Module 14 - Intracellular Signaling and Transduction
1/39
Earn XP
Description and Tags
included on exam 4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
intracellular receptors
receptors that are recognized by signal molecules
many are transcription reculators
signal molecules can be steroids or amino acid-derived hormones, e.g., which are small and lipid-soluble
response requires expression of hormone-activated genes
cortisol
a steroid hormone
produced by the adrenal cortex (near the kidneys)
targets many organs
effects: adaptation to long-term stress by raising blood glucose levels and mobilizing fat
can bind to an intracellular receptor to bind protein to DNA
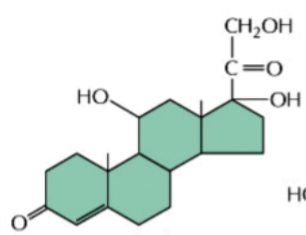
steroid hormones
lipid-soluble hormones derived from cholesterol
e.g. cortisol, estradiol, testosterone
contain 3 6-membered rings and 1 5-membered ring
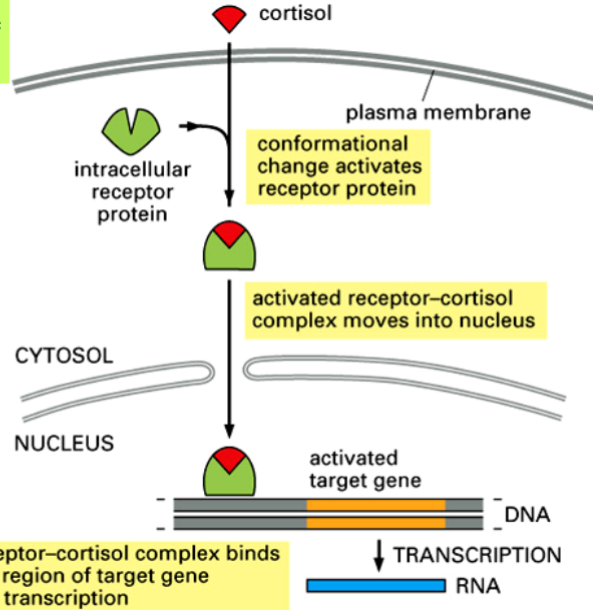
the cortisol receptor, and binding of cortisol
cortisol receptor is a transcription factor
in absence of cortisol, receptor remains inactive in the cytosol
cortisol enters cell and binds to receptor → conformational change occurs → receptor becomes an active transcription regulator
active protein goes to cell nucleus and binds to specific regulatory DNA sequences of target genes
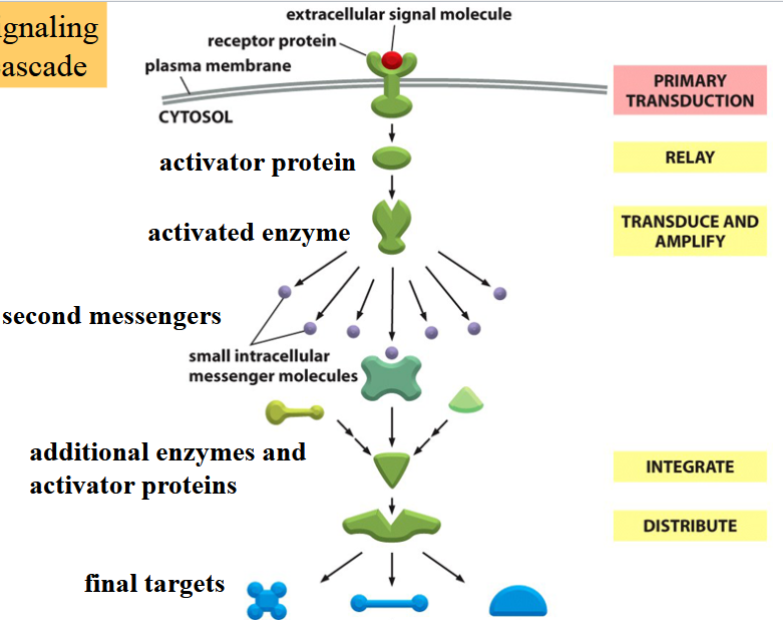
signal transduction
the relay of an outside signal through an intracellular signaling cascade involving a number of intermediate molecules with different activities
leads to final targets
can be in pathways
