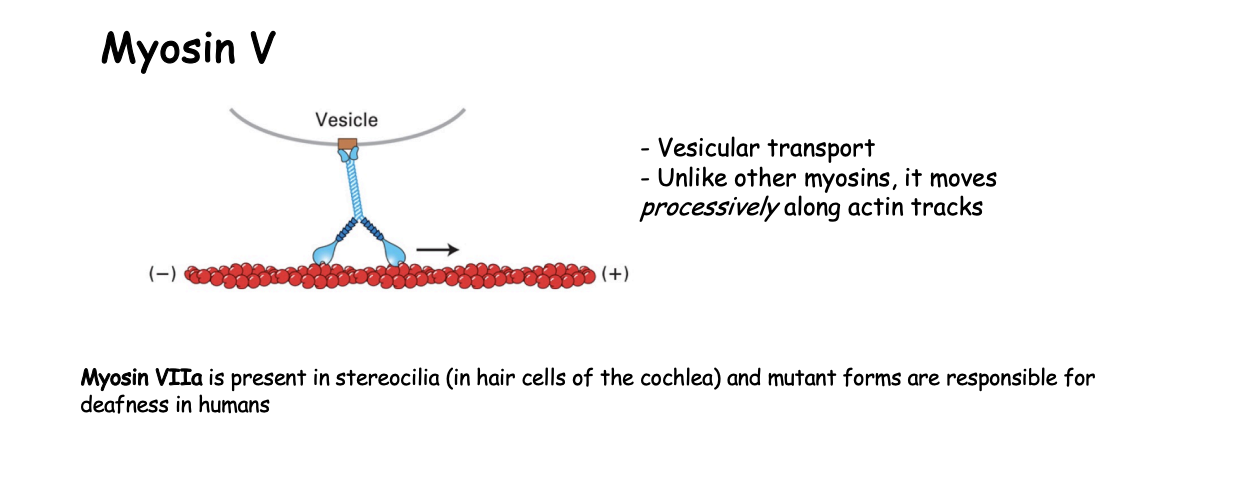
Example 2: Myosin V what is it involved in
vesicular transport
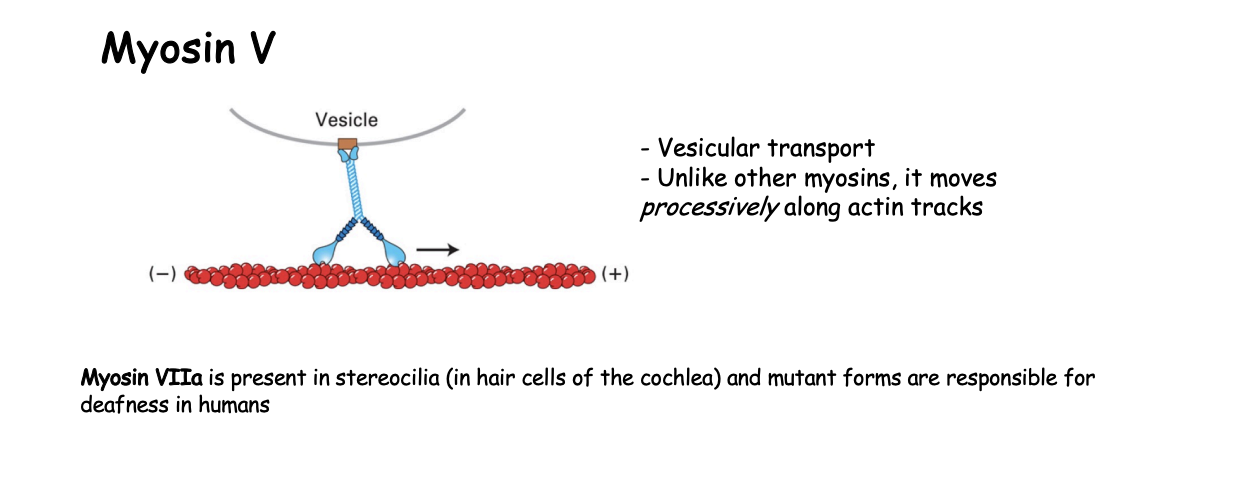
How does Myosin V work
continuous hand-over-hand fashion along filament
processive motor that travels long distances without detaching from the track
must make sure that it is coordinated movement so that there is always one head atached→ ensures it does not fall off
ATP cycle in a continuous manner
only lets go when at destination
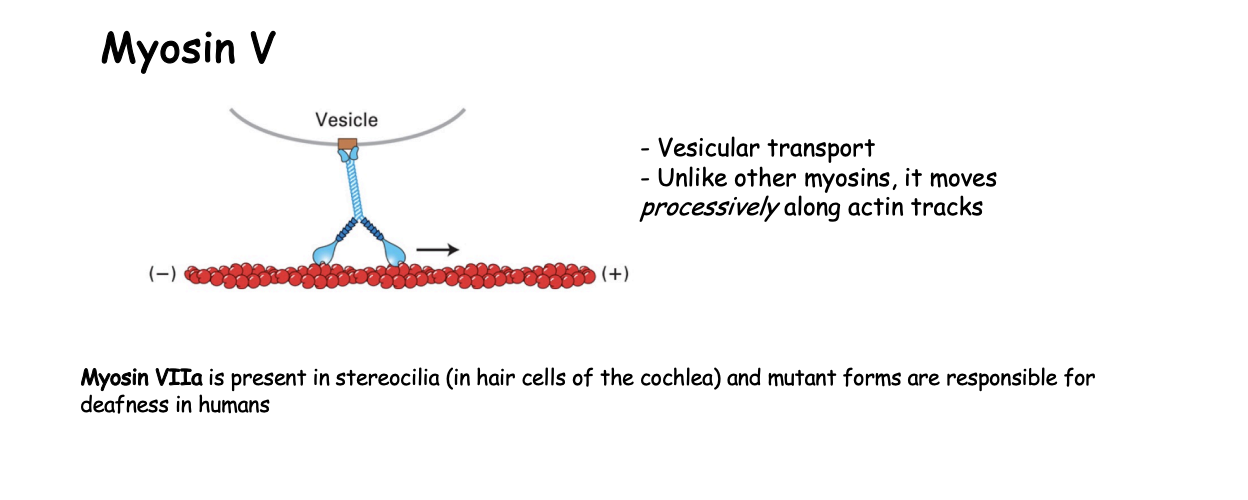
Myosin V compared to Myosin II
Myosin II spends a major fraction of its cycle in a detached state
around 300 motor heads for rapid sliding of actin filaments as the muscle is signalled to contract
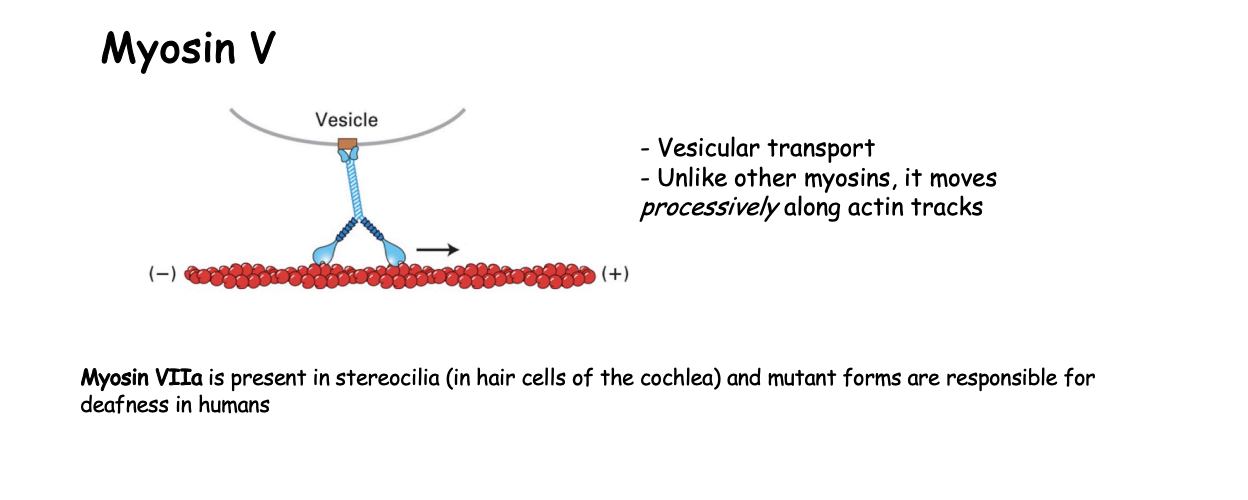
Example 2.1→ Myosin VIIa where present
stereocillia
in hair cells of the cochlea
mutants→ responsible for deafness in humans
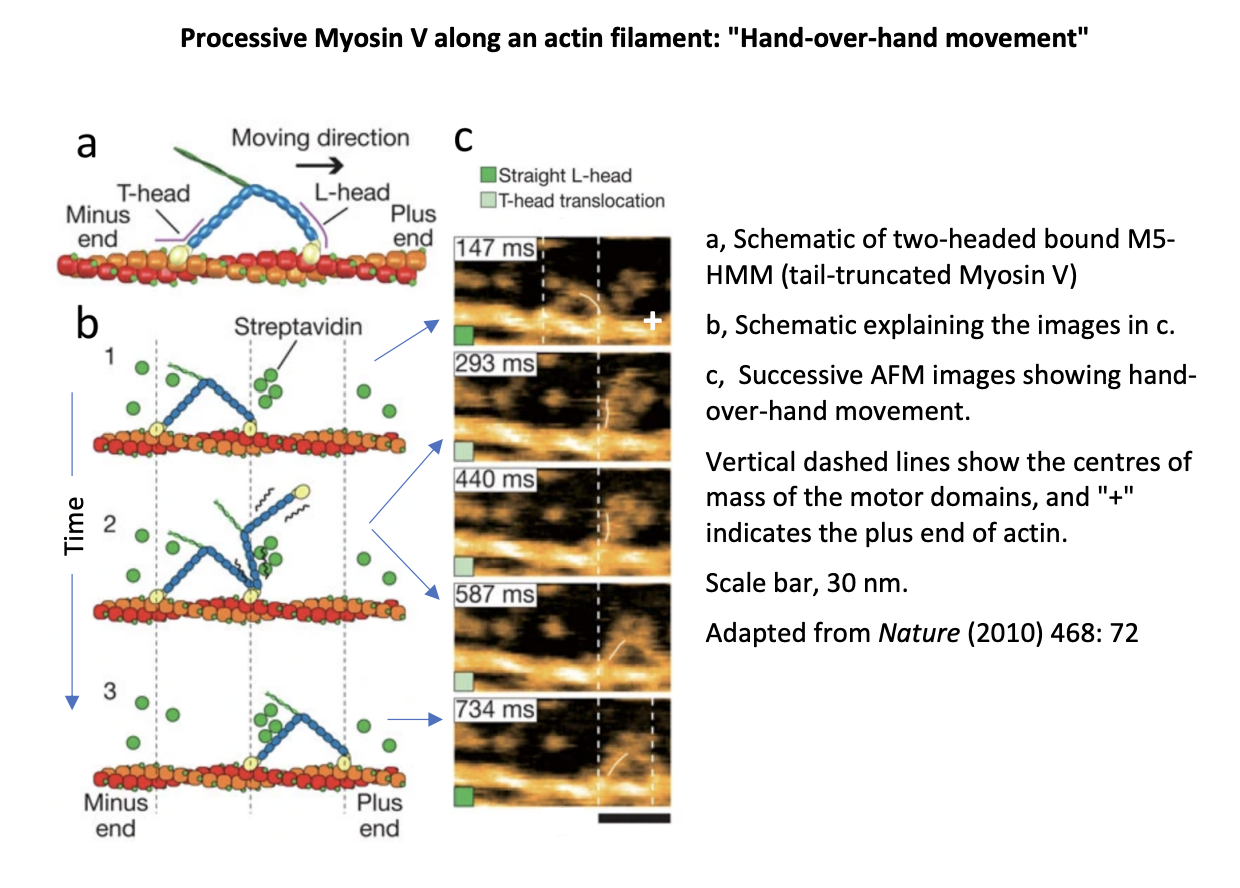
How to do video imaging of walking myosin V
High-speed atomic force microscopy
single-molecule fluoresence miscroscopy (e.g using TIRFM- total internal reflection fluorescence microscopy)
observe individual fluorescent spots (from fluorophore attached to a protein)
not the proteins themselves
