PT 536 Progressive Lesions Physiology
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
112 Terms
1. growth rate
2. nuclear atypia
3. angiogenesis
Tumor aggressiveness is based on what 4 criteria?
glutamate toxicity, increasing ICP
Brain tumors are mass lesions that kill neurons through what mechanisms?
proto-oncogenes, tumor suppressor genes
what two proteins are responsible for controlling cell division?
Proto-oncogenes
which protein promotes the cell cycle (cyclins and cyclin-dependent kinases (Cdks) phosphorylate proteins to progress the cell cycle)
tumor suppressor genes
what proteins prevent the cell cyce?
DNA damage → p53 → p21 → inhibits cyclin-Cdks
how can tumor suppressor genes inhibit the cell cycle shorter term?
DNA damage → p53 → p21 → apoptosis
how can tumor suppressor genes inhibit the cell cycle long term?
oncogenes
mutations in proto-oncogenes lead to the formation of what?
lead to aberrant progression through the cell cycle
how can oncogenes contribute to the development of cancer?
unable to prevent cell cycle
how can mutations in the tumor suppressor genes contribute to the development of cancer?
p53
50% of cancers have mutations in what cancer gene?
anaplasia
mutations in the proteins that regulate cell division lead to what change in the cells?
anaplasia
presence of multiple cell differentiation state
high
anaplasia indicates a high or low grade tumor?
pleomorphic nuclei
having variation in the size and shape of cells or their nuclei.
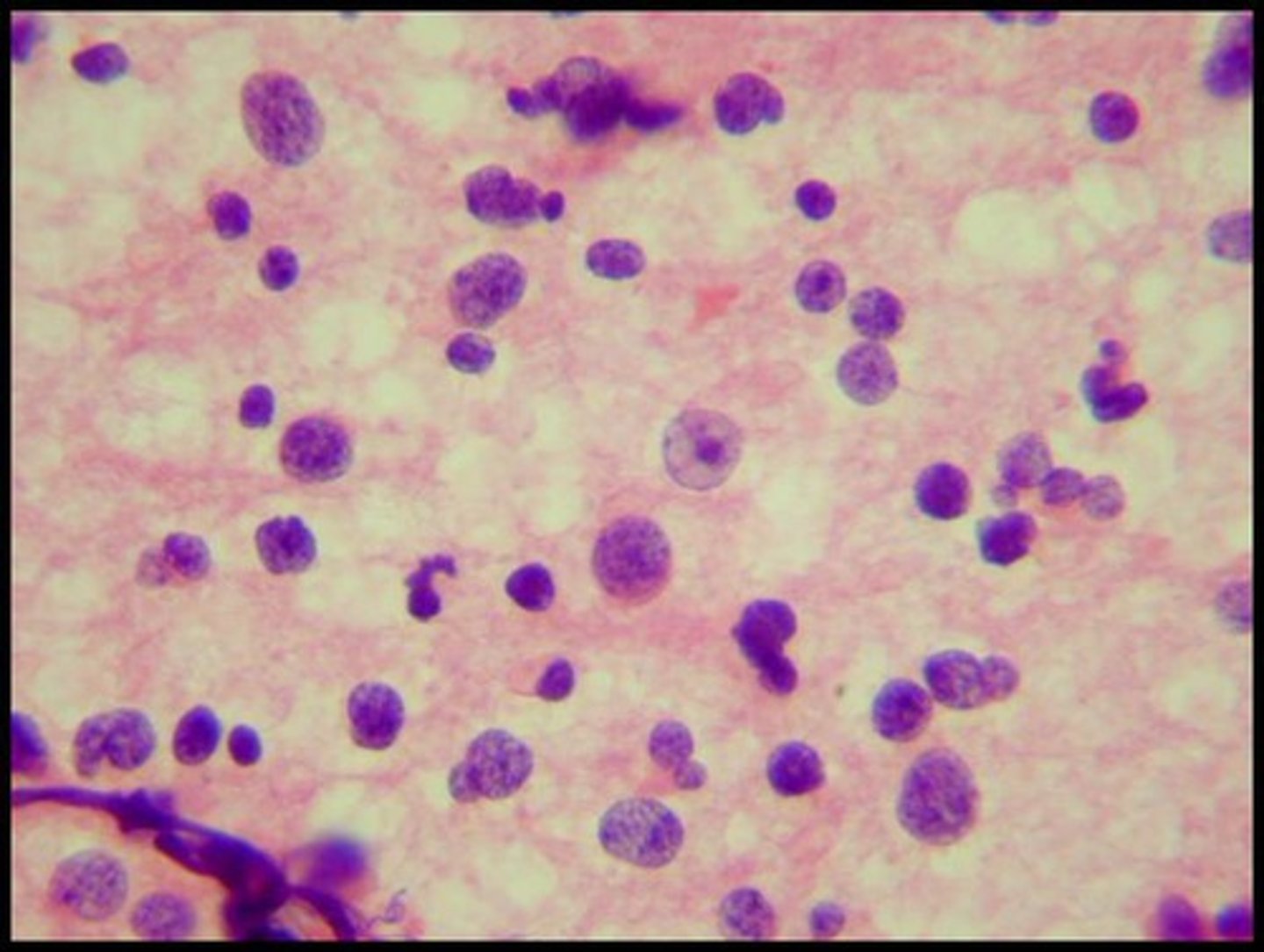
diffuse astrocytoma; grade 2
a type of brain tumor that originates from astrocytes, with no pleomorphism present
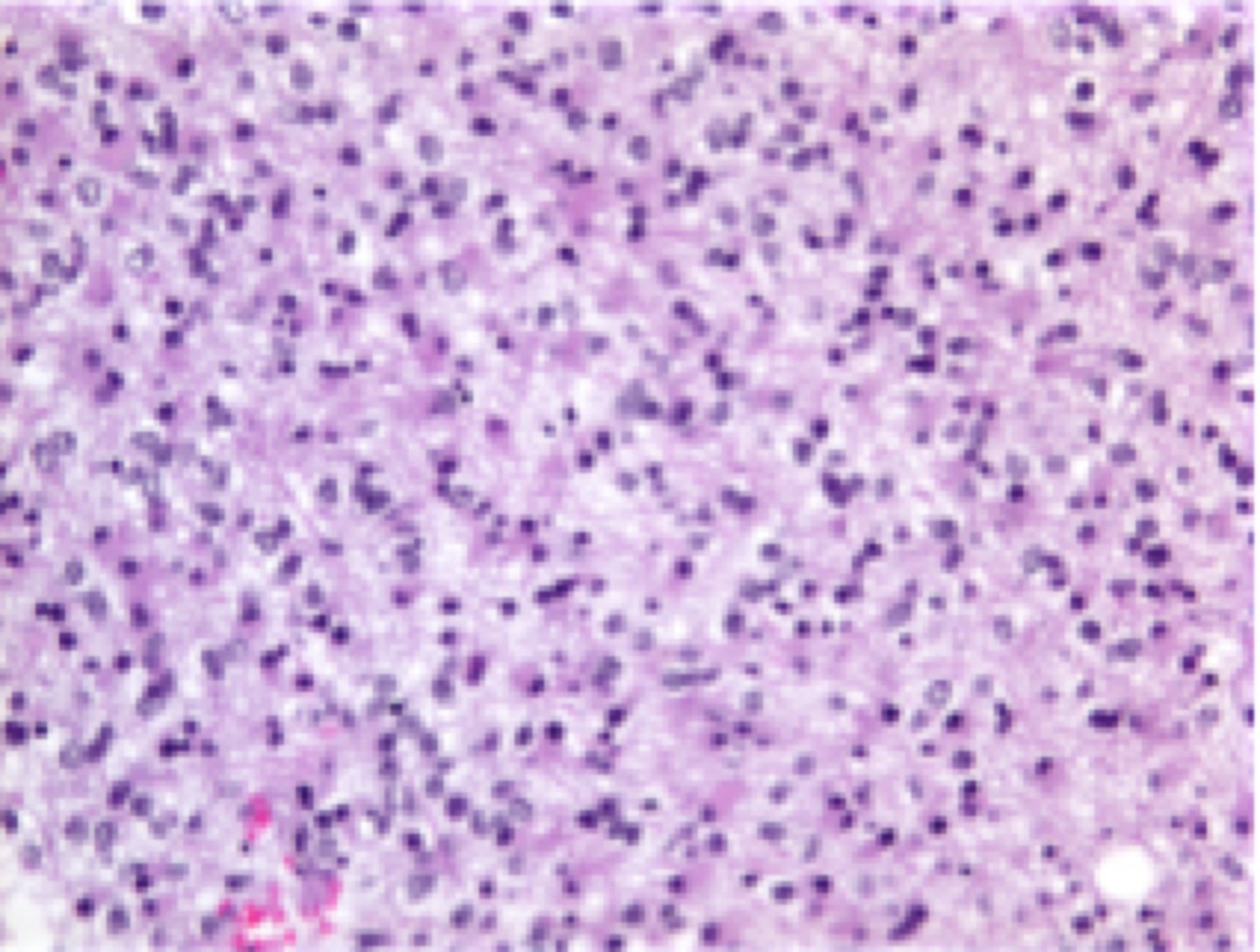
anaplastic astrocytoma; grade 3
a type of brain tumor that originates from astrocytes, with pleomorphism present
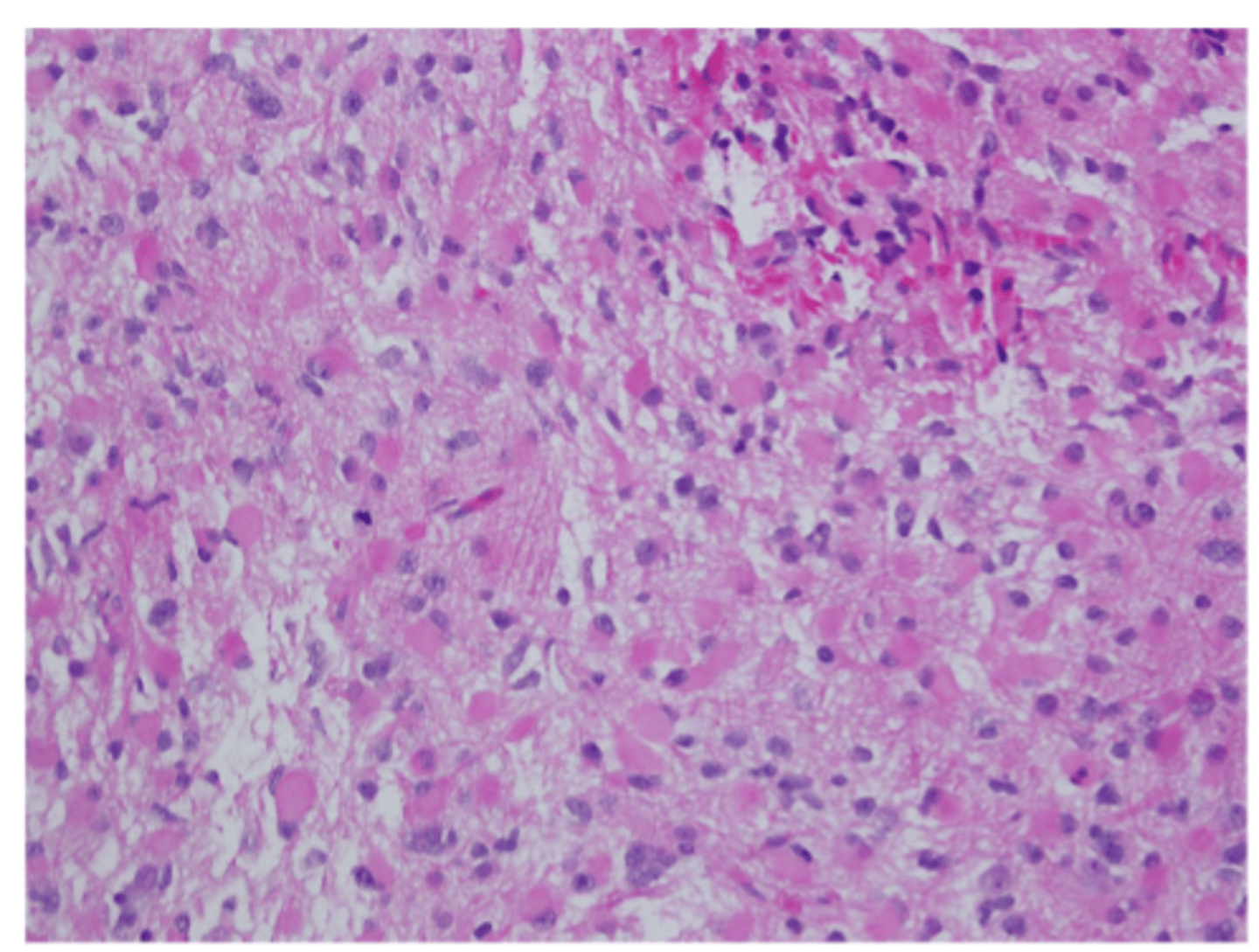
glioblastoma; grade 4
high grade astrocytoma present with necrosis and microvascular proliferation
benign tumors
tumors that have a slow growth rate and are noninvasive; but can still grow and damage normal tissue and become malignant
malignant tumors
tumors that have a high growth rate and are more likely to metastasize
20/100,000
annual incidence of primary brain tumors