signal transduction pathways
triggered when an external signal molecule binds to a surface receptor → becomes activated
activated receptor relays signal to cell’s interior → initiate intracellular cascade of molecular events leading to a response
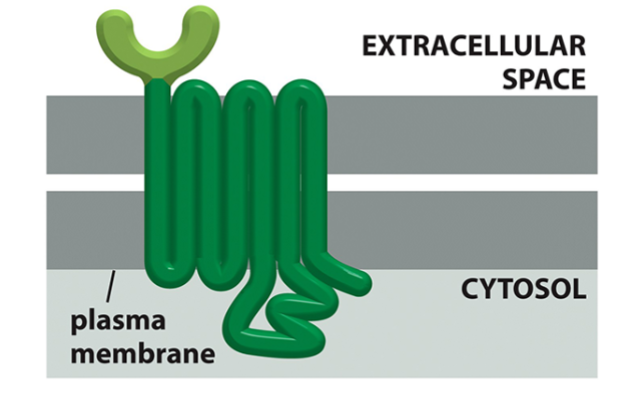
domains of a G protein-coupled receptor
receptor (extracellular domain)
7 transmembrane domains
activator of G protein (cytoplasmic domain)
G protein
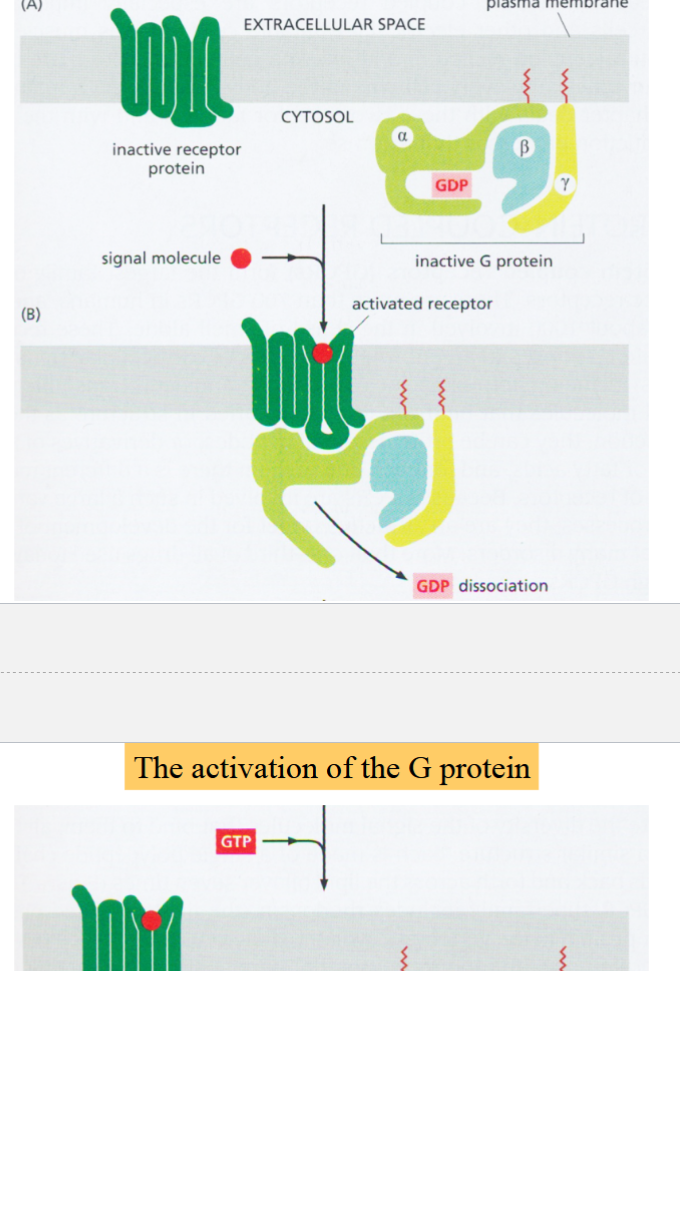
a membrane-bound protein complex with three subunits
activated by GTP
when inactive, all three subunits are associated, alpha subunit has a GDP bound to it
when receptor is activated, G protein binds to it:
GDP dissociates from alpha-subunit, replaced with GTP
alpha-subunit dissociates from beta and gamma subunits
beta and gamma subunits remain associated and act as a single unit (beta-gamma complex)
both alpha-subunit and beta-gamma complex are active, can interact with different targets
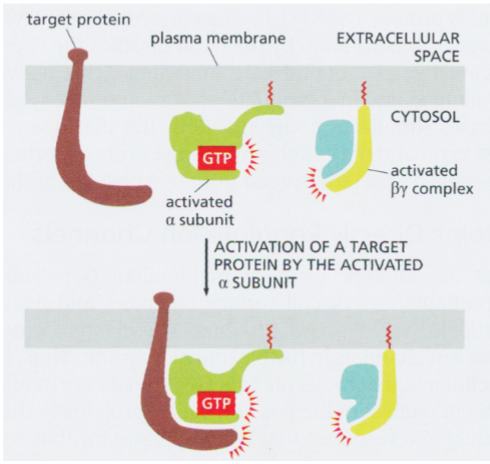
activated alpha subunit of a G protein
can interact with and regulate activity of membrane-bound target proteins
target proteins are often enzymes
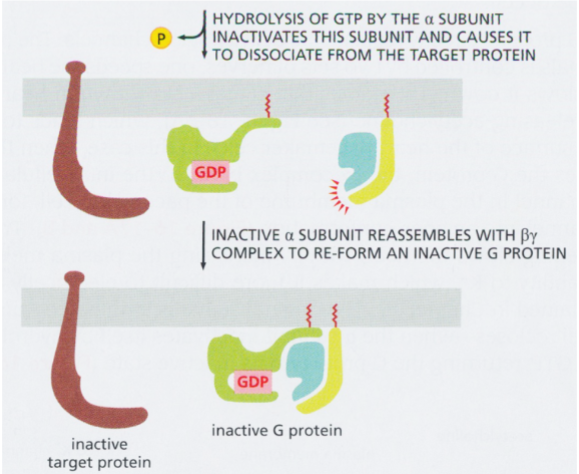
inactivation of G protein
hydrolysis of GTP → re-association of alpha with beta and gamma → inactivation of G protein
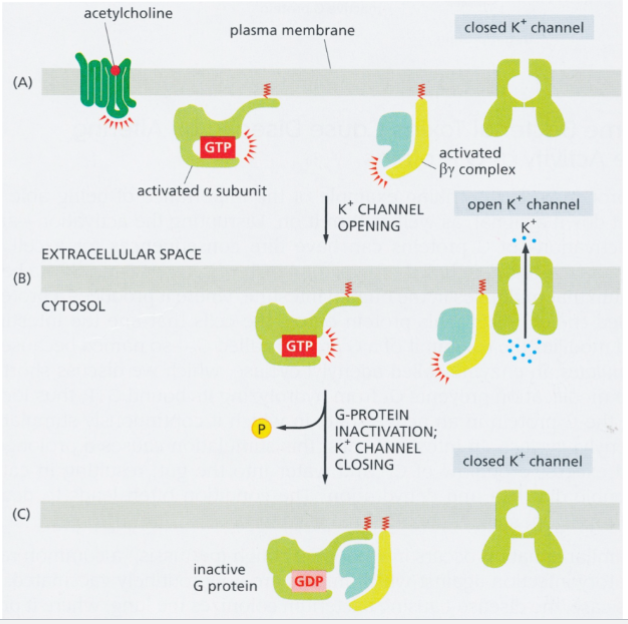
activated beta-gamma complex
in heart muscle cells: directly opens a K+ channel in response to ACh from parasympathetic neurons (target protein is an ion channel)