Compared to other methods (e.g EM or X-ray crystallography)
show only a static view
high-speed atomic (AFM)→ direct observation of the structure and dynamics of biomolecules simultaneously
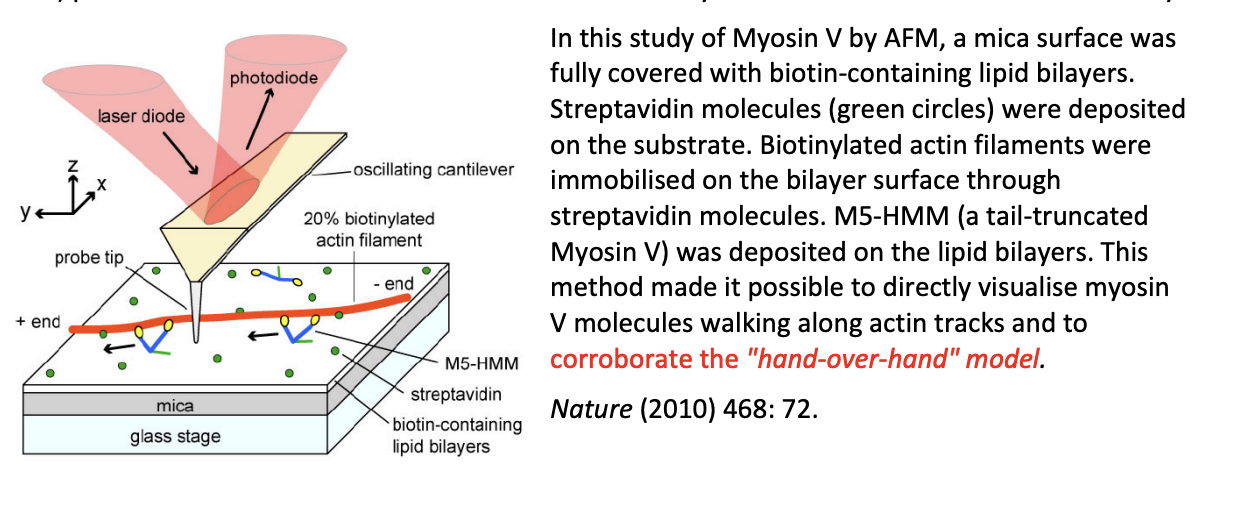
Example of imaging myosin V by AFM
mica surface sully covered with biotin-containing lipid bilayers
Streptavidin molecules (green circles) deposited on the substrate
biotinylated actin filaments immobilised on the bilayer surface through streptavidin molcules
M5-HMM (a tail-truncated Myosin V) deposited on the lipid bilayers
Overall: Made is possible to directly visulaise myosin V molecues walking along actin tracks and to corroborate the ‘hand-over-hand’ model
Actin polymerisation-Driven Motility: why is cell migration important
needed for
development→ growth cones
repair→ fibroblasts to repair wounds, osteoclasts to reach sites for bone remodelling
defence processes→ WBC
spread of tumour cells→ metastasis
almost all cell locomotion occurs by actin-driven crawling
except sperm cells
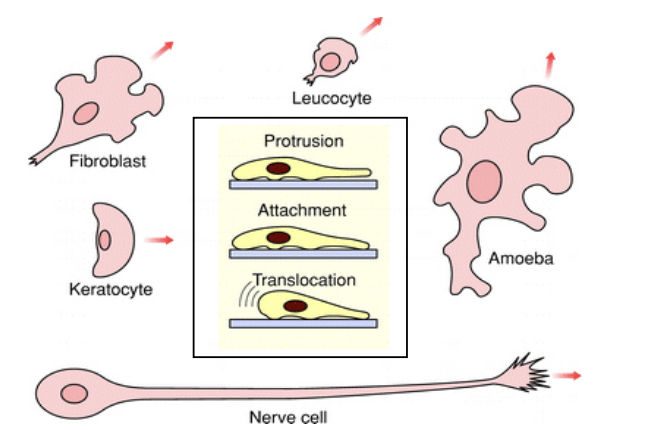
Principles of cell movement
protrude a front
attach it to its substrate
retreat its rear
All driven by actin cytoskeleton
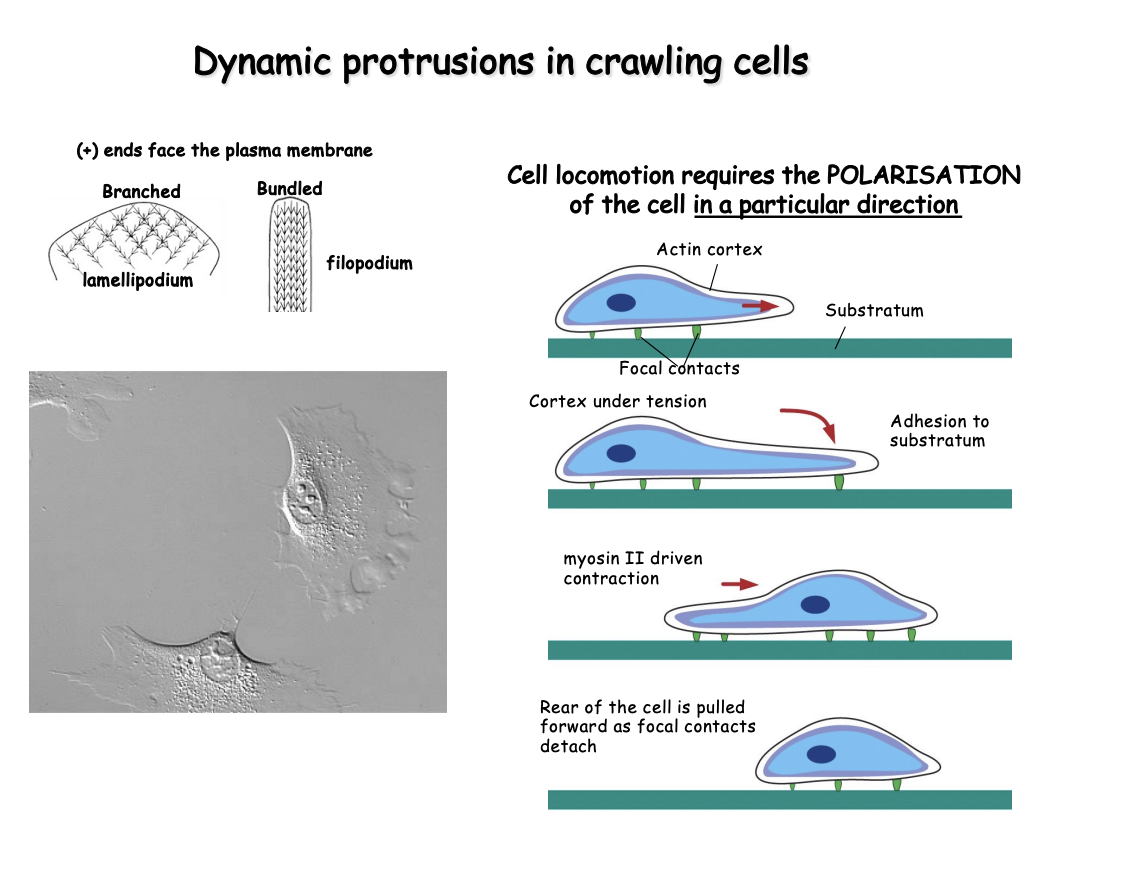
What does the cell need to be for movement and how
