Hydrocarbons: Crude oil and Fuels
1/16
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What is a hydrocarbon
A compound that only contains carbon and hydrogen atoms
What is crude oil
Mixture of many different hydrocarbon compounds and is finite. Most hydrocarbons in crude oil are alkanes.
What is nomenculature
Naming organic compounds:
Monkeys: 1 carbon → Meth-
Eat: 2 carbons → Eth-
Peanut: 3 carbons → Prop-
Butter: 4 carbons → But-
Pent-
Hex-
Hept-
Oct-
Non-
Dec-
How do u draw hydrocarbons
Write out no. of carbons
Identify bond required and write
Carbon can only have 4 bonds
Add in hydrogen
What is the formula for alkane
CnH2n+2
Only single covalent C-C bonds
What is the formula for alkene
CnH2n
At least ONE double bond
Why does CH2 not exist
Only 1 carbon so double bond cannot work even if the formula fits.
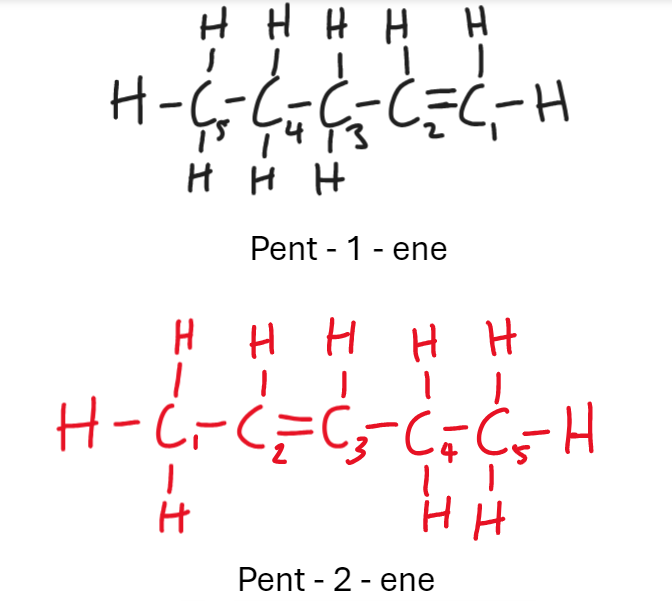
How do u start numbering carbons in hydrocarbon alkenes
From side double bond is closest to
What are the properties of long - chained hydrocarbons
High boiling point
Low volatility (how quick it is to evaporate)
Thick/High viscosity (flow)
Low flammability (smoky)
What are the properties of short - chained hydrocarbons
Low boiling point
High volatility
Runny/low viscosity
High flammability (clear/less smoky)