result: reduced frequency of cardiac muscle contraction
when alpha subunit reassociates with the complex, K+ channel closes
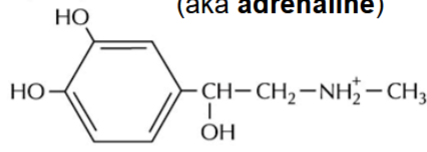
epinephrine (adrenaline)
produced by the adrenal medulla
stimulates glycogen breakdown in anticipation of muscular activity
stops glycogen production
used as a neurotransmitter by sympathetic neurons as part of fight-or-flight response
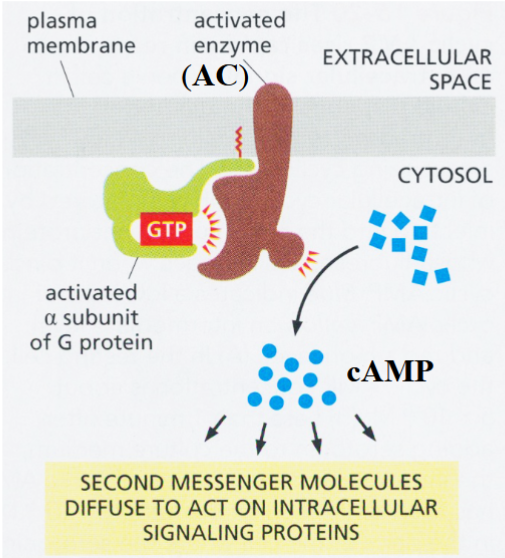
epinephrine receptor
a G protein-coupled receptor
activated G protein activates adenylyl cyclase (AC) → AC catalyzes conversion of ATP → cAMP
result: elevated cAMP levels → activation of protein kinase A (PKA)
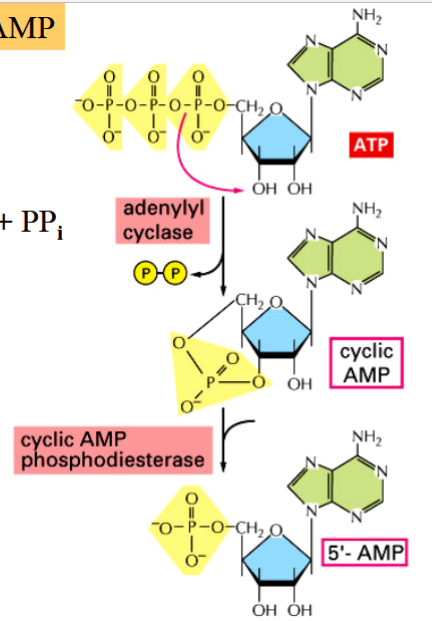
cAMP
cyclic AMP
a second messenger, activates PKA
ATP → cAMP + PPi (activation, catalyzed by AC)
cAMP → AMP (deactivation)
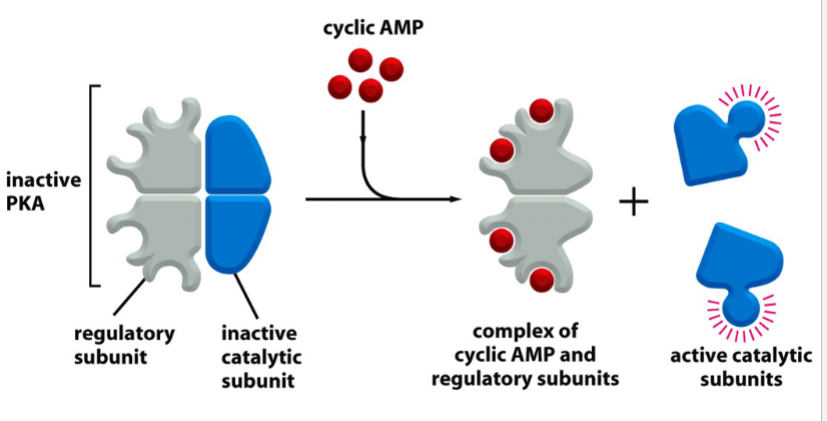
PKA
protein kinase A
cAMP-dependent
promotes glycogen metabolism
when inactive, is a tetramer with 2 regulatory (R) subunits bound to 2 catalytic (C) subunits
activated by cAMP: cAMP binds to R subunits → C subunits are released
kinase activity of released C subunits phosphorylates multiple effector proteins
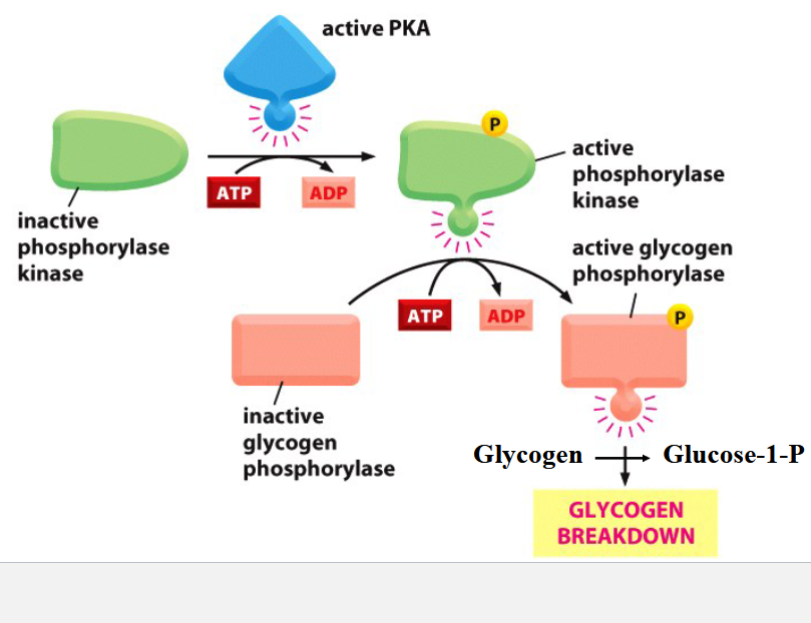
glycogen breakdown in skeletal muscle cells
a fast response to PKA activation
PKA phosphorylates/activates phosphorylase kinase
phosphorylase kinase phosphorylates and activates glycolgen phosphorylase
glycogen phosphorylase breaks up glycogen into glucose units (as glucose-1-P)
phosphoglucomutase isomerizes G1P into G6P → G6P enters glycolysis