Cell must be polarised→ so it can persistently extend projections on one edge and reduce its net protrusive activity elsewhere
How→ Barbed + end features
Lamellipodium→ branched
Filopodium→ Bundled
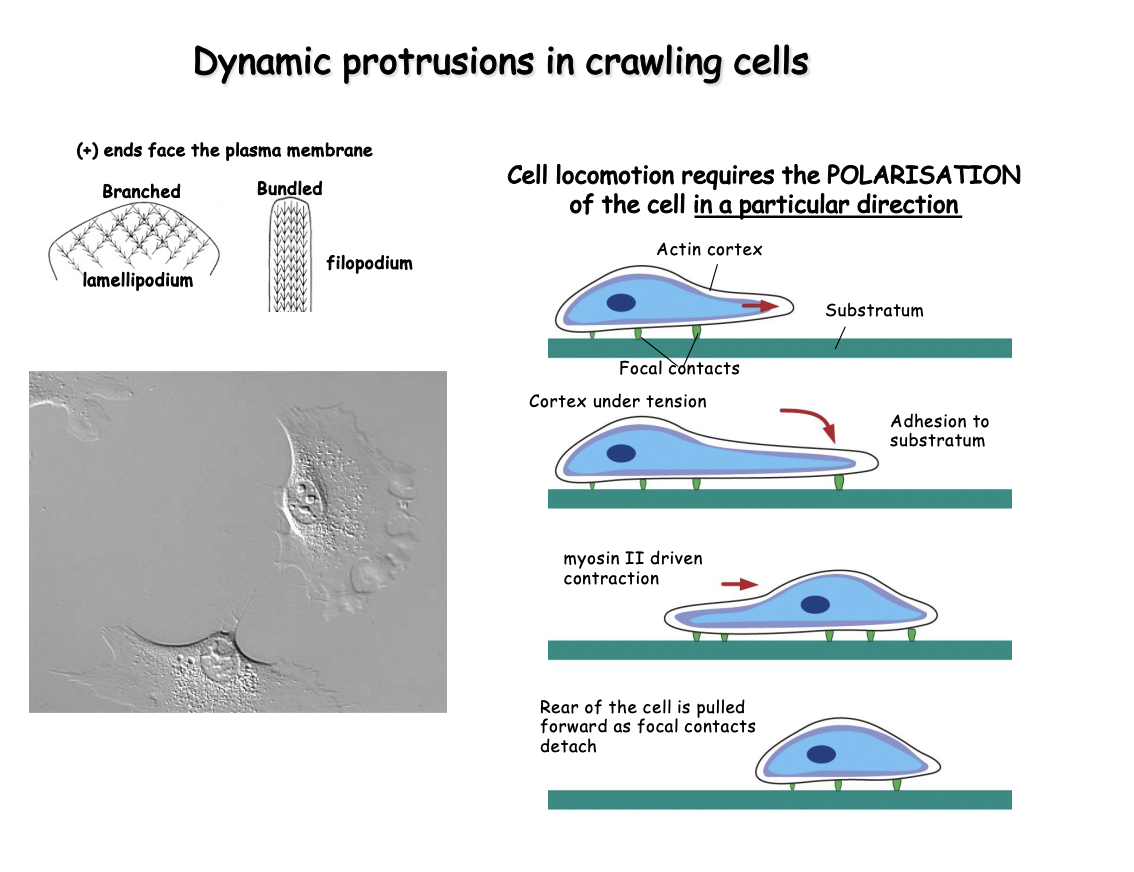
Process of how a cell moves
Protruding either
thin leaflet of cytoplasm→ lamellipodium
Finger-like projection→ filopodium
(based on polymerization of an actin filament network
Actin and myosin make stress fibres
contractile pundles ending at focal contacts
Retraction of the rear of the cell driven by myosin II
Reorgnaisation of the actin cytoskeleton during crawling is coupled to cel adhesion to the underlying substratum
Adhesion is achieved by
linkage of parts of the actin cytoskeleton to transmembrane receptors
for extracellular matrix (integrins)
at specialized focal contacts
these must be dissolved from the back→ to ensure continued in the forward direction
Note: when observing movement and then mitosis
shows that during mitosis→ movement stops
suggsts→ the two processes clash
This hints to how in meiosis and the making of oocyte→ how it must be seriously regulated
ensure not moving at the same time as meiosis or embryo
The cells are polarised but what directs the movement?