What are the products of incomplete combustion
Carbon monoxide and water. Is toxic, colourless and odourless.
What is the test for CO2
Turns limewater cloudy
What is the test for water
Turns blue cobalt chloride paper pink
Turns white anhydrous copper sulfate blue
What is the test for saturation with halogen Bromine (alkanes)
Saturated
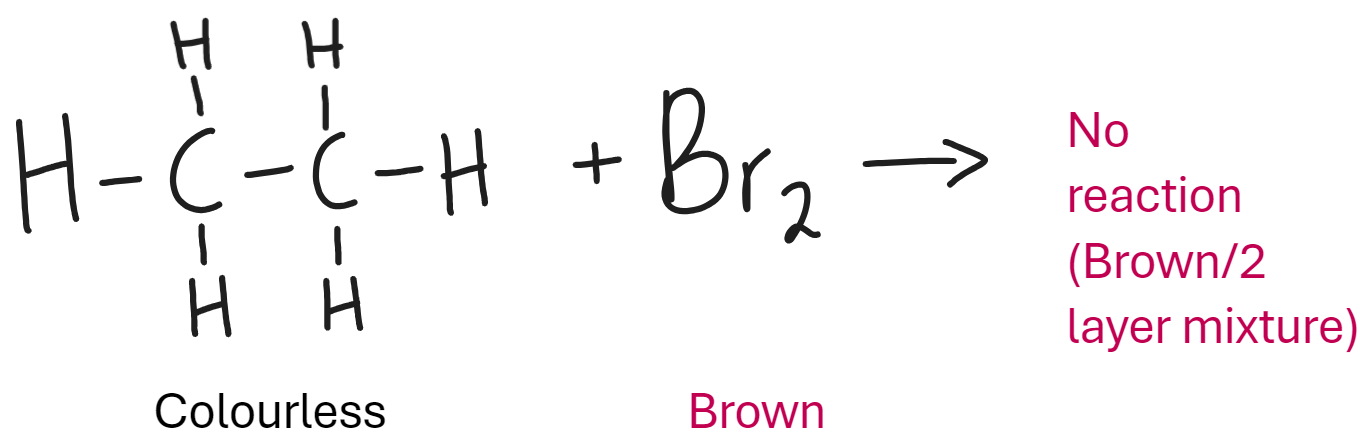
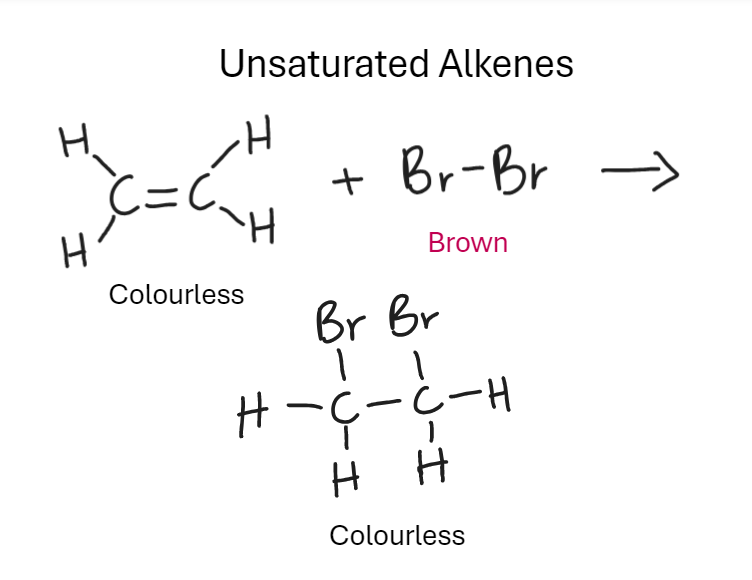
What is the test for saturation with halogen Bromine (Alkenes)
Unsaturated as more hydrogen atoms can be added still
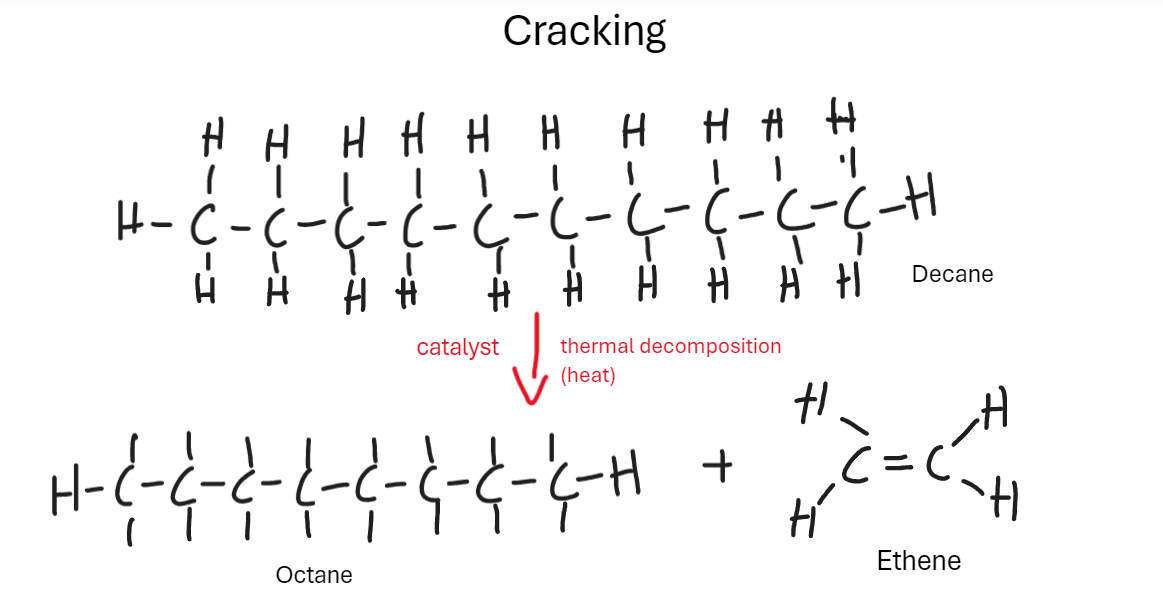
Explain the process of cracking [6 Marks]
Long-chained hydrocarbons are broken down using heat and a specific catalyst to form smaller, more useful, short chained hydrocarbons.
How is petrol separated from crude oil using fractional distillation [6 Marks]
Crude oil is heated to approximately 300`C at the bottom of the column to vaporise it
As the gases move up through the column, the temperature drops and becomes cooler
Different boiling points mean gases condense at different points in the column. Petrol condenses near the top as it is a shorter chained hydrocarbon.
This gets syphoned off while the rest of the vapour continue up.
Longer chained hydrocarbons near the bottom and short chained near the top as lower BP.