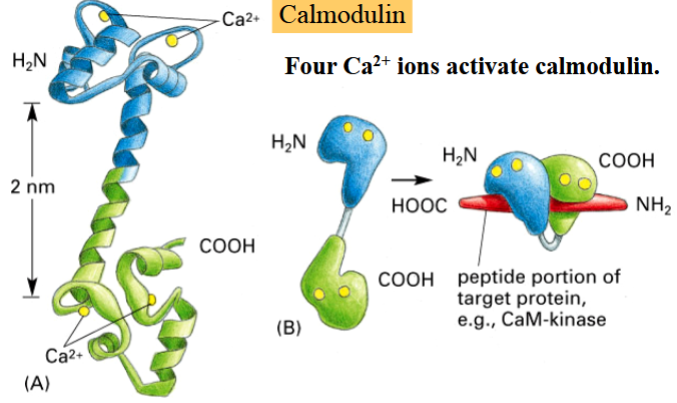
calmodulin
a small Ca2+-binding protein that is involved in modulation of many cellular processes by activating many calmodulin-dependent kinases and phosphatases
activated by 4 Ca2+ ions
activated by a pathway
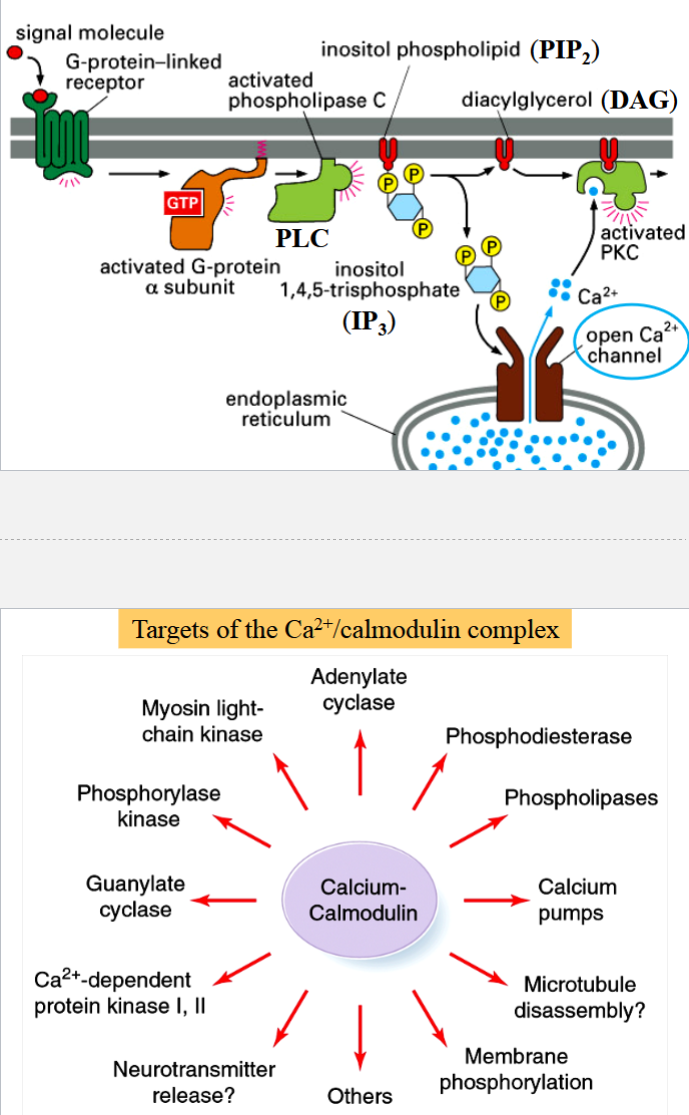
calmodulin activation pathway
begins with activation of a G protein
activated alpha subunit activates phospholipase C (PLC)
PLC catalyzes hydrolysis of phosphatidyl inositol biphosphate (PIP2) into diacyl glycerol (DAG, membrane bound) and inositol triphosphate (IP3, released as free cytoplasmic messenger)
DAG and IP3 both can activate other cellular functions
IP3 is ligand for IP-gated Ca2+ channels on ER
released Ca2+ ions bind to calmodulin → activates Ca2+/calmodulin-dependent (CaM) kinases
Wnt proteins
a large family of secreted signals
name is fusion of wingless gene in Drosophila + integrated, a vertebrate homolog
involves a signaling pathway
the Wnt signaling pathway
involved in cell differentiation, establishment of embryonic axes, and cell adhesion
activates beta-catenin (a transcription factor, important for transcription of cell differentiation genes in neighboring cells)
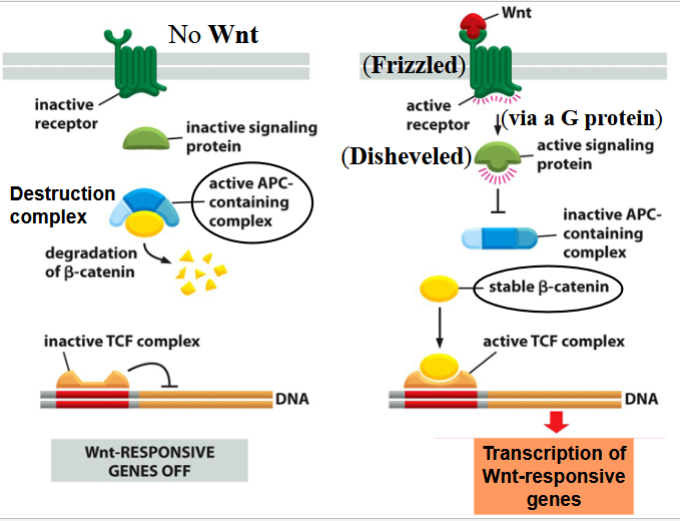
canonical pathway:
if no Wnt signal present: destruction complex forms in the cytoplasm
a complex containing APC (a tumor suppressor) is active → targets beta-catenin for proteolytic degradation
if Wnt signal is present: Wnt binds Frizzled (a GPCR) → Frizzled activates Disheveled through a G protein