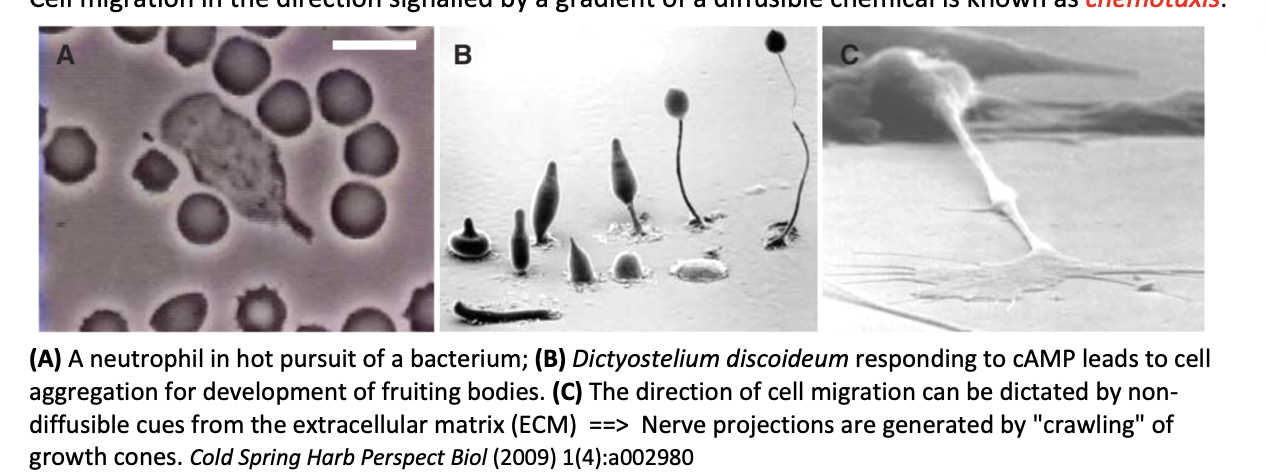
Chemotaxis
direction signalled by a gradient of a diffusible chemical
Non-diffusible cells
Examples of chemotaxis
Neutrophil in hot pursuit of bacterium
Dictyostelium discoideum responding to cAMP→ leads to aggregation for development of fruiting bodies
Neutrophile migration towards the site of a wound in a zebra fish larva
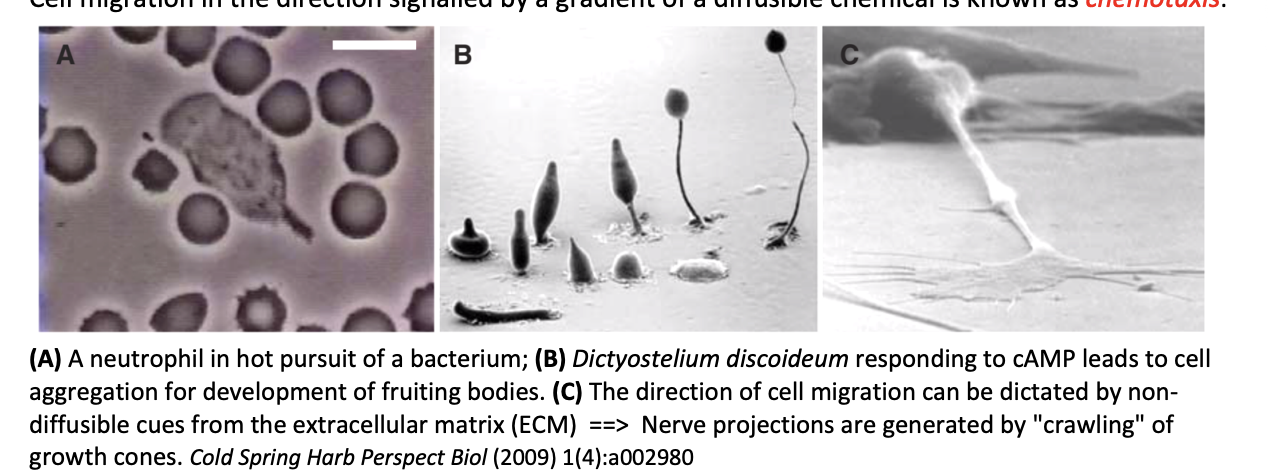
Direction of cell migration by non-diffusible cues
from the extracellular matrix
example:
nerve projections are generated by ‘crawling’ of growth cones
Outstanding question
Chemotaxis is good for short distances
but how does it guide cell migration over long distances
e.g during embyronic development
e.g spreading metatstic cancer cells
and complex paths branched paths
Does chemotaxis allow for this?
gradients over long distances would be too shallow for guidance
What is the proposed explanation as to how cells can do this then?
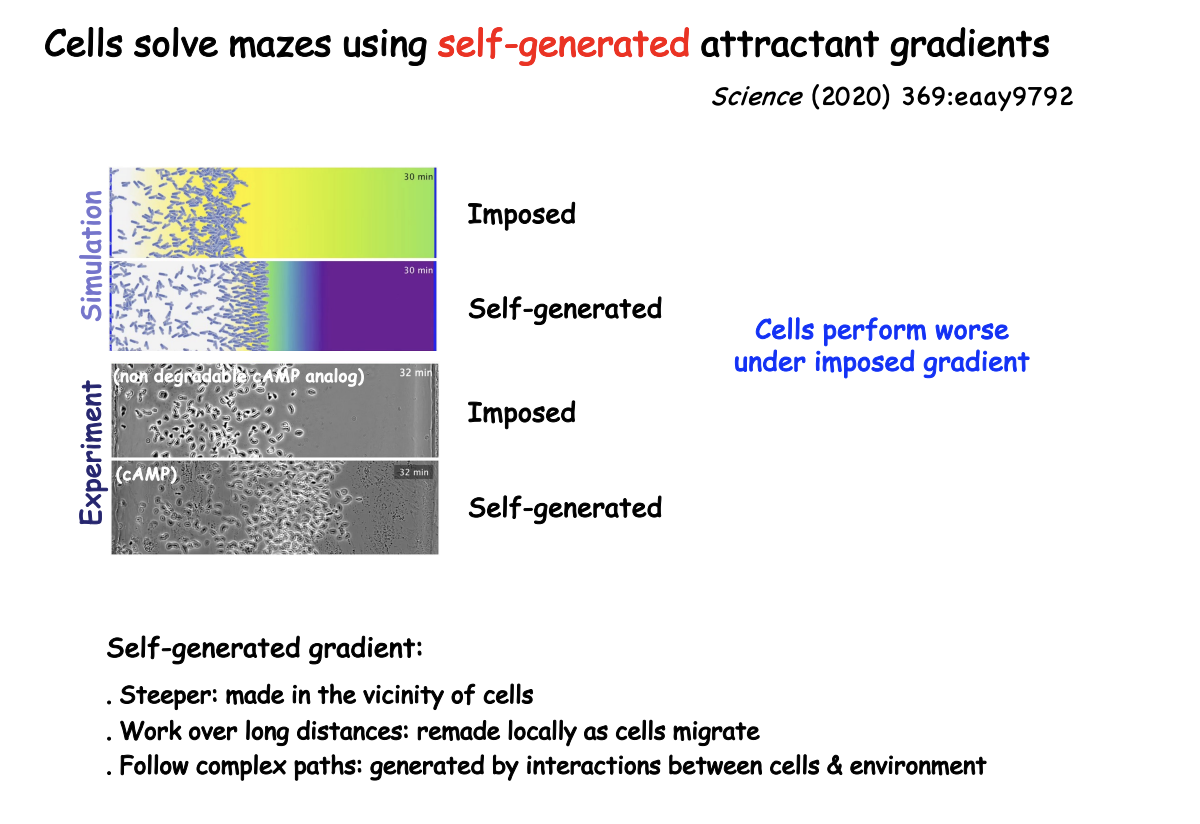
Self-generated gradient
cells may break down attractant
by cell surface enzymes or depleted by receptor-ligand endocytosis
remains sufficiently steep near the cell to sustain long-distance migration
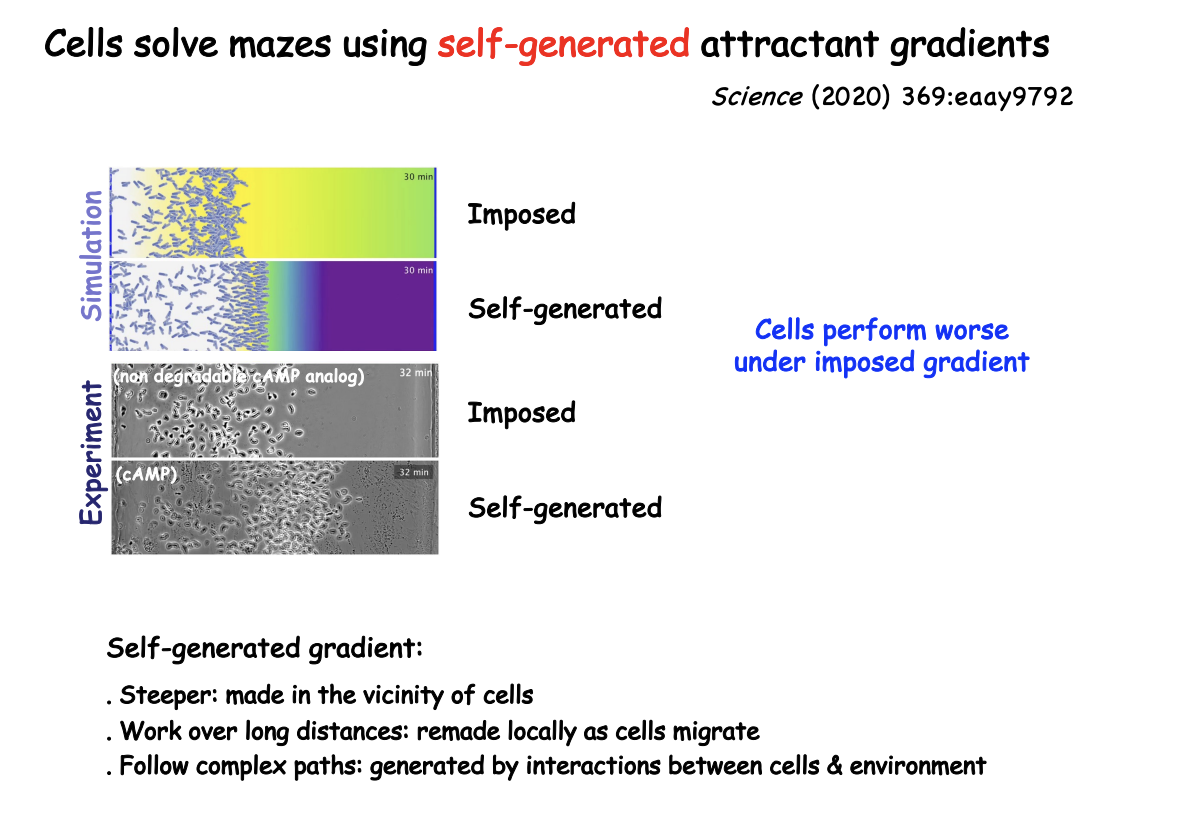
Why are self-generated gradients advantageous
steeper→ made in vicinity of cells
Work over long distances→ remade locally as cells migrate
Follow complex paths> generated by interactions between cells and environment
This has been proven by
modelling and simulations
factoing into attractant depletion and diffusion
What did it predict
self- constructed chemoattractant gradeints may allow cells to navigate complex pathas
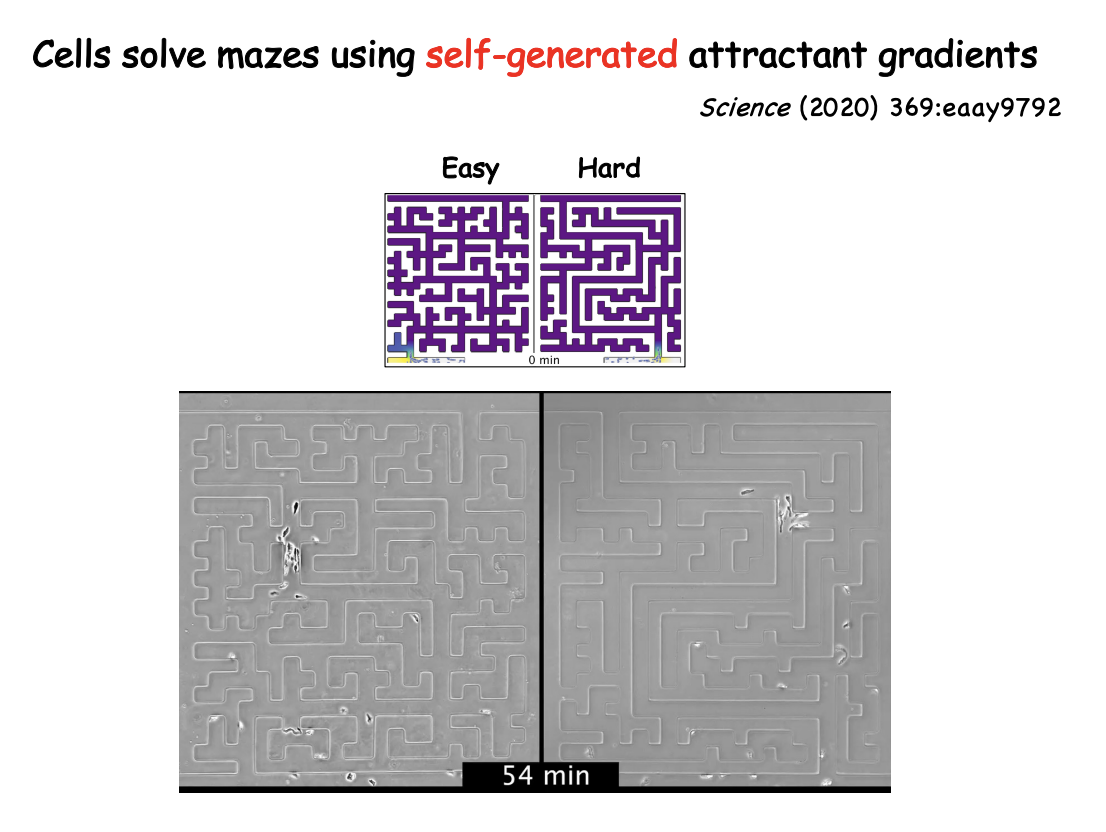
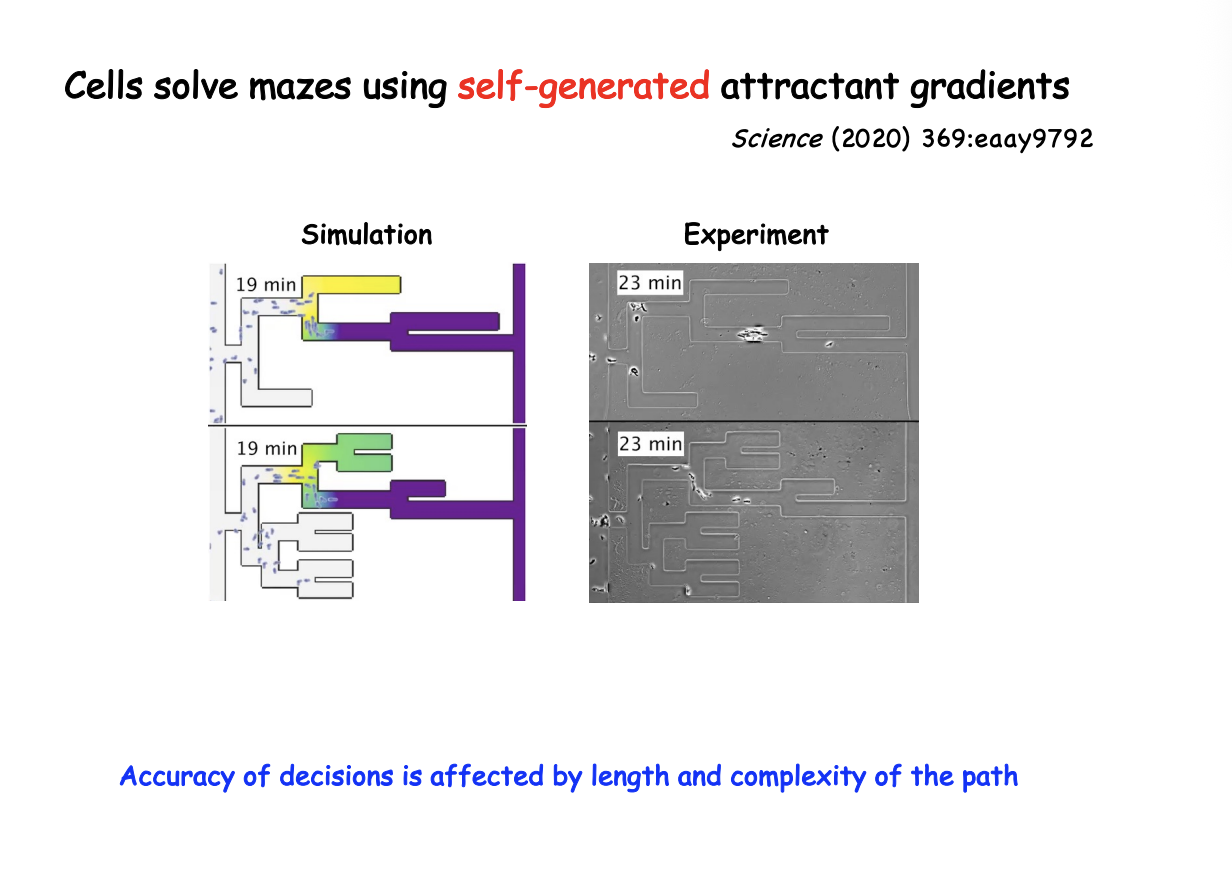
How was this tested
Microfluidic mazes of variable complexity
Dictostelium cells led by cAMP self-generated gradient