age, exposure to ionizing radiation, family history, exposure to certain infections (EBV)
risk factors for primary brain tumors
gliomas
most common primary brain tumor that affects the glia
high grade astrocytoma
most common type of glioma with the worst prognosis
low grade astrocytomas
glioma common in the frontal lobes with a prognosis of 6-8 years and prevalence of 10-12% of primary tumors
neuroma (schwann cells)
what type of glioma has the best prognosis and may not even be treated in the elderly?
12 years
prognosis of a oligodendroglioma grade II
3.5 years
prognosis of a oligodendroglioma grade III
ependymoma
type of glioma that is more likely to obstruct blood flow, involving a prognosis of >10 years
medulloblastomas
gliomas that often develop in the vermis (affecting balance and movement), involving a prognosis of 3 years for children and 6 years for adults
meninges
most common site of non-gliomas
meningioma
most common non-glioma that produces ICP signs and has a typical 10 year survival rate
pituitary adenoma
non-glioma that includes visual signs and hormonal irregularities with a typical 10-year survival
craniopharyngioma
non glioma that includes visual signs and hypopituitarism with a typical 10 year survival rate
epidermoid, dermoid
non glioma that is benign and occurs in development, with a 20 year survival rate
hemangioblastoma
non-glioma that is well-vascularized with >80% in cerebellum and a 10 year survival rate
primary CNS lymphoma
non-glioma that is deep periventricular and involves controversial surgery and only has a 1 year survival rate with RT and CT
intramedullary
rarest spinal cord tumor that gives burning diffuse pain
age, exposure to ionizing radiation, family history, exposure to certain infections (EBV)
risk factors for spinal cord tumors
extramedullary
spinal cord tumor outside of the cord and stimulating the dura; gives sharp and radiating pain
extradural spinal cord tumors
spinal cord tumors that tend to be in the posterior regions of the spine due to ease of metastasizing near valveless blood vessels
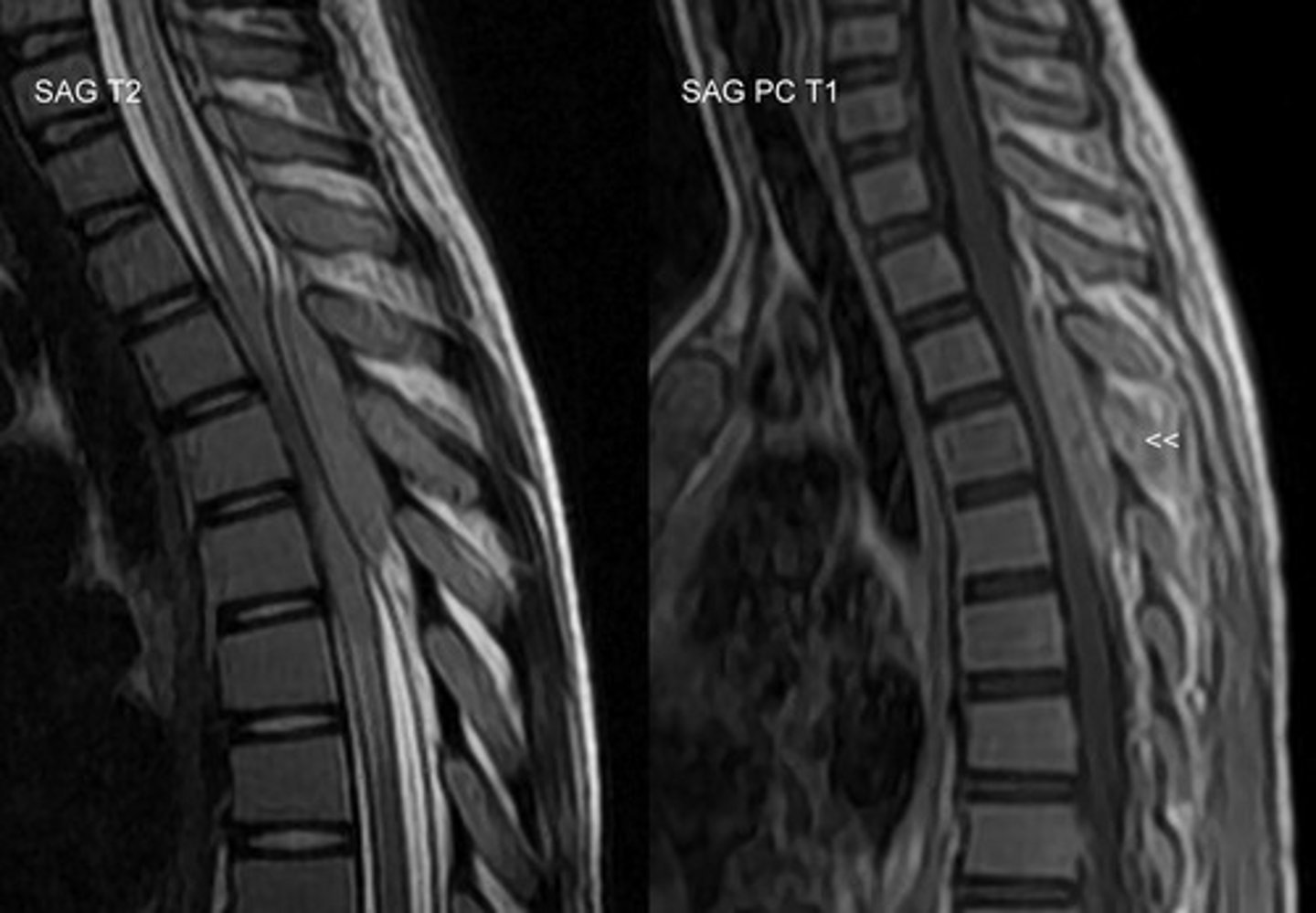
14/100,000
annual incidence of metastatic brain tumors
age, exposure to ionizing radiation, family history, exposure to certain infections (EBV)
risk factors for primary brain tumors
metastatic
do primary or metastatic brain tumors generally have a worse prognosis?
middle cerebral artery
what artery is usually associated with metastatic brain tumors?
lung
most common site of origin for metastatic brain tumors
myelin
When brain tumors are present, the pressure first "pops" what structure of a nueron?
conduction block
electrical impulses go down but encounter blocks and delays in the demyelinated internodes
insert ion channels to propagate APs
how can neurons recover from demyelination?
increase metabolic stress and free radicals
risk of inserting ion channels to propagate APs
true
true or false? if we removed the source of pressure, we can remyelinate via the reproduction of oligodendrocytes
late neuronal loss
in the presence of brain tumors, initial demyelination causes what process?
1. oxidative stress + apoptosis
2. mechanical pressure + excitotoxicity
3. tumors release glutamate + excitotoxicity
4. incomplete functional recovery
4 ways late neuronal loss can occur after initial demyelination
cystine-glutamate antiporter inhibitor (SAS)
what do tumors use to release glutmate?