Disheveled inactivates the APC-containing complex
stable beta-catenin enters nucleus, joins and activates a transcription factor (TCF) complex, promotes transcription of cell differentiation genes
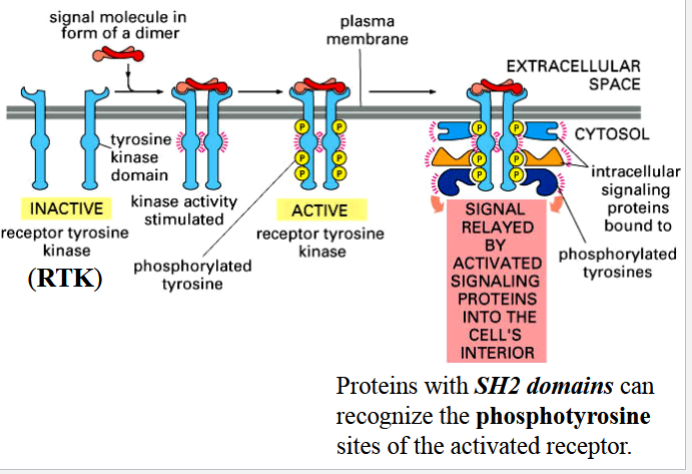
RTKs
receptor tyrosine kinases
enzymic receptors with single transmembrane domains that form dimers upon binding of a signal
3 steps in activation
steps in activating an RTK
1) binding of signal as a dimer causes formation of receptor subunit dimers
2) cytoplasmic TK domains of the receptor subunits phosphorylate each other
3) phosphorylated tyrosines become binding sites for signaling proteins that contain an SH2 domain
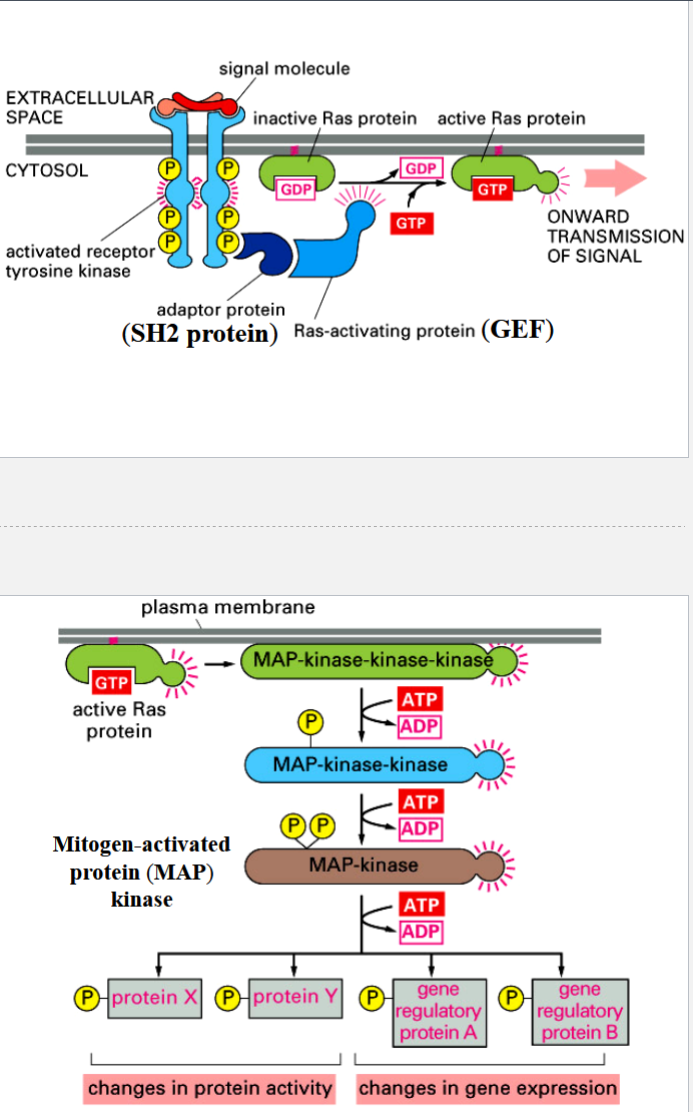
the Ras-MAP pathway
a pathway that controls cell proliferation and differentiation in response to growth factors
signal molecule: mitogen (promotes mitosis)
activated RTK is recognized by adapter protein (an SH2 protein)
adapter protein recruits Ras-activating protein (a GEF)
GEF activates Ras (a monomeric GTP-binding protein) by exchanging its GDP for a GTP
Ras-GTP activates a MAP kinase-kinase-kinase → triggers MAP kinase cascade of activation of protein kinases by phosphorylation
Ras can hydrolyze its GTP → GDP to deactivate itself (Ras has GTPase activity)
importance of involving many intermediates before a cellular response
1) signal can be amplified
2) each step ban be a target for regulation/modulation of the response
2 is especially important in controlling cell proliferation
MAP kinase and Ras: importance of many intermediates