cancer cells directed by LPA (lysophosphatidic acid)
could solve mazes→ sense upcoming junctions, accurately choose live channels over dead ends and identify optimum paths as shown in the figure
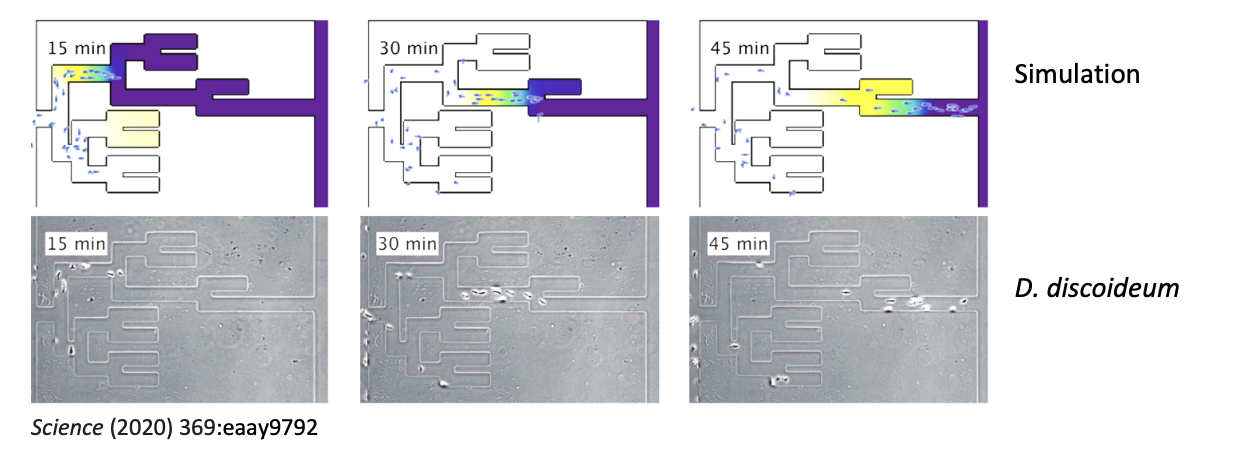
Add more info on the trakcs pls
Now that we have the signals, how do they get tansduced to direct movement→ what they use
Rho-like GTPases transduce signals to promote the polarised organisation of specific actin structures
Rho, Rac and Cdc42→ members of the Ras superfamily of small GTPases
21kDa proteins
with weak intrinsic GTPase activity
act as molecular switches to signal transduction at the plasma membrane
by cycling between GTP and GDP bound forms
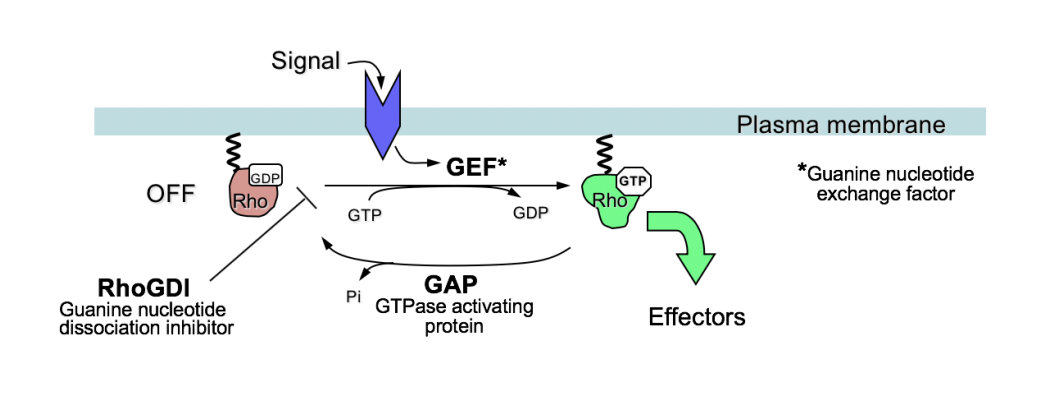
Why is GTPase acitivity significant for this
Small GTPases are central in transducing external signals or internal cues to effector ABPs
to impart directionality to cytoskeletal structures
Also underlie the basis of pattern formation and embryonic development asymmetric partition of fate determinants or organelles in polarised asymmetric divisions
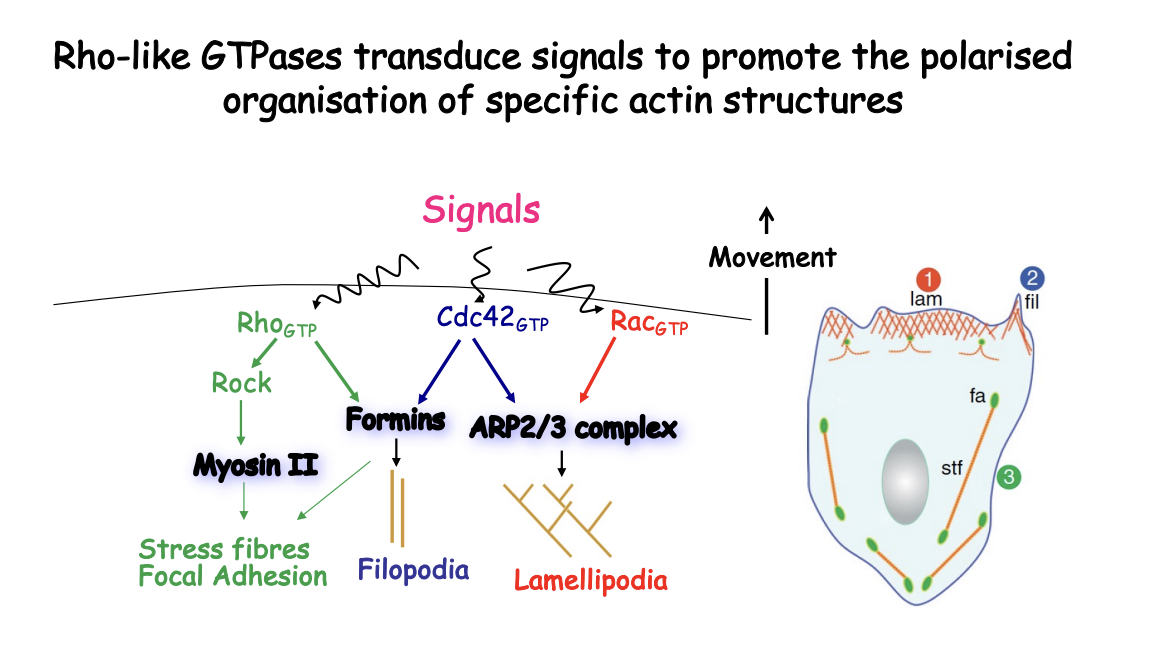
How do Rho-like GTPases transduce signals to promote the polarised organisation of specific actin structures
Directional migration arises from the asymmetrical activation of small GTPases at the cell surface according to the extternal single gradent
When GTP-bound→ Induce distinct membrane protursions:
Rac activate Arp2/3
causes Lamellipodia (branched F-actin networks)
Cdc42 activate Arp2/3
causes filopodia (linear bundles)
Rho promtes contraction by activating actin organisation by formins and myosin II
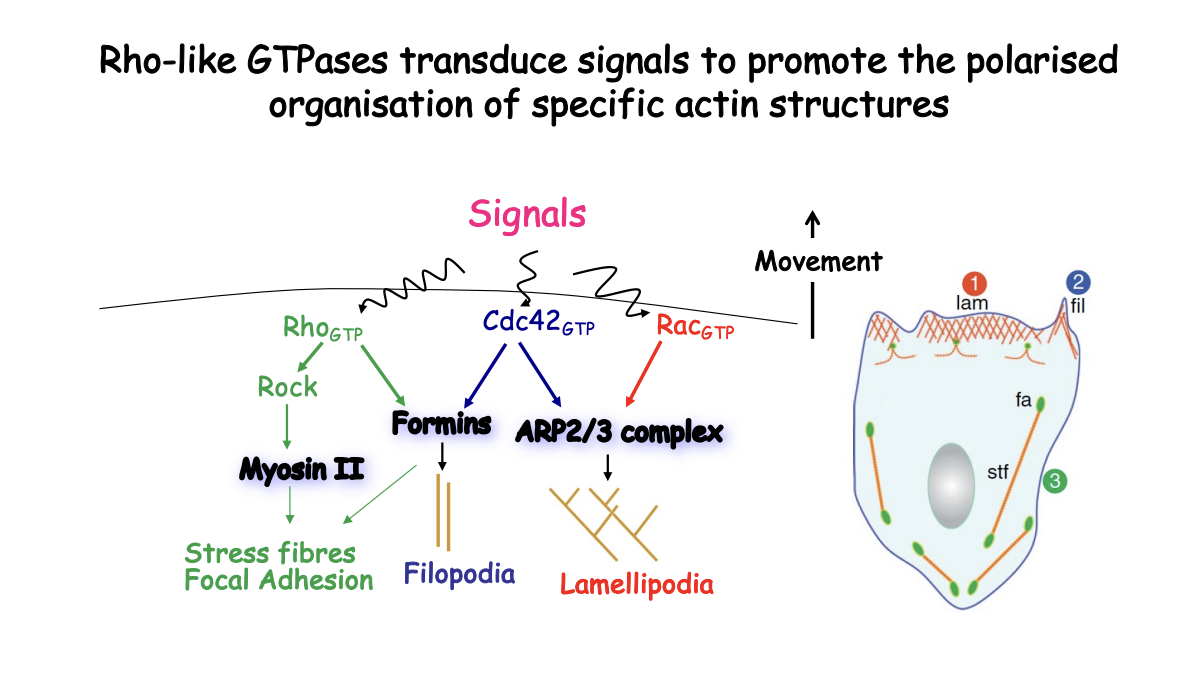
What happens when signal is received and Rho GTP is made
MLC→ two regulatory light chains of MLC that polymirses into thick filaments associating with actin filaments
RhoGTP
Activates Rho kinase (ROCK)
