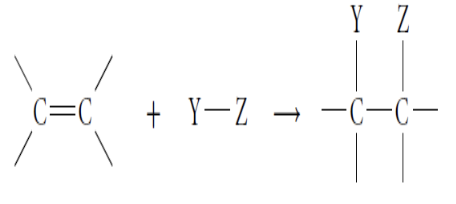Chapter 1: Organic Compounds
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Freidrich Wohler

Organic Compounds

Inorganic Compounds

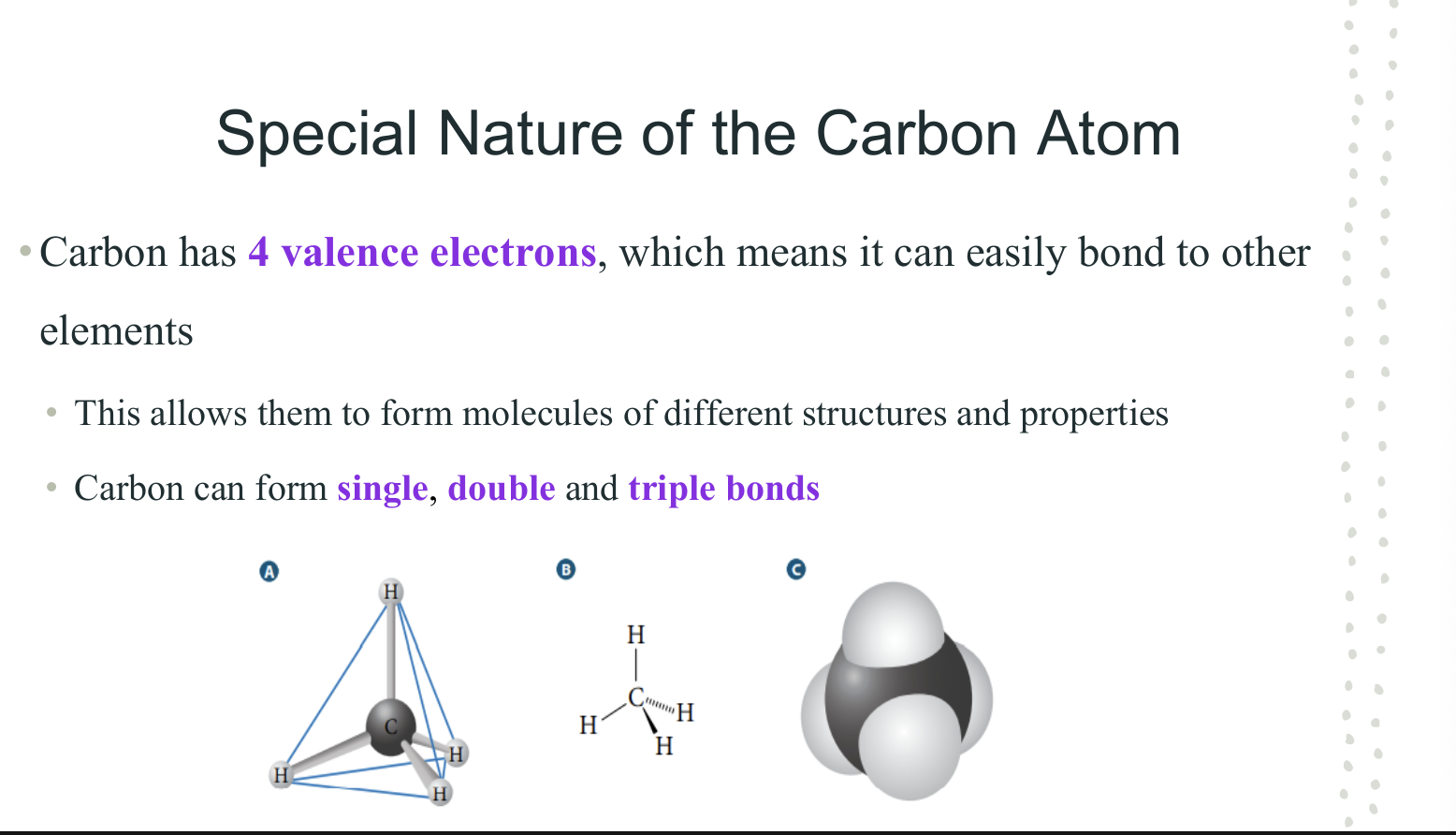
Special Nature of Carbon Atoms
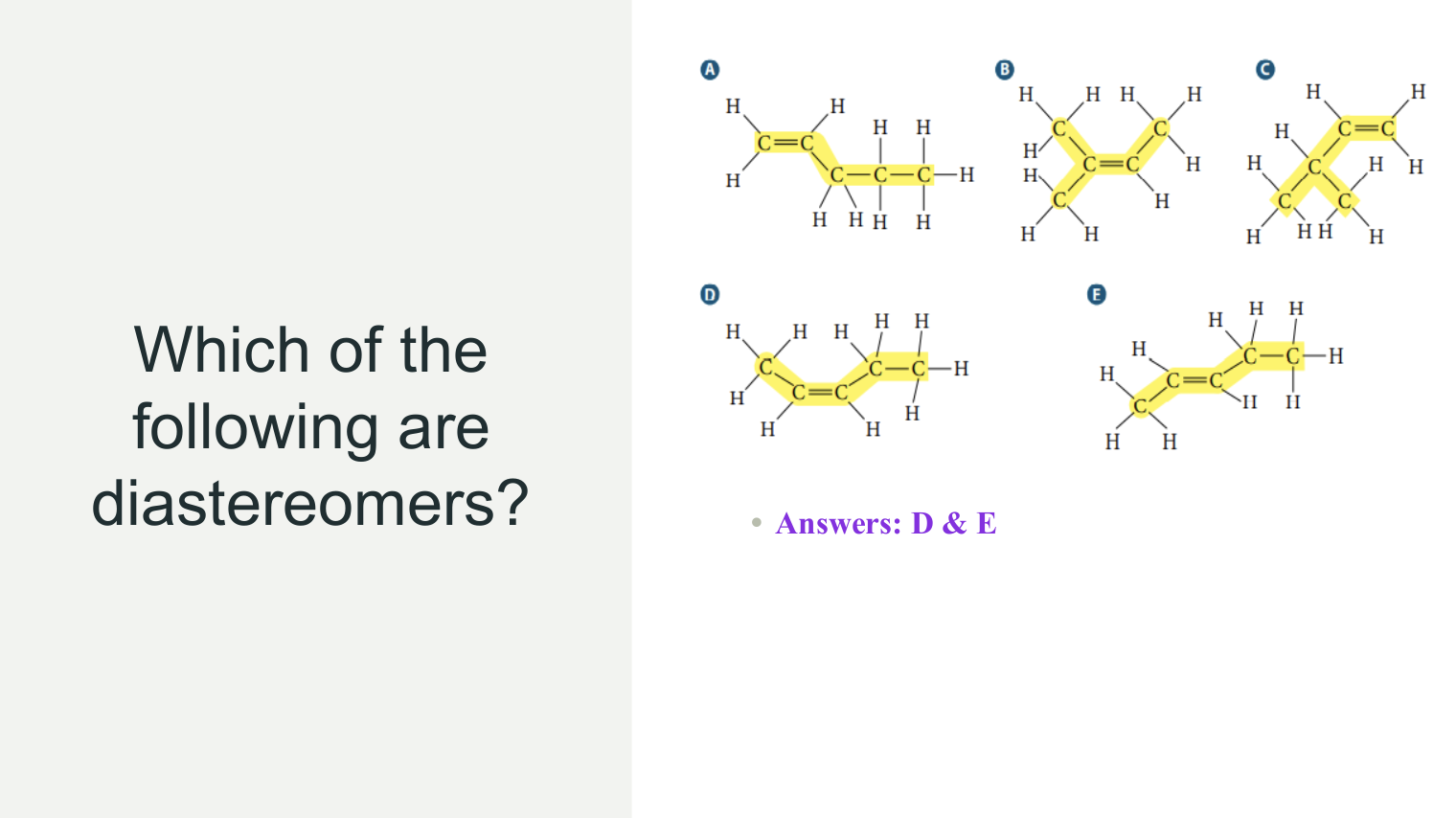