MAP kinases and Ras are coded by proto-oncogenes
some mutations turn them into oncogenes because activity of gene products becomes independent of GTP or phosphorylation → independent of growth factors → result: abnormal cell proliferation
(mutated lack of independence on intermediates → abnormal proliferation → cancer)
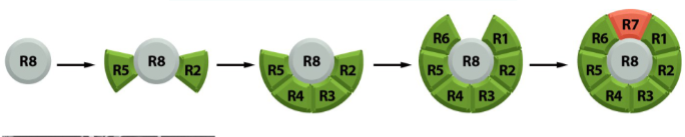
Drosophila compound eye
eye has 800 ommatidia
each ommatidia consists of 8 photoreceptor cells and 12 lens cells
all 20 cells differentiate from eye epithelial cells
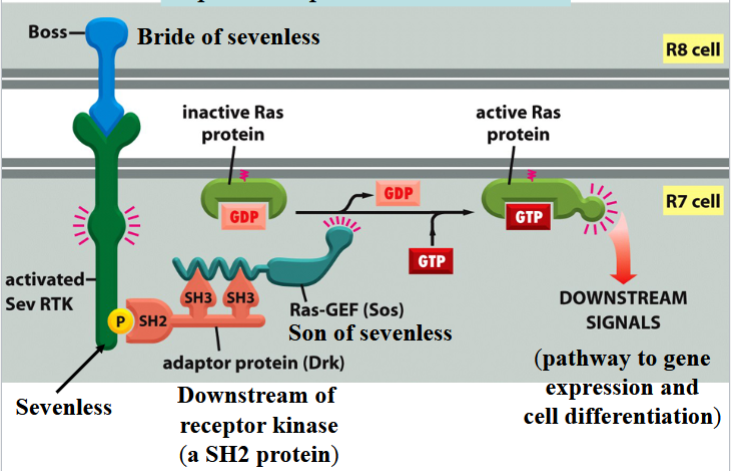
R8 differentiates into a photoreceptor cell first → then induces 7 neighboring cells to become photoreceptor cells → end with cell R7
R7 differentiation involves Boss, Sevenless, Drk, and Sos
remaining 12 cells of the ommatidia (1 eye unit) become lens cells by default
Boss
bride of sevenless
a signal protein on the surface of cell R8
involved in R7 differentiation in Drosophila eye
blue in the picture
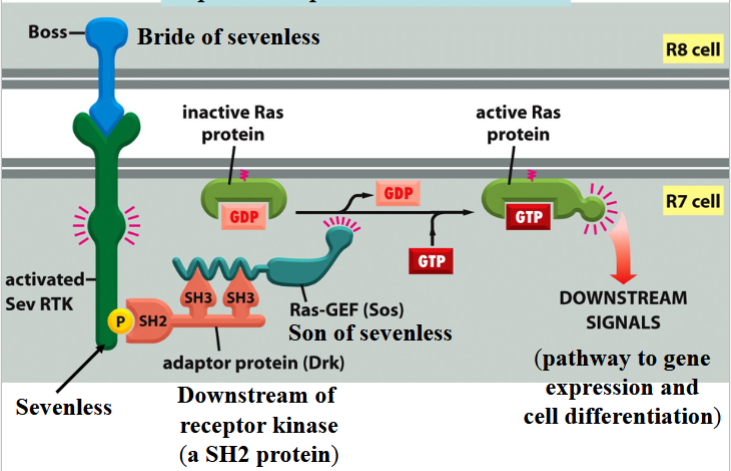
Sevenless
an RTK
Boss receptor on the surface of R7 in Drosophila
activates a Ras pathway
green in the picture
can be activated by phosphorylation to connect to Drk
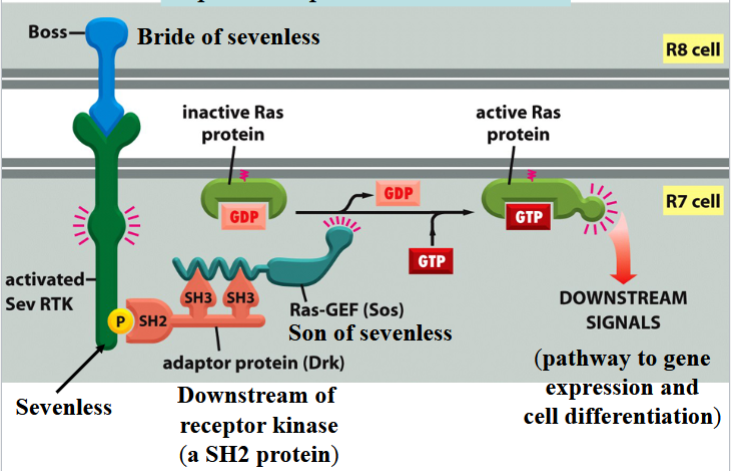
Drk
downstream of receptor kinase
an SH2 protein
connects to phosphorylated Sevenless to activate Ras
red/orange in the picture
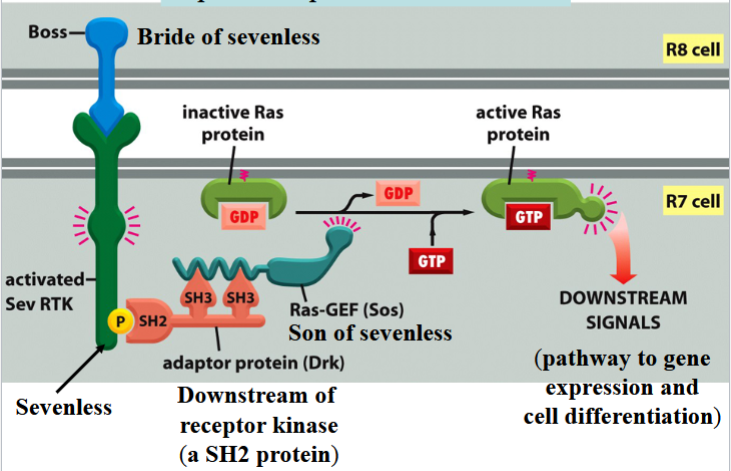
Sos
son of sevenless
a Ras-GEF
binds to Drk to activate Ras by swapping Ras’s GDP for a GTP
teal in the picture
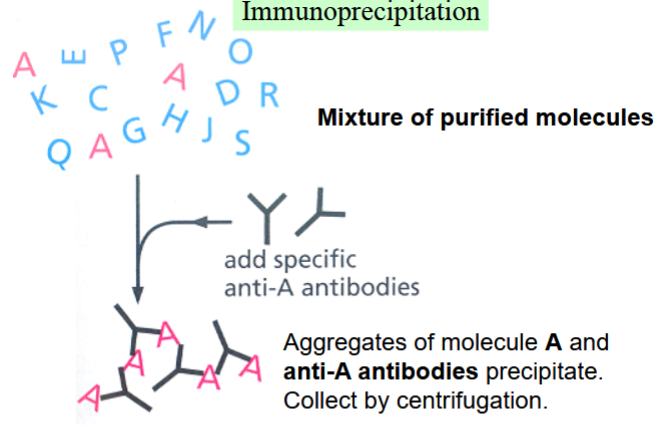
