Chemical Bonding
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
What are two or more chemically combined elements called?
compounds
What tells you the elements and how many of each element there are in a compound?
chemical formula
What is a charged atom called?
ion
What is the charge of an ion called?
oxidation number
What is a covalently bonded group of atoms that is charged called?
polyatomic ion
Al2O3
Aluminum oxide
K3PO4
Potassium Phosphate
HgClO3
Mercury (I) Chlorate
N2O
Dinitrogen monoxide
CuSO4
Copper (II) sulfate
Strontium bromide
SrBr2
Zinc (II) phosphide
Zn3P2
Sulfur hexachloride
SCl6
Dinitrogen tetroxide
N2O4
Lead (IV) carbonate
Pb(CO3)2
Why do elements form compounds?
to be stable
What will magnesium do to become stable?
lose 2 electrons
What is the overall charge of an ionic compound?
0
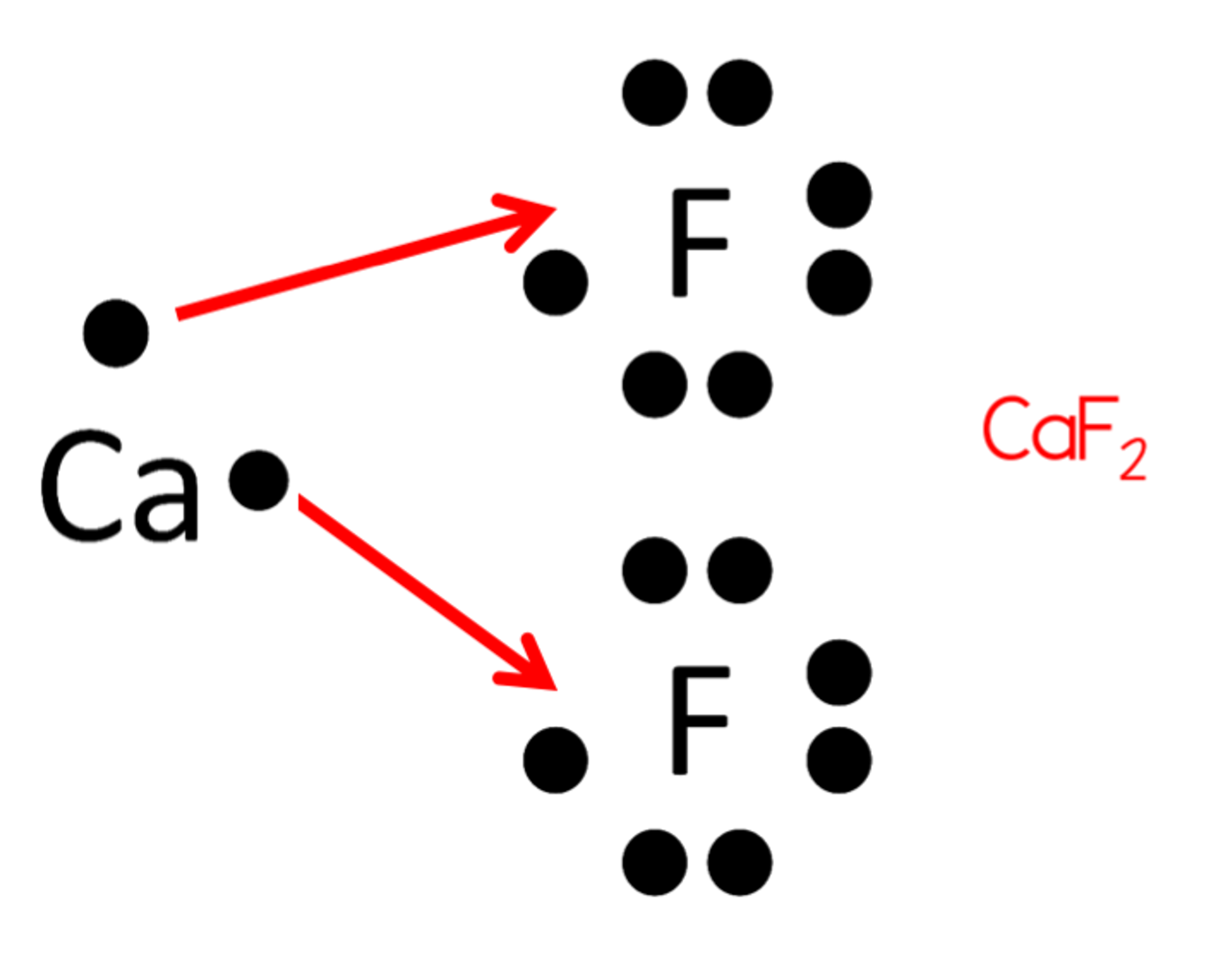
Draw an electron dot structure for calcium fluoride.