Isomers & Cyclic Isomers


Stereoisomers
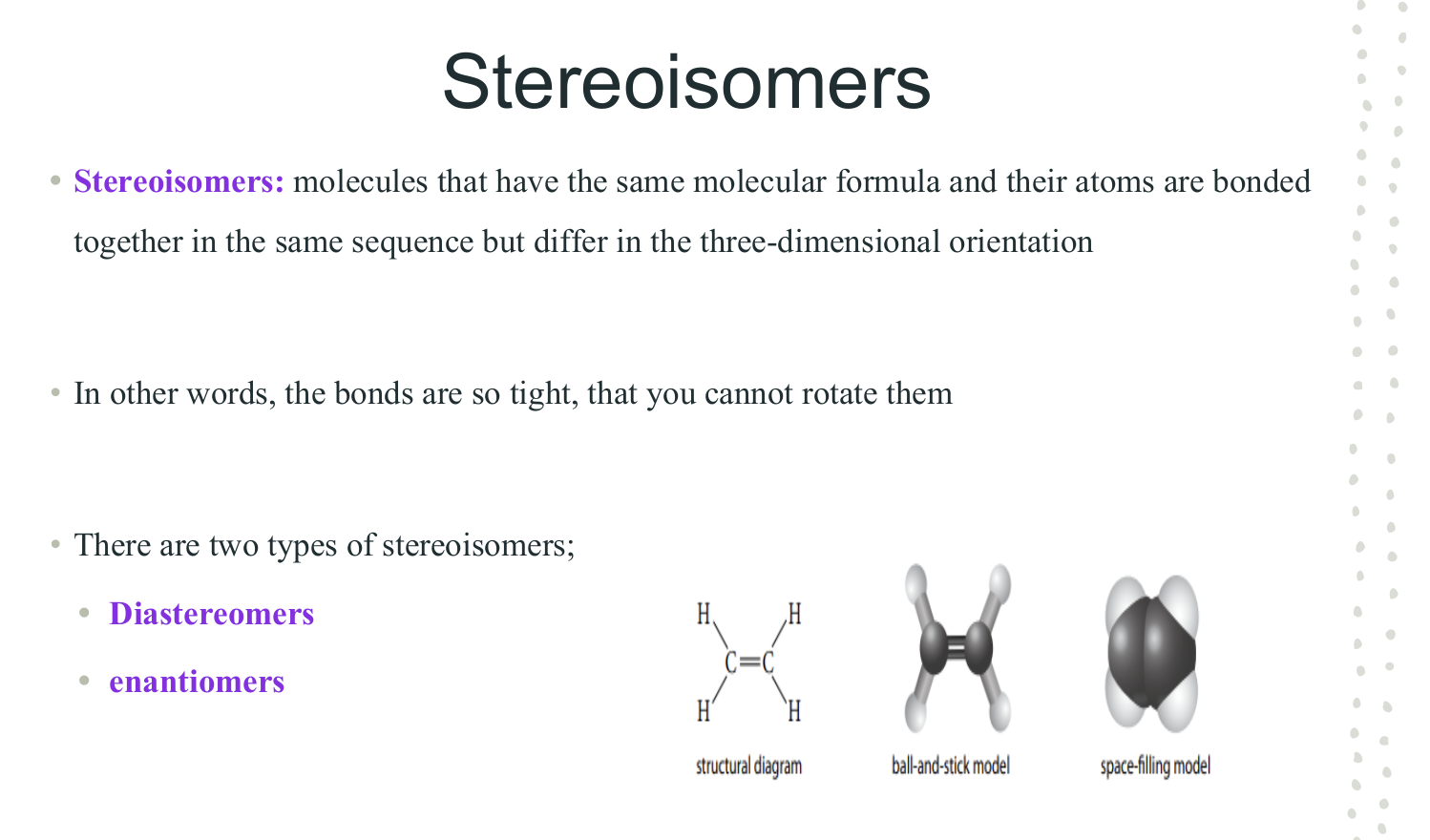
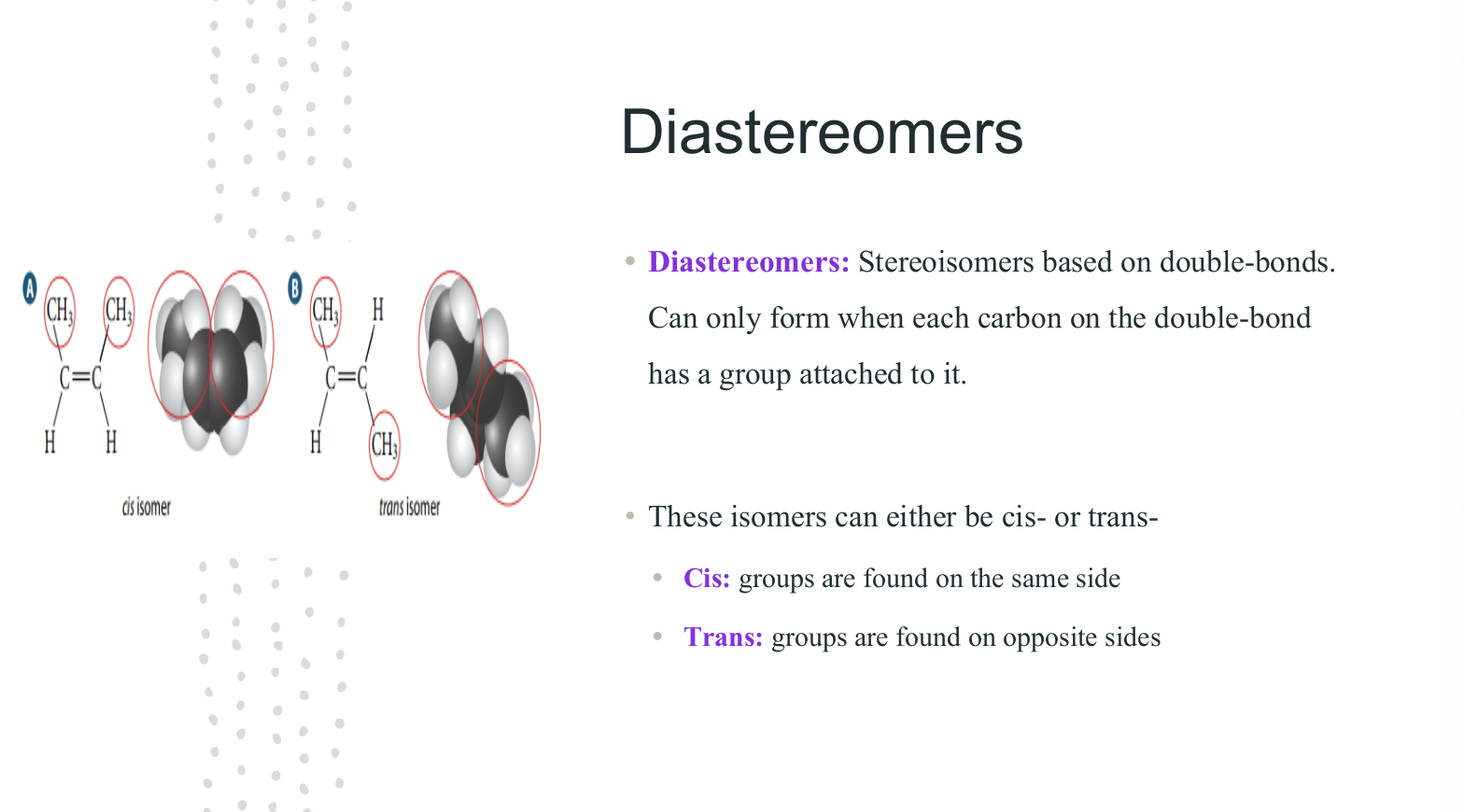
Diastereomers
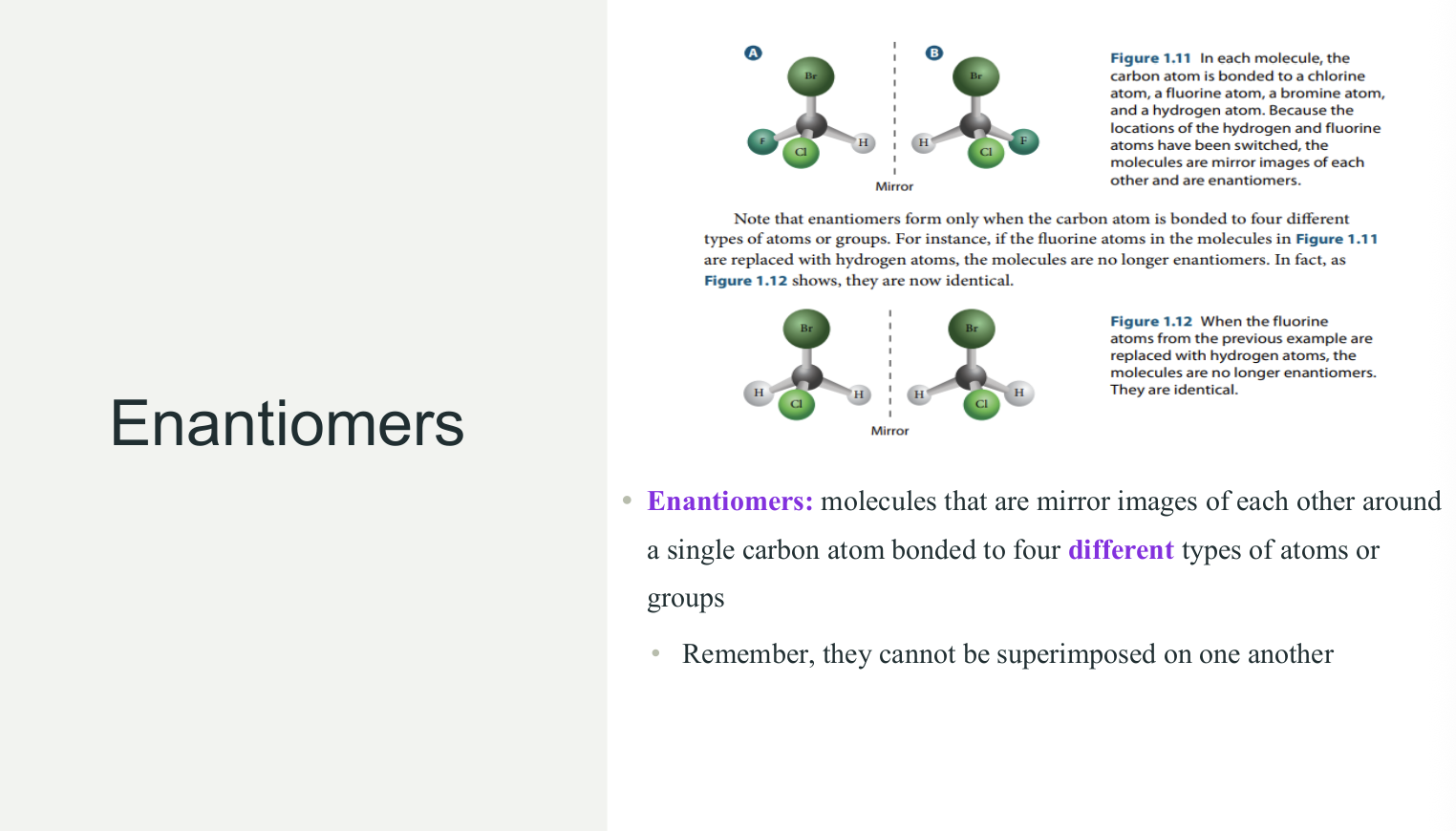
Enantiomers
Hydrocarbons
Alkanes
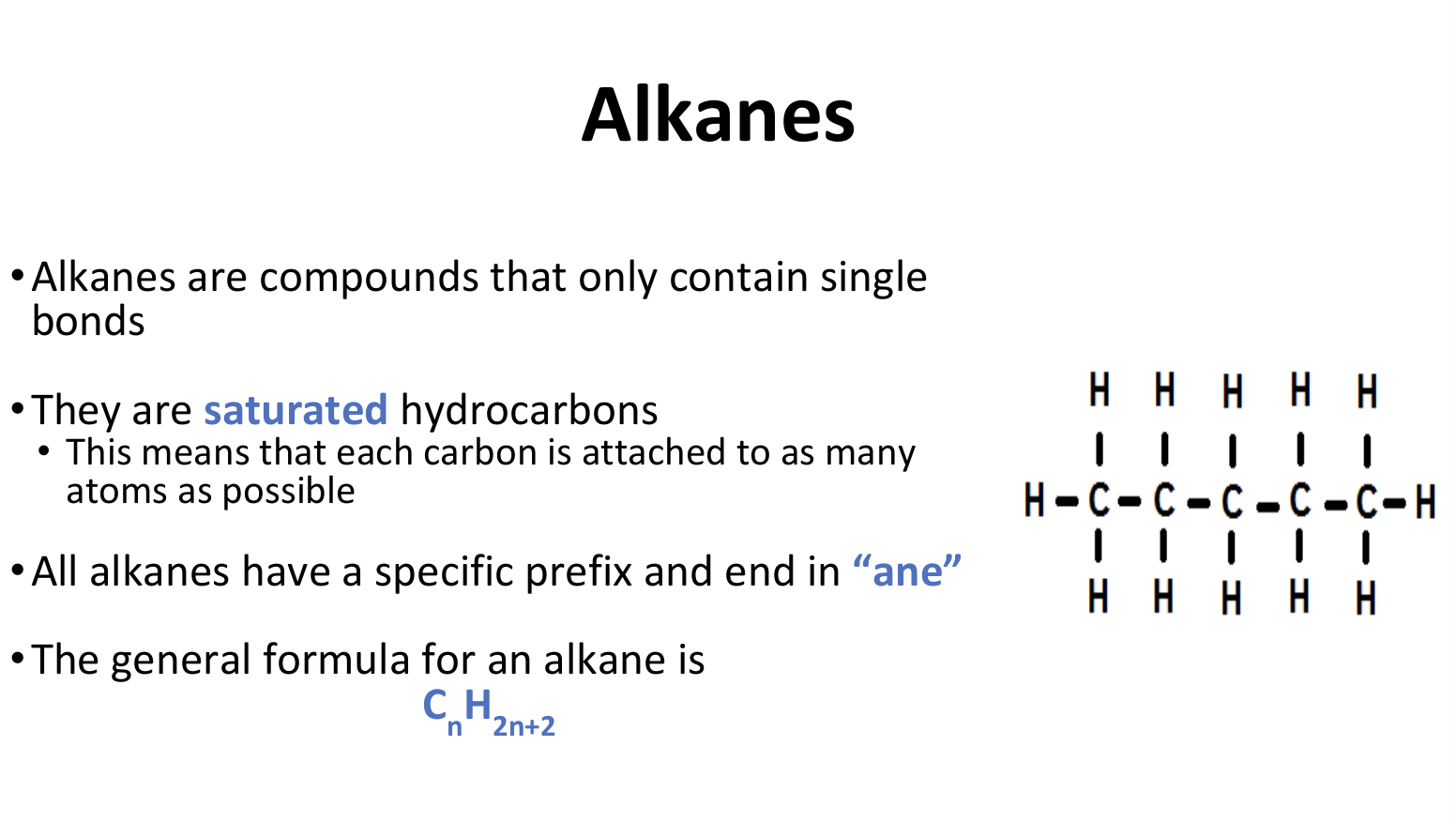
Alkane Properties