ROCK phosphorylates both MLC and MLC phosphatase (inactivating it)
MLC phosphorylation= myosin II filaments can slide along actin filaments and exert contractile force
Therefore: ROCK drives contraction both by activating MLC phosphoylation and inhibiting its deposphylation
Forms stress fibres
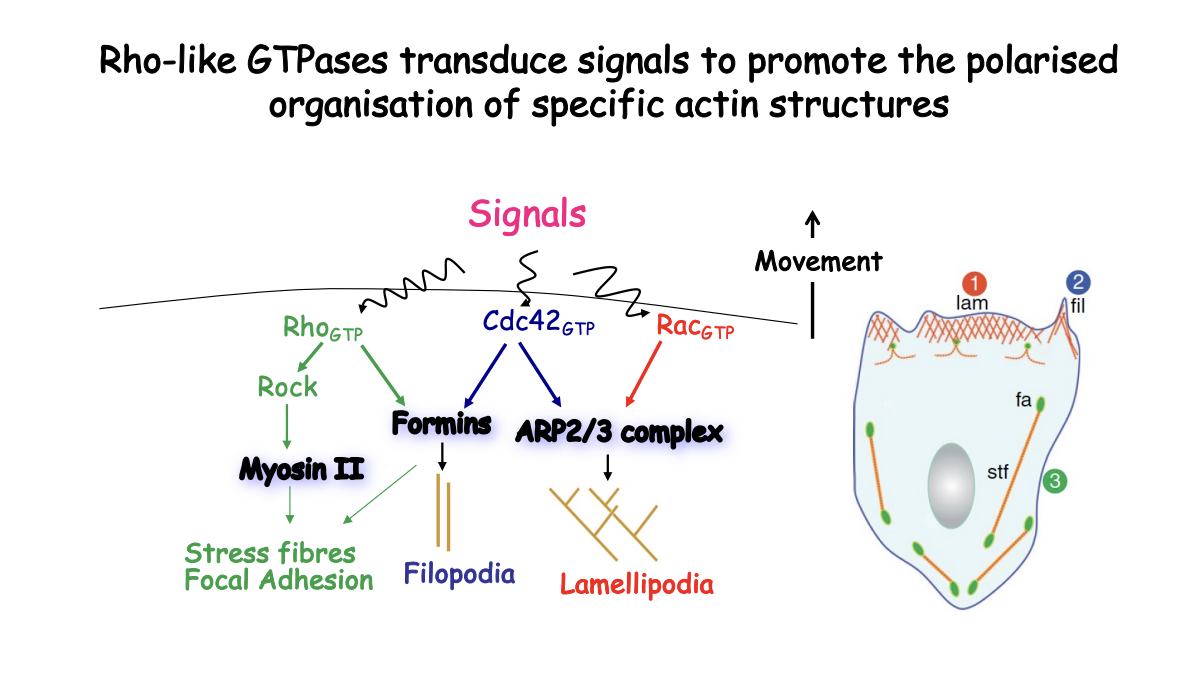
How is focal-adhesion developed and maintained
linked to myosin-II induced contractility
stimulated by Rho
With traction at focal contacts to transport the cell body forawrd
Therefore: directional movement is coupled to polaried adhesion-site turnover as well
Overall what do the Rho-like GTPases do
Lamellipodia
Filopodia
Stress fibres and focal adhesion
Extracellular signals that locally activate Rho, Rac and Cdc42 as plasma membrane trigger what
highly polarised F-actin nucleation
therefore→ promoting the ‘leading edge’
Branched (Arp2/3) and linear formins F-actin polyers aim their + ends towawrd the leading edge
further back in the cell→ as actin filaments age, cofilin cataluses depolymerisation
results in continuous treadmilling of actin subunits from front to back coupled to progression forward of the leading edge as shown in the model (below)
docal contacts also follow an asymmetric pattern
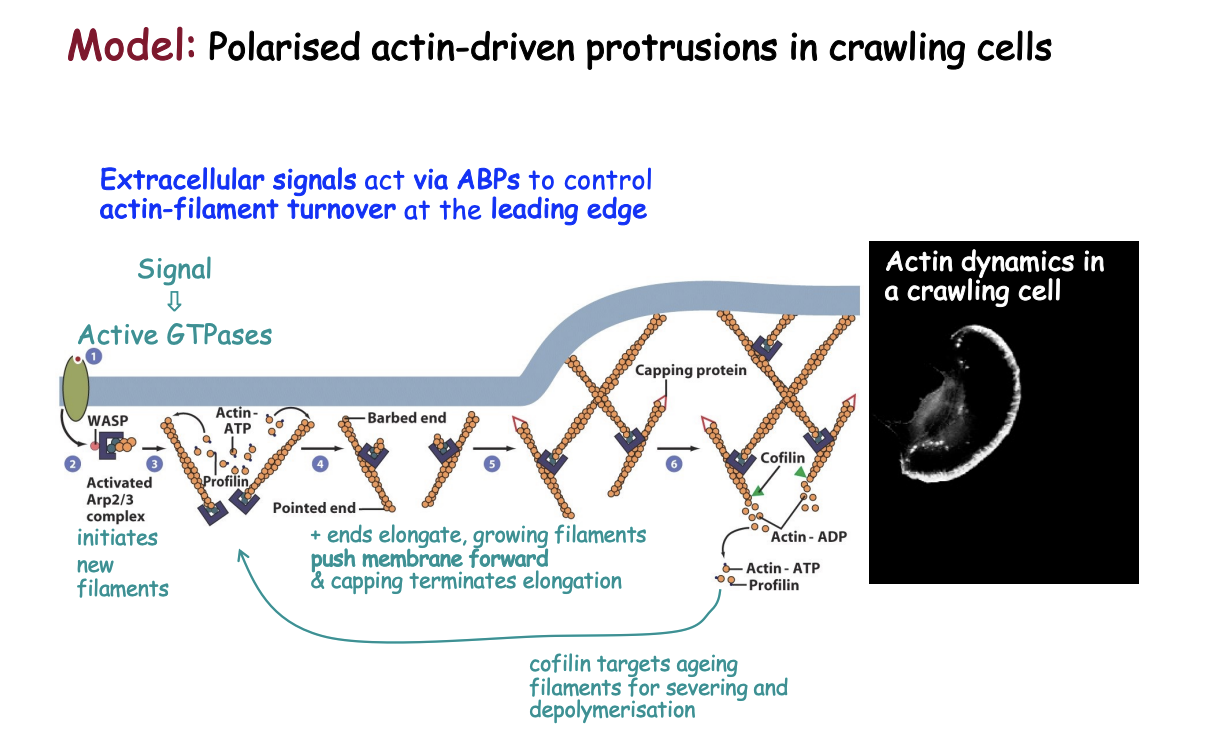
Model: Polarised actin-driven protrusion in crawling cells: the actin network at the leading edge of motile cells may consist of
a branched array of F-actin pusing the plasma membrnae
with + ends either facing or reaching tangentially the mmebrane
- ends making Y-junctions with other filaments linked by Arp2/3 complex
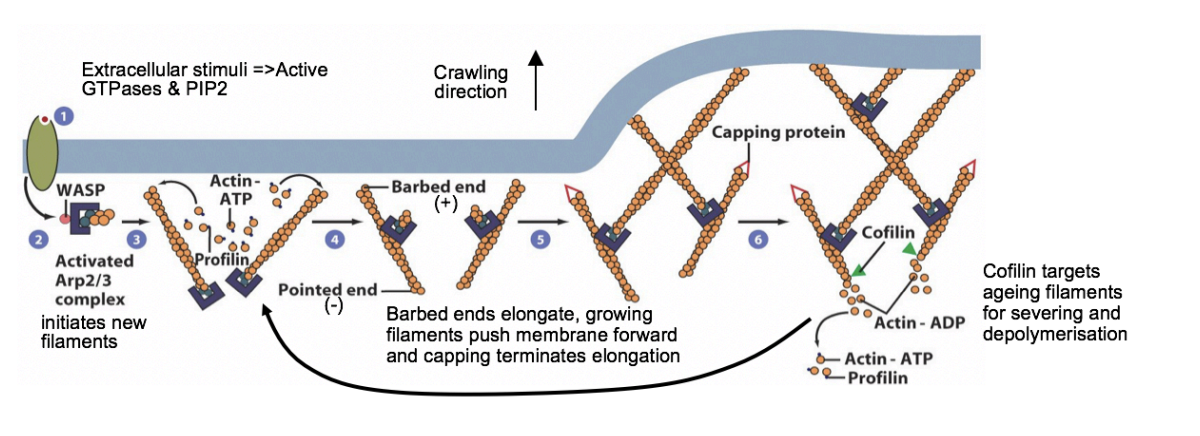
MODEL: What happens when stimulants bind to the plasma membrnae
bind to receptors activating signalling pathways via small GTPases
activate WASP and related proteins by freeing autoinhibition
activates Arp2/3 complex