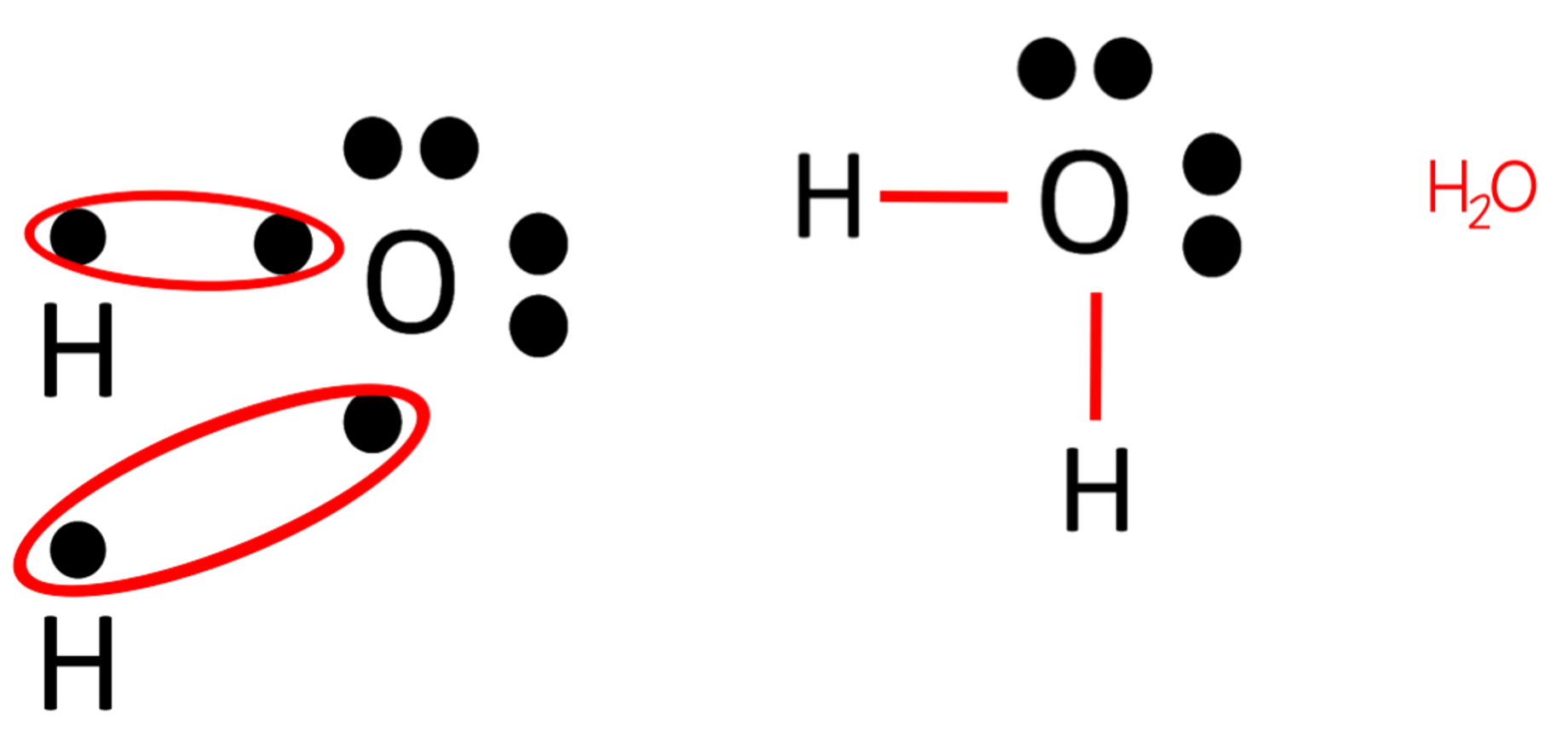
Draw an electron dot structure for the bond that would form between hydrogen and oxygen.
The properties of a compound are the properties of the elements that make it up.
Different from
What types of elements form covalent bonds?
Nonmetals
What happens to the electrons in an ionic bond?
They are transferred
When an atom loses an electron its charge is and it is called a(n) .
Positive; cation