immunoprecipitation
one way to test whether protein-protein interactions are occurring
combine a mixture of purified molecules (e.g. cell extract) with antibodies specific to one molecule (molecule A, in the picture)
antibody and molecule A will aggregate and precipitate, collected by centrifugation
anything bound to A will also precipitate
elucidating signal transduction pathways
can identify receptor binding sites, or order of signaling proteins and Ras
different methods to elucidate both
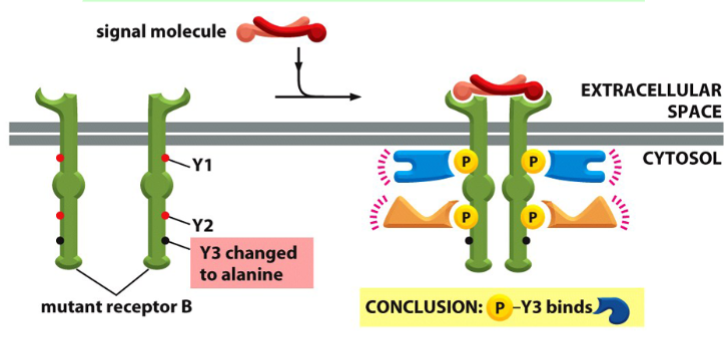
elucidating the identification of receptor binding sites in a signal transduction pathway
mutate specific sites, and use co-immunoprecipitation of receptors and SH2 proteins to see what protein did not bind
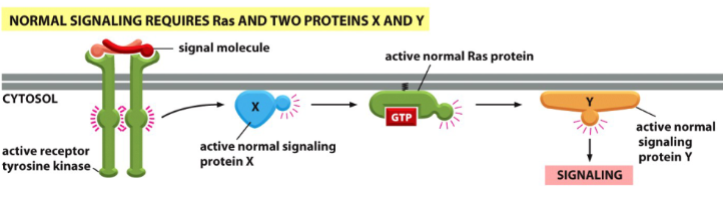
elucidating the order of signaling proteins in a signal transduction pathway
a protein upstream from Ras (e.g. X) regulates Ras
a protein downstream from Ras (e.g. Y) is regulated by Ras
if X is mutated and Ras is normal, no signaling will occur
if X is normal and Ras is mutated (oncogenic active), signaling will occur (Ras rescues the pathway)
if Y is mutated and Ras is normal, no signaling will occur
is Y is normal and Ras is mutated (oncogenic active), no signaling will occur (Ras cannot rescue the pathway)
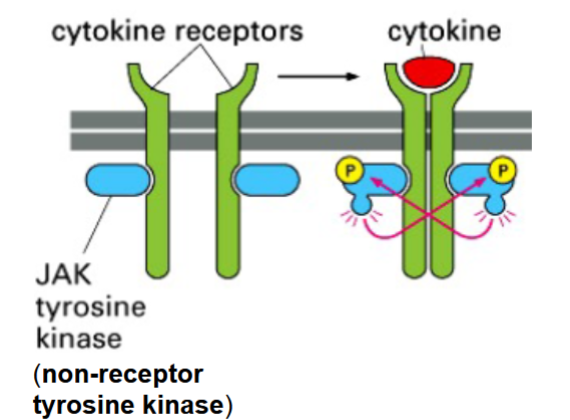
cytokines
soluble signals secreted by the lymphoid system (part of the immune/defense system)
receptors activate transcription factors, but do not have intrinsic enzymatic activity → receptors depend on associated protein kinases (they are non-receptor tyrosine kinases (NRTKs))