Arp2/3 initiates a branch on the side of an existing filament
new filaments grow rapidly at the + end, fed by profilin-bound actin
Capping proteins bind to the growing ends, terminating elongation
Small GTPases also activate formins to form filopodia
As filaments age→ cofilin binds to ADP-actin subunits and severs or depolymerises the ADP filaments
Profilin re-entes the cycle at this point→ promoting ADP-ATP exchange
Rho family GTPases also activate p21-activated protein kinase PAK
stimulates LIM kinase to phosphorylate and inhibit cofilin
Strong support to this model comes from
biochemical and
EM studies
what was shown:
ability of Arp2/3 complex to promote actin filament formation by generating branch points on existing actin filaments
demonstrated in vitro asays
acitivty has been correlated with EM images of cytoskeleton
show a richly branched actin network underlying the protruding plasma membrane
Protrusive activity may also play an important role in
sampling gradients for guidance
However→ certain aspects of this dendritic network model have been challenged by studies based on
electron tomography
and
alternative fixation protocols
What has been questioned
The importance of Arp2/3-dependent nucleation in promoting the network (relative to alternative actin nucleators such as formins)
controversy hinged on the existence and density of branch points in the network
and relative stability of branched strucutres
as later studies detected fewer branches and indicated instead the the presence of long linear fibres
Overall consensus
actin filaments grow at the lemellipodium tip
forming a treadmilling network
with filaments polymerising at the front and under continuous turnover toward the rear of the lamellipodium
Contrasting views regarding the mechanistc impact of filament severing by cofilin on the dynamics of the actin network