Alkenes
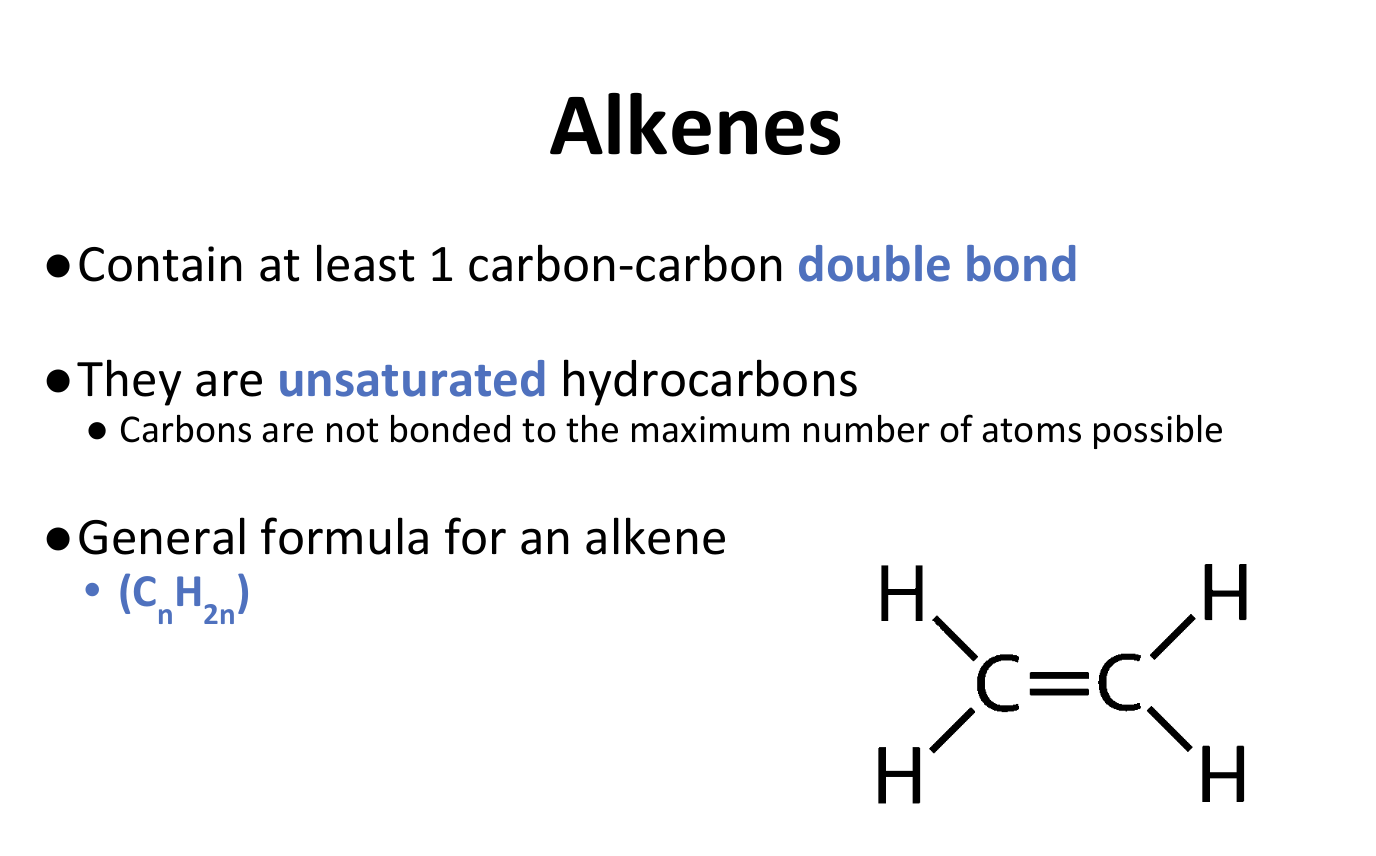
Alkene Properties

Alkynes
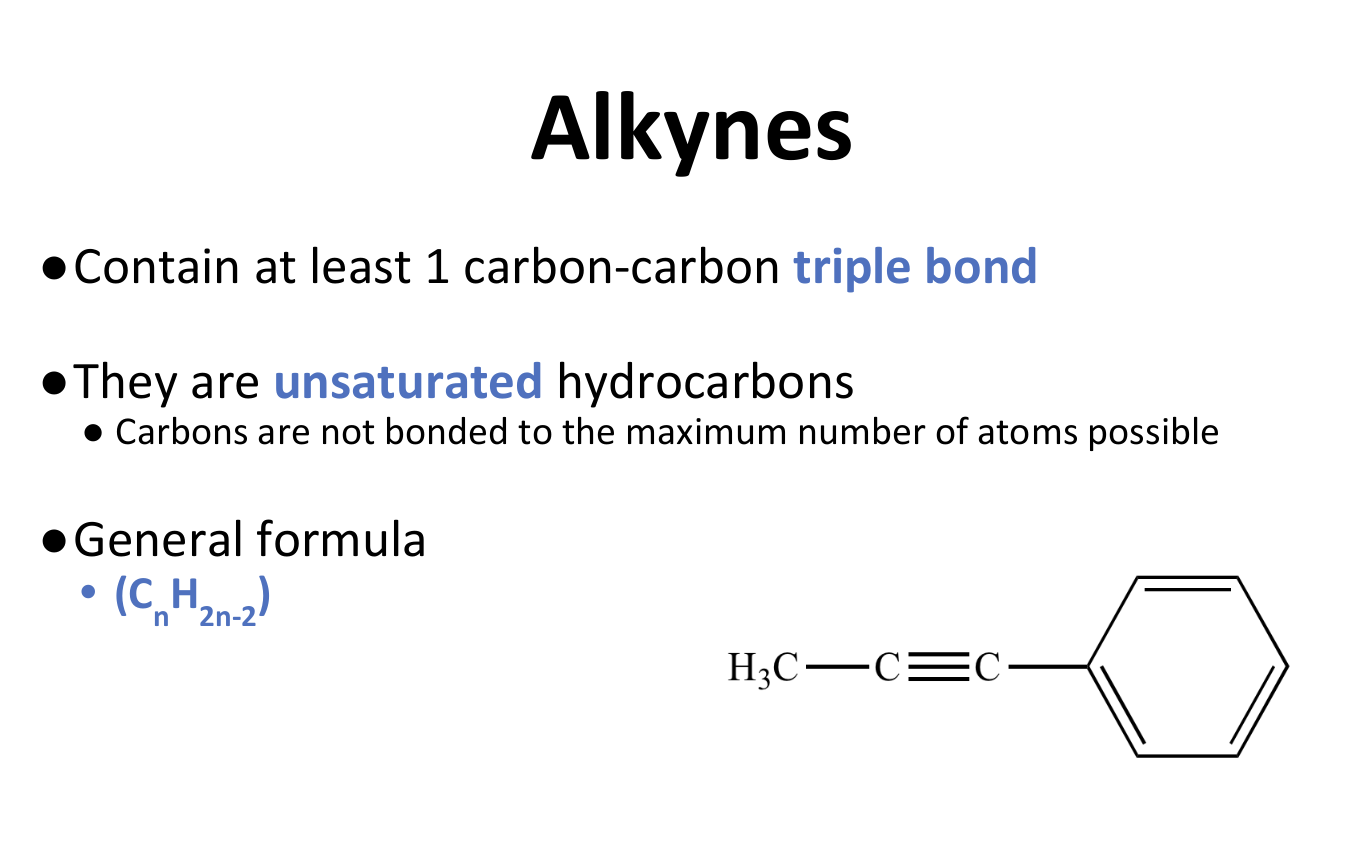
Alkyne Properties

Cyclic Hydrocarbons
Hydrocarbon molecules that form rings
Can be alkanes, alkenes, or alkynes
Found in many biological molecules including hormones and steroids