activated by dimerization of receptor subunits
activation of cytokine receptors
binding of signal causes receptor subunit homodimers to form
JAKs (non-RTKs) into close proximity so they can phosphorylate and activate each other
JAKs
janus kinases
non-receptor tyrosine kinases
phosphorylate each other, then the cytokine receptor subunits they are associated with
phosphorylated receptors are then recognized by STATs
part of the cytokine receptor and JAK-STAT pathway
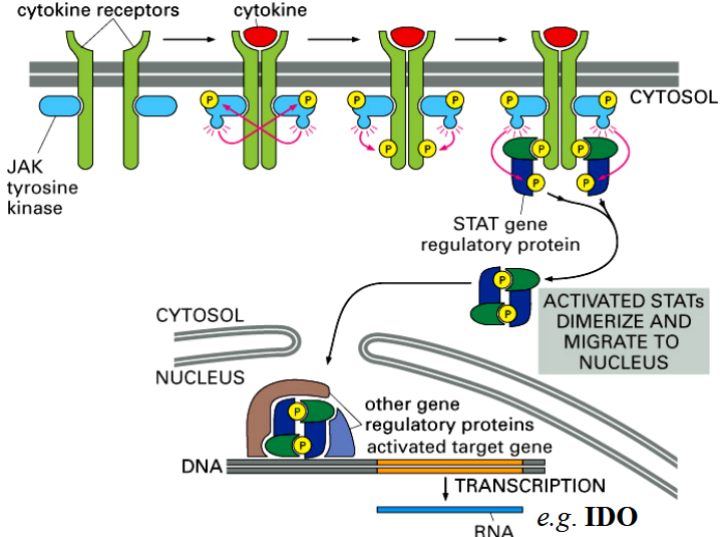
STATs
signal transducers and activators of transcription
DNA-binding proteins
phosphorylated and activated by JAKs
part of the cytokine receptor and JAK-STAT pathway
2 STATs form a dimer → go to nucleus → activate cytokine-responsive genes
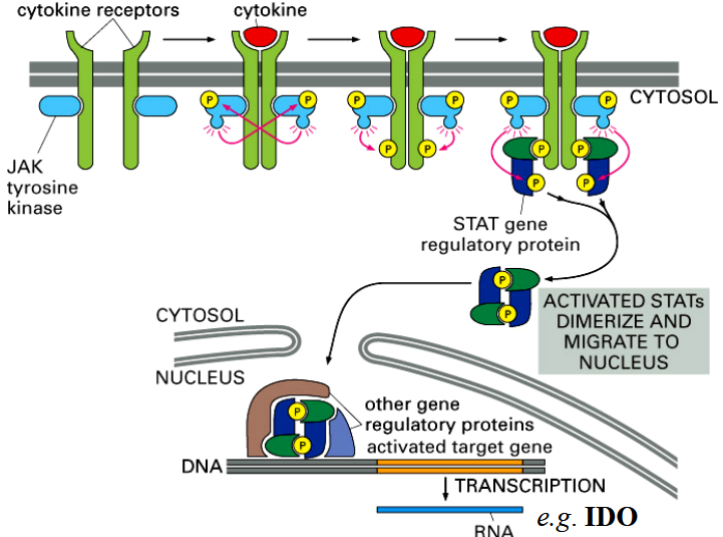
the interferon-gamma response pathway
natural killer cells can distinguish malignant from health cells, kill malignant cells by cell-mediated immunity
NK cell has different activating receptors, healthy vs malignant cells have different activating ligands
when NK receptors bind to malignant ligands, cytokines (IFN-gamma) is released
IFN-gamma binds to a receptor → triggers a JAK-STAT pathway in target cell → activates expression of the IDO gene → causes cell death via tryptophan starvation
IDO
idoleamine dioxygenase
an enzyme that catalyzes the degradation of tryptophan → causes a target cell to die of tryptophan starvation
released by NK cells when a malignant cell binds