CSF
liquid cushion in the brain that is in equilibrium with extracellular fluid (ions, NTs, nutrients, & wastes)
hydrocephalus
accumulation of CSF in the ventricles, compressing the midbrain and cerebral aqueduct
blockage of cerebral aqueduct from herniated brain tissue
typical causation of hydrocephalus
increased ICP
big negative consequence of hydrocephalus
ischemia
Whenever tumors occupy space and herniate brain tissue, an increase in ICP can ensue and lead to what issue with blood?
increased blood brain barrier
Other than ischemia, what specific pathophysiology of brain tumors that involves blood can happen?
edema, hemorrhage (increased ICP overall)
with increased BBB permeability, blood secreting factors leak through and lead to what two issues?
1. blood has higher levels of glutamate and K+, so leakage into brain with hyper-excite neurons
2. reactive gliosis and reduces K+ and glutamate pick-up
how can an increase in BBB permeability lead to excitotoxicity in the brain
directly, increasing ICP
how can infections kill neurons?
necrosis
physical injury involves what kind of cell death
damage-associated molecular patterns
Endogenous molecules released by damaged or dying cells. They serve as signals to alert the immune system to tissue injury and initiate an inflammatory response (stuff that should be inside the cell that is not outside the cell)
pathogen-associated molecular patterns
Molecular structures released in infections that increase inflammation to bring blood to the area of infection
increased BF, vasodilation, increased permeability, increased blood flow and blood cells
results of infection
toll-like receptors
recognize molecular patterns unique to pathogens, detecting DAMPs and PAMPs
sheddases, transcription factors
activated TLRs activate what molecules to stimulate the release of pro-inflammatory cytokines
cytokines
secreted signaling proteins that stimulate inflammation
1. VD, increase permeability via COX and prostaglandins causing smooth muscle to relax
2. chemotaxis (attracting macrophages to the area)
3. reactive gliosis
how can cytokines facilitate inflammation?
meninges
layers of connective tissue that cover the CNS
prevent nervous tissue from directly contacting bone
how do the meninges work as a physical barrier?
prevent diffusion from blood into the brain
how do the meninges work as a chemical barrier?
dura mater, arachnoid mater, pia mater
three different meninges
dura mater
tough, outermost layer of the meninges; acting as a physical barrier
arachnoid mater
CSF filled intermediated meninge; acting as a diffusion barrier
pia mater
delicate & permeable, innermost meninge; acting as an anchor
2.5/100,000
annual incidence of bacterial meningitis
11/100,000
annual incidence of viral meningitis
exposure to neurotrophic pathogens, lack of vaccination, compromised immune function, nervous system damage, age <5
risk factors of meningitis
virus/bacteria enters CSF in subarachnoid space → triggers immune response from epithelial cells in meninges
how can infection cause meningitis
edema (increased ICP) and ↑BBB permeability
infection in the meninges can lead to inflammation and cause what two main issues?
brain herniation, hydrocephalus, ischemia, mechanical depolarization
consequences of edema and increased ICP in meningitis
edema, WBC entry
consequences of increased BBB permeability in meningitis
encephalitis
inflammation of the brain
3.5-7.4/100,000
incidence of encephalitis
exposure to neurotrophic pathogens, lack of vaccination, compromised immune function, nervous system damage, age <5
risk factors of encephalitis
retrograde
in what manner do infections enter the central nervous system? (anterograde or retrograde)
increase ICP, increase BBB permeability
how can inflammation with encephalitis affect the CNS?
explode the cell membrane
how do new viruses leave their hosts?
motor neurons, thalamus, brainstem, cerebellum
where are cells targeted with the west nile virus?
motor neurons
where are cells targeted with the poliovirus ?
limbic system, temporal lobes, frontal lobes
where are cells targeted with the herpes simples virus?
prions
infectious proteins that act as potent neurotoxins
0.1/100,000
annual incidence of prion disease
ingesting infected meat or brain matter
risk factors for prion disease
- ataxia, myoclonic jerks, weakness or paralysis
- hallucinations
- cognitive/memory impairments
- emotional disturbances
- sleep abnormalities
symptoms of prion disease
none
treatment for prion disease
death within 1 year
prognosis of prion disease