View 1→ indirect contribution to treadmilling by increase in actin monomer pool
broought about by depolymerisation
view 2→ cofilin stimulates network formation through its severing activity that may give rise to short filaments
providing novel sites for initiation of Arp2/3-dependent actin branches
it follows that the detailed molecular action of the various players in setting up this strucutre is far from understood
Actin organisation in cells→ key concepts
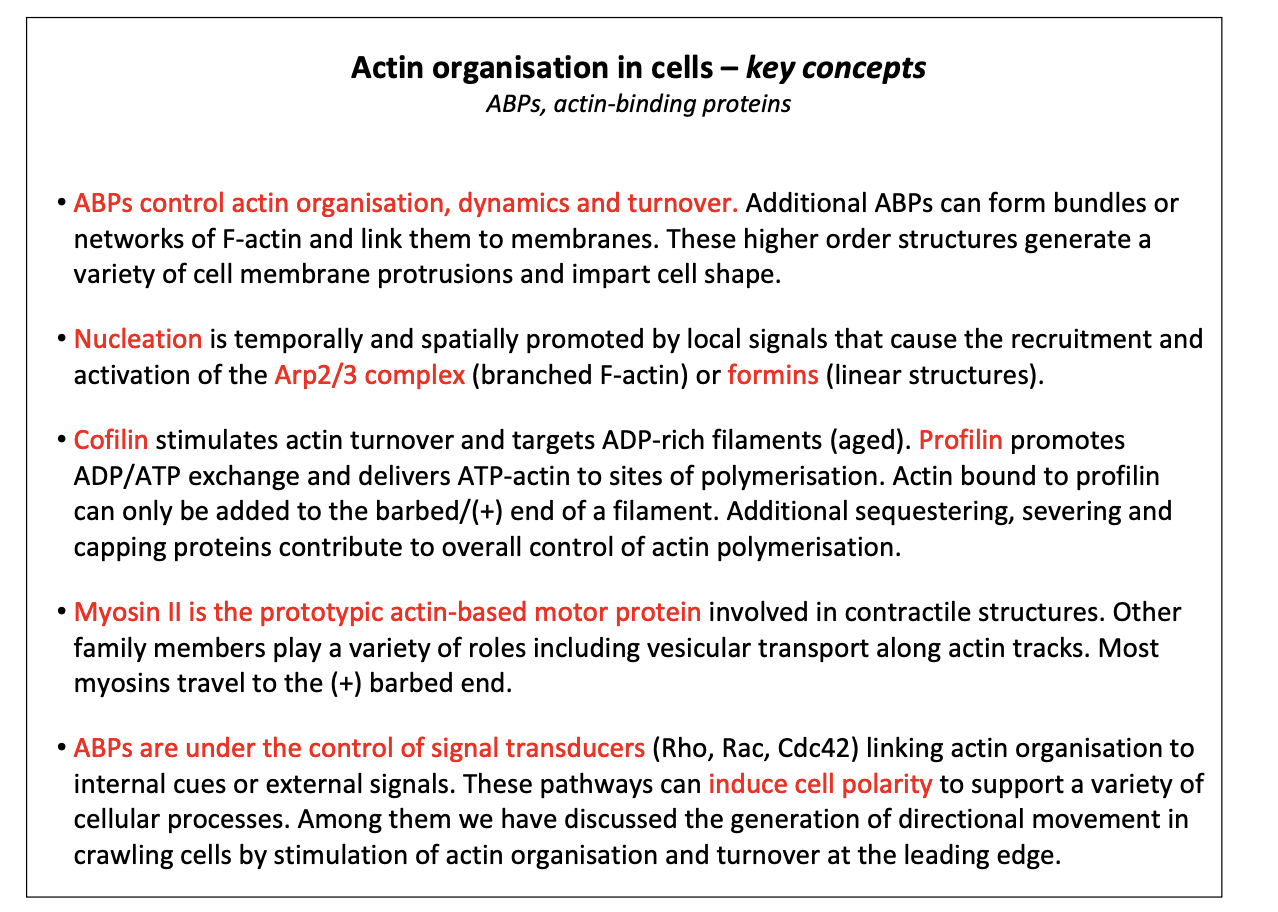
ABPs control actin organisation, dynamics and turnover. Additional ABPs can form bundles or networks of F-actin and link them to membranes. These higher order structures generate a variety of cell membrane protrusions and impart cell shape.
Nucleation is temporally and spatially promoted by local signals that cause the recruitment and activation of the Arp2/3 complex (branched F-actin) or formins (linear structures).
Cofilin stimulates actin turnover and targets ADP-rich filaments (aged). Profilin promotes ADP/ATP exchange and delivers ATP-actin to sites of polymerisation. Actin bound to profilin can only be added to the barbed/(+) end of a filament. Additional sequestering, severing and capping proteins contribute to overall control of actin polymerisation.
Myosin II is the prototypic actin-based motor protein involved in contractile structures. Other family members play a variety of roles including vesicular transport along actin tracks. Most myosins travel to the (+) barbed end.
ABPs are under the control of signal transducers (Rho, Rac, Cdc42) linking actin organisation to internal cues or external signals. These pathways can induce cell polarity to support a variety of cellular processes. Among them we have discussed the generation of directional movement in crawling cells by stimulation of actin organisation and turnover at the leading edge.