Aromatic Hydrocarbons
Molecules with a ring structure
Derive from benzene
A 6-carbon ring which has alternating single and double bonds
Referred to as resonance
Why does resonance occur?
Electrons in aromatic hydrocarbons are spread out (delocalized) and shared equally between the carbon atoms
Spreading makes the molecule more stable, so the electrons are less likely to react with other molecules
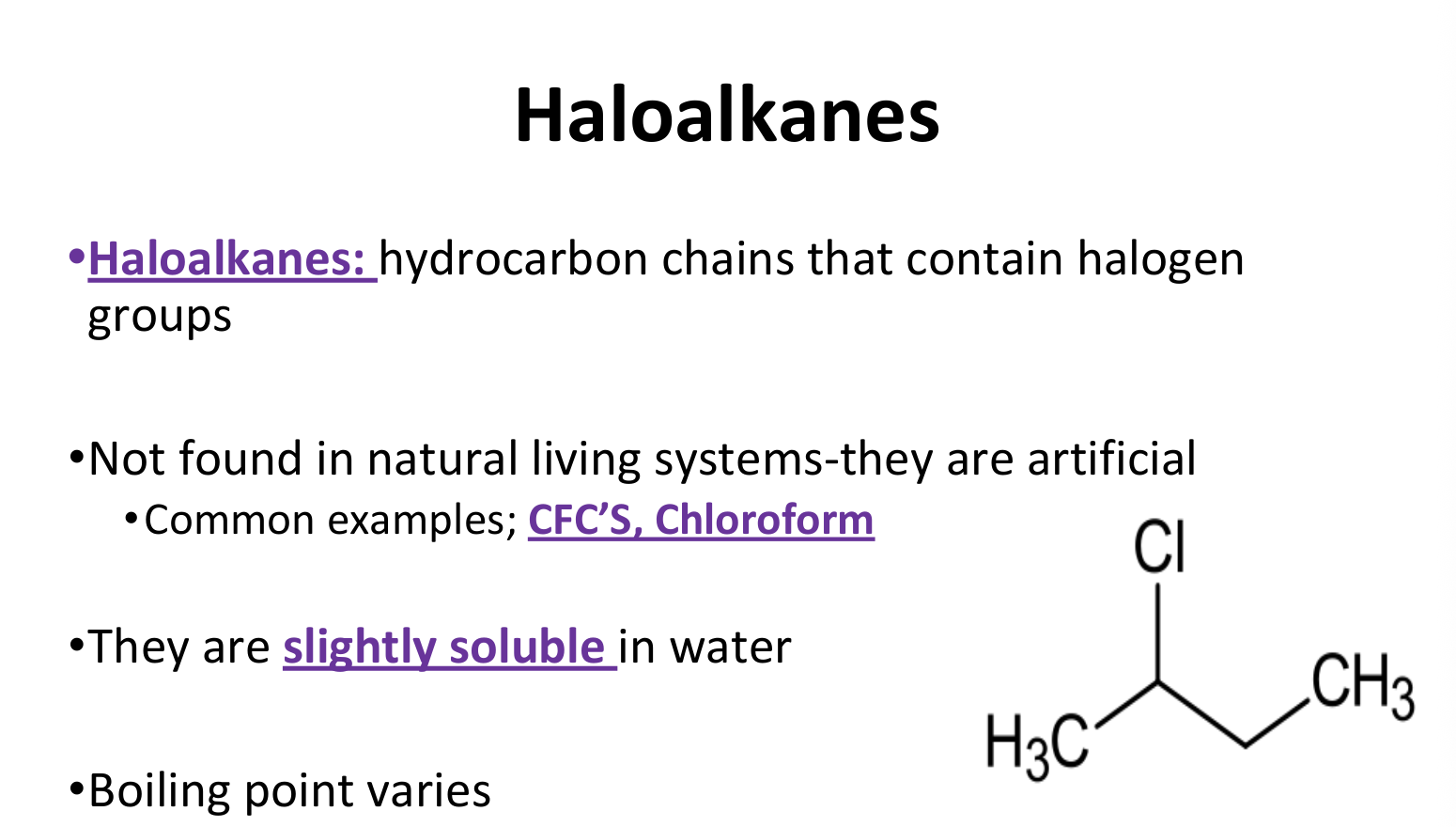
Haloalkanes
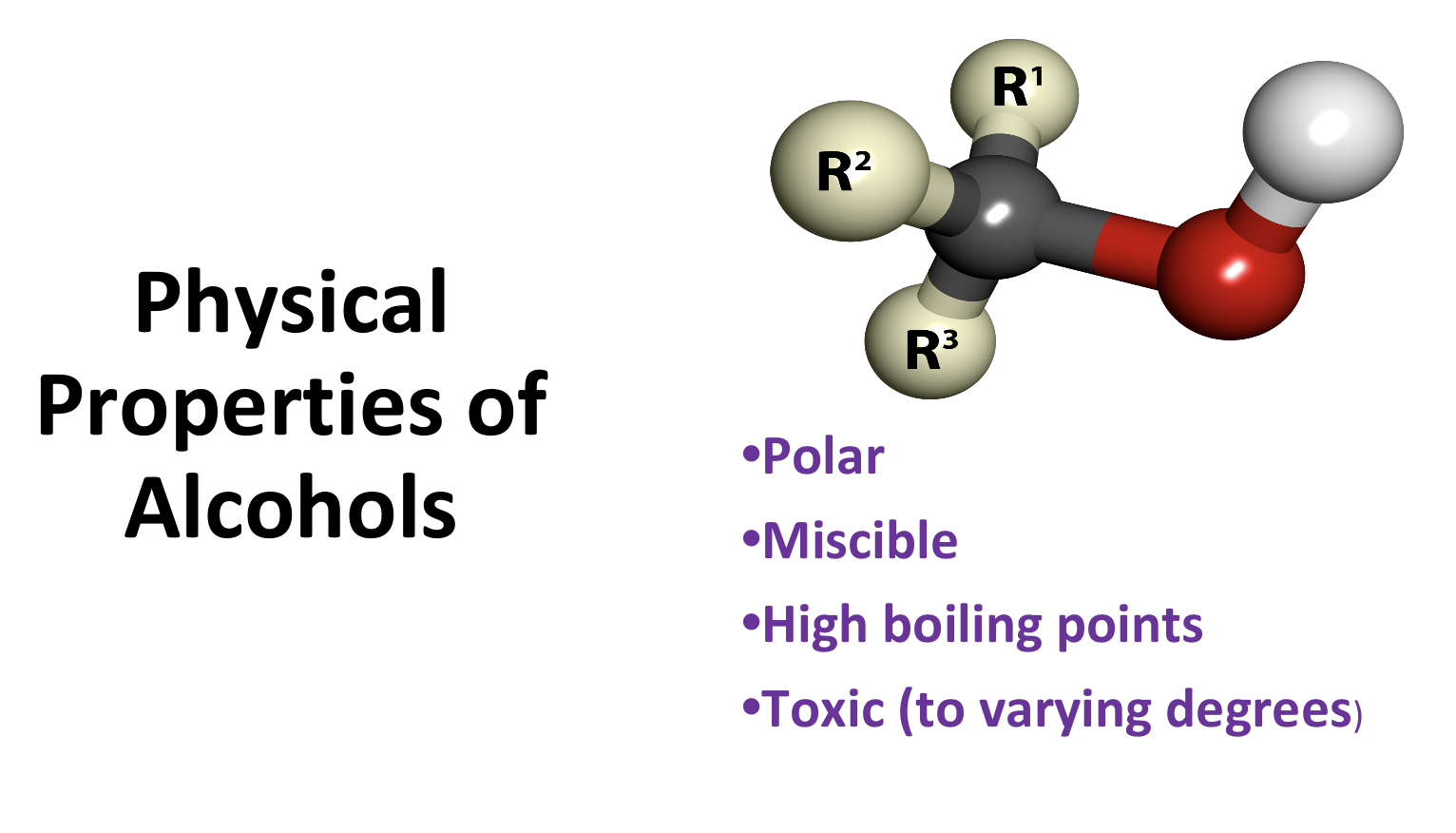
Physical Properties of Alcohols
Aldehyde Properties

Physical Properties of Ketones

How are esters made?
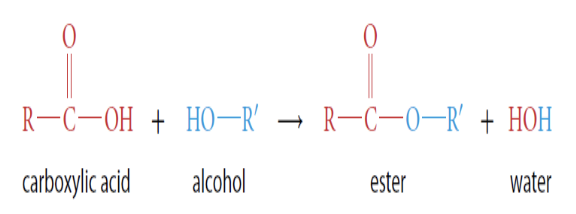
Formed when a carboxylic acid reacts with an alcohol
Physical Properties of Esters
Somewhat polar due to acid (OH makes it weaker)
Low boiling points
Longer chains waxy at room temp
Very volatile
Physical Properties of Ethers
Slightly polar
Larger ethers are liquid at room temp
Smaller ethers are gases at room temp
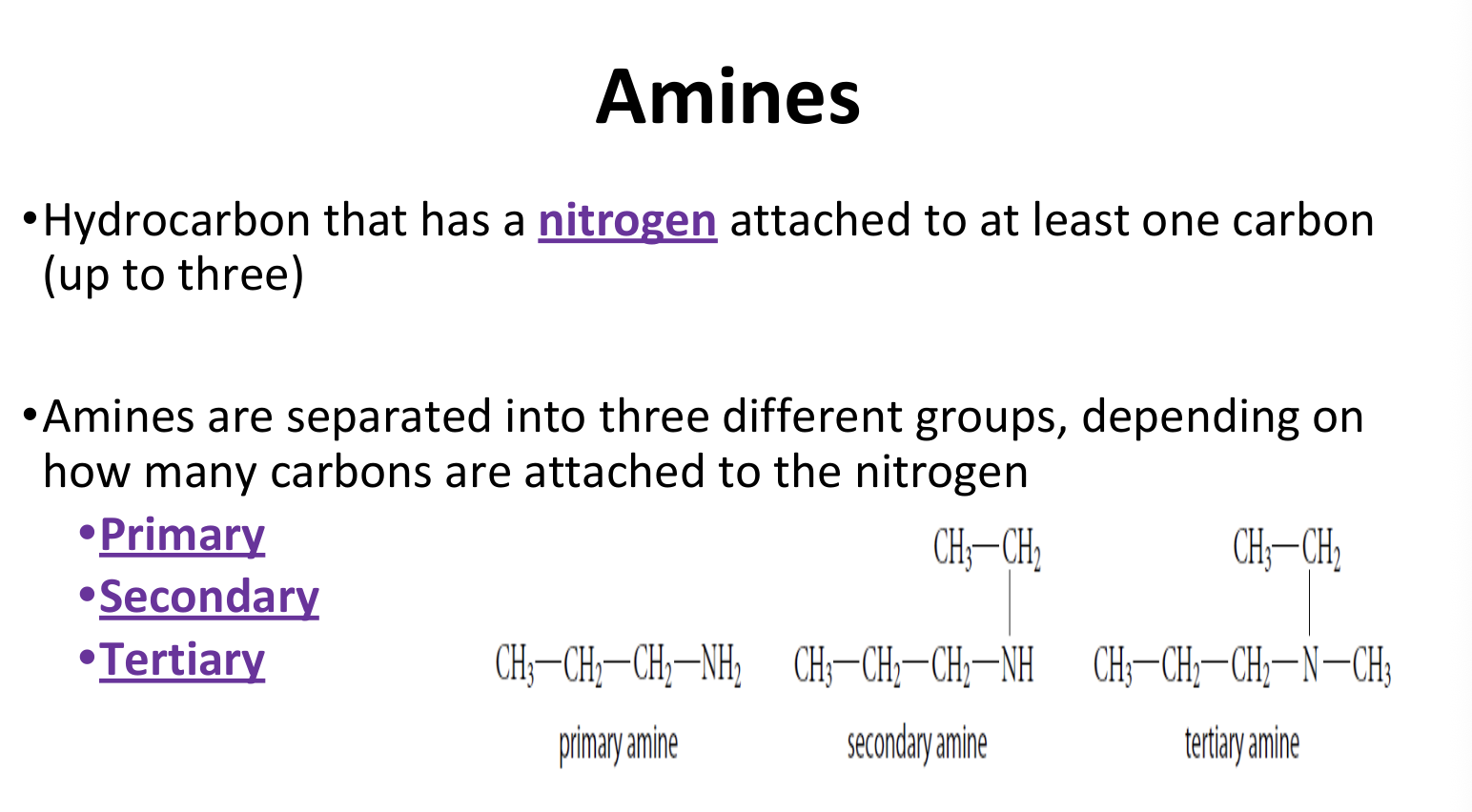
Amines
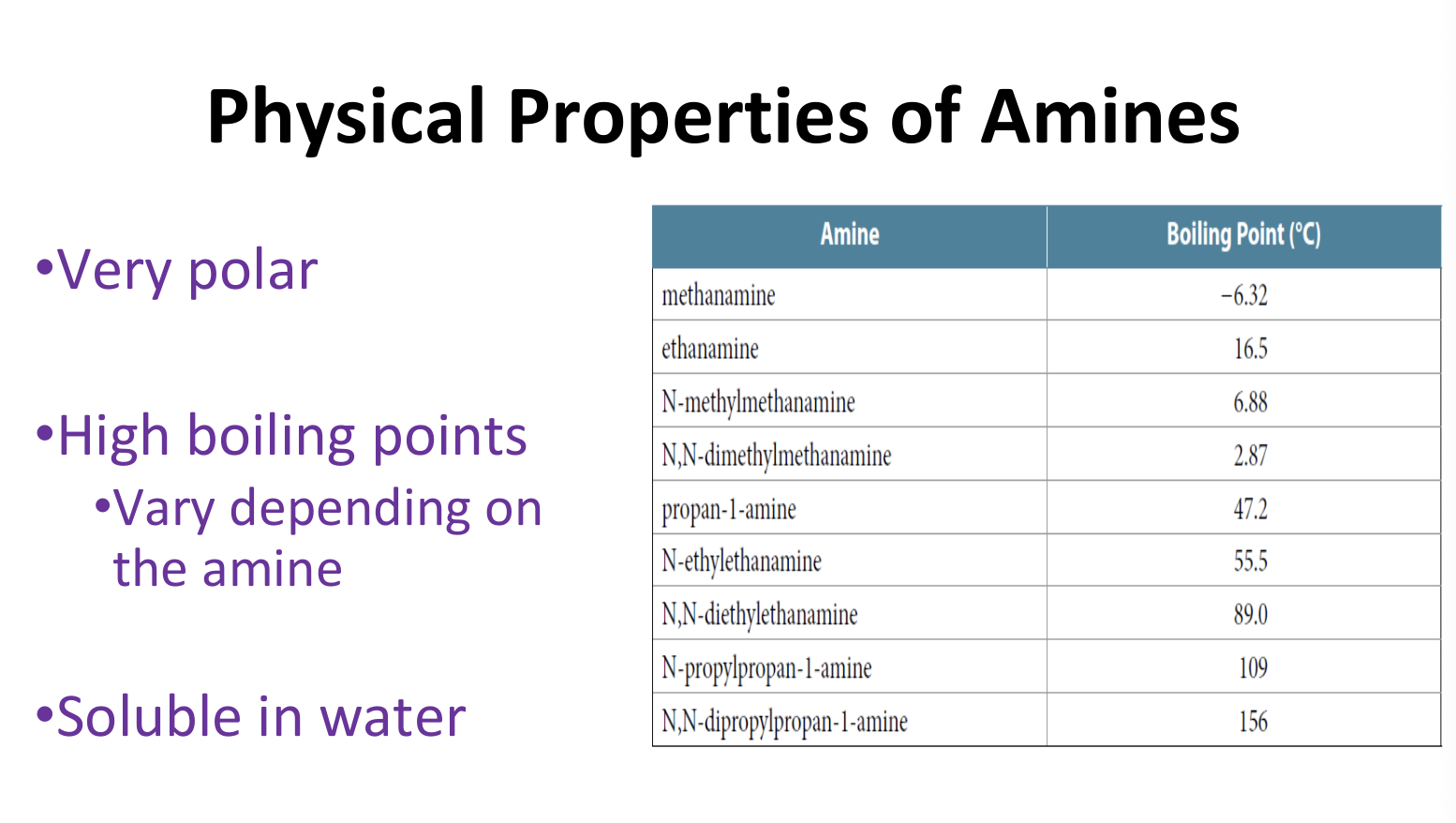
Physical Properties of Amines
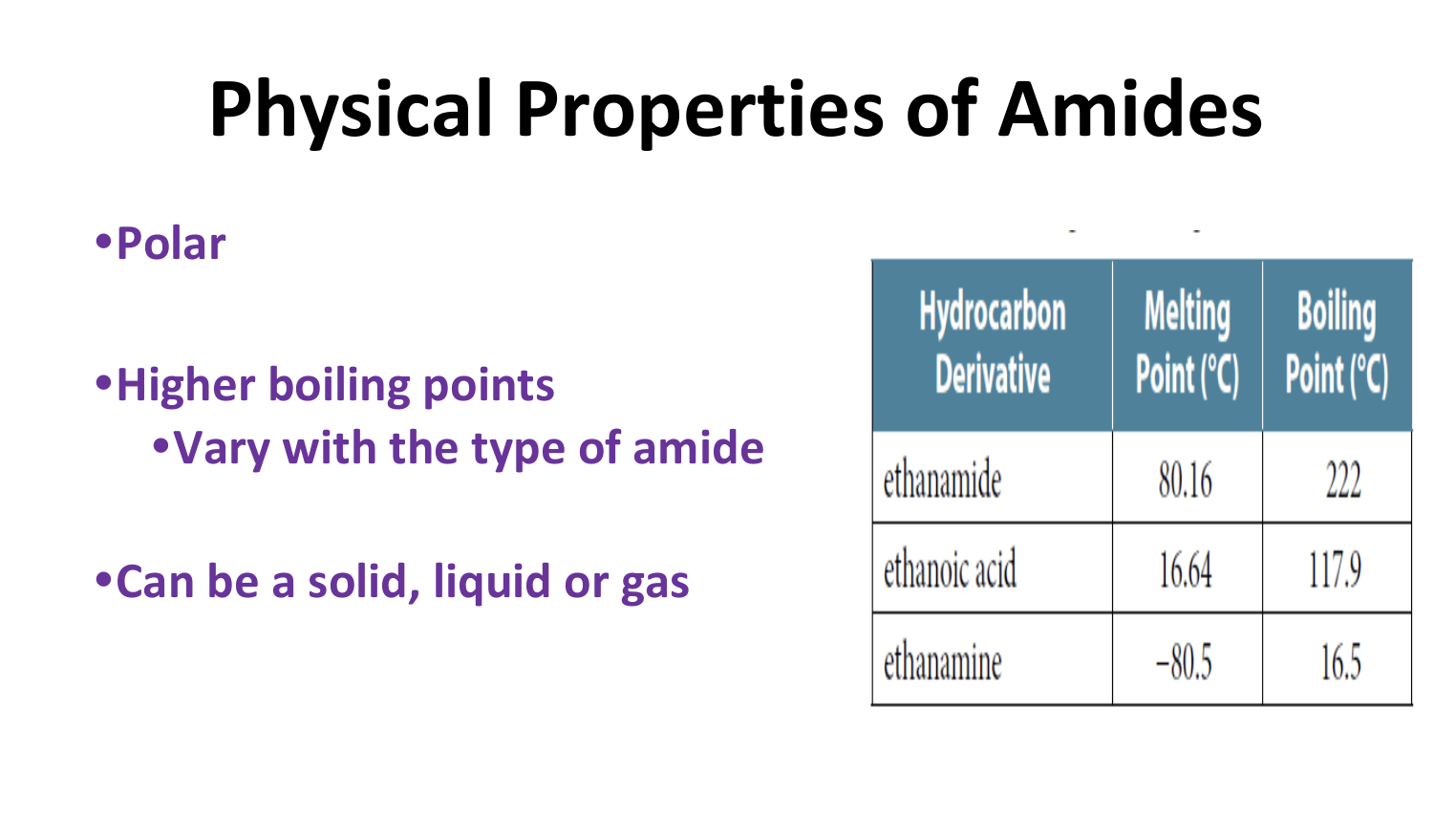
Physical Properties of Amides
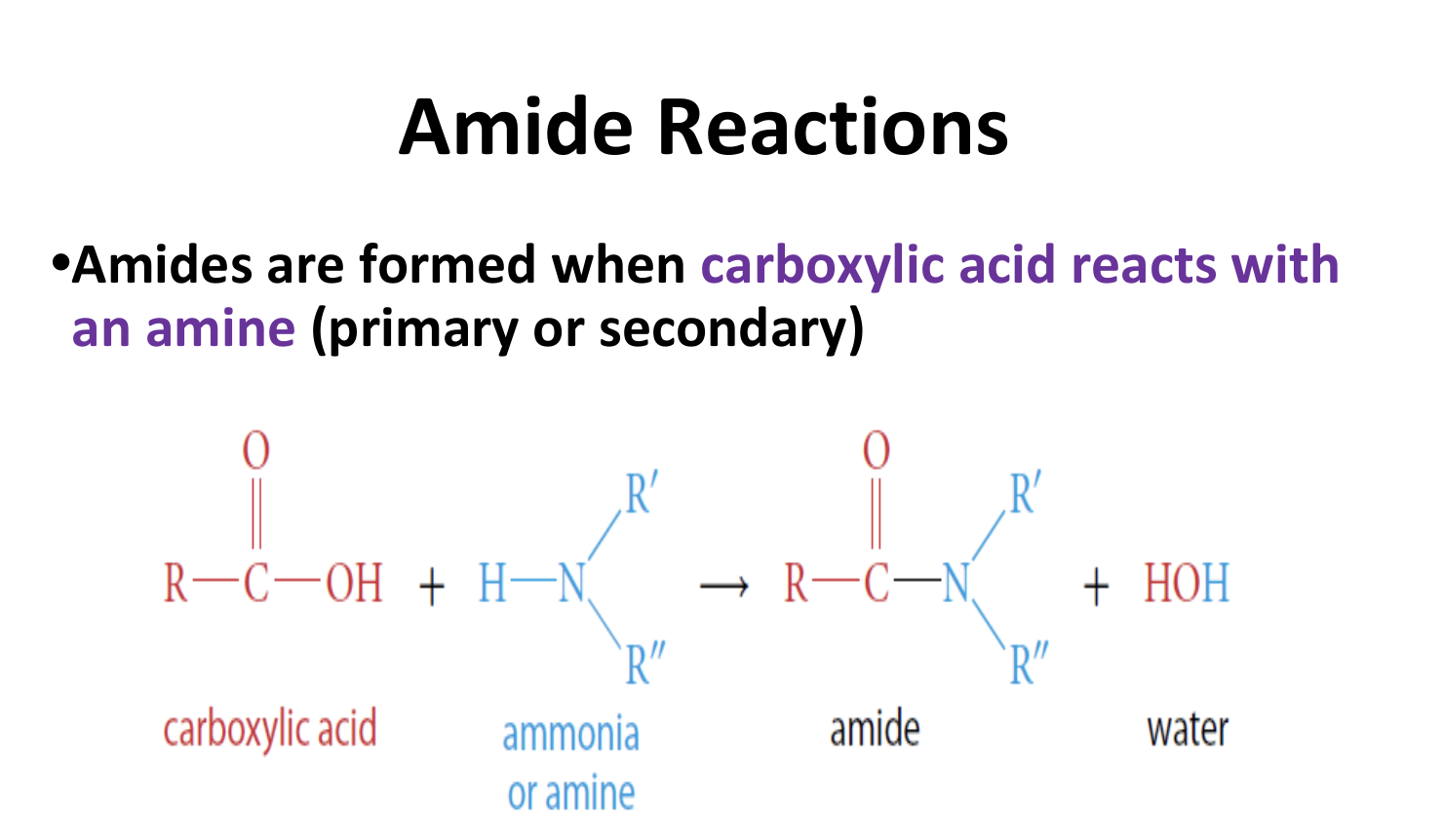
How are Amides formed?
Addition Reactions
Involve the addition of an atom to a carbon-carbon double or triple bond
(Will break double/triple bond and form a newly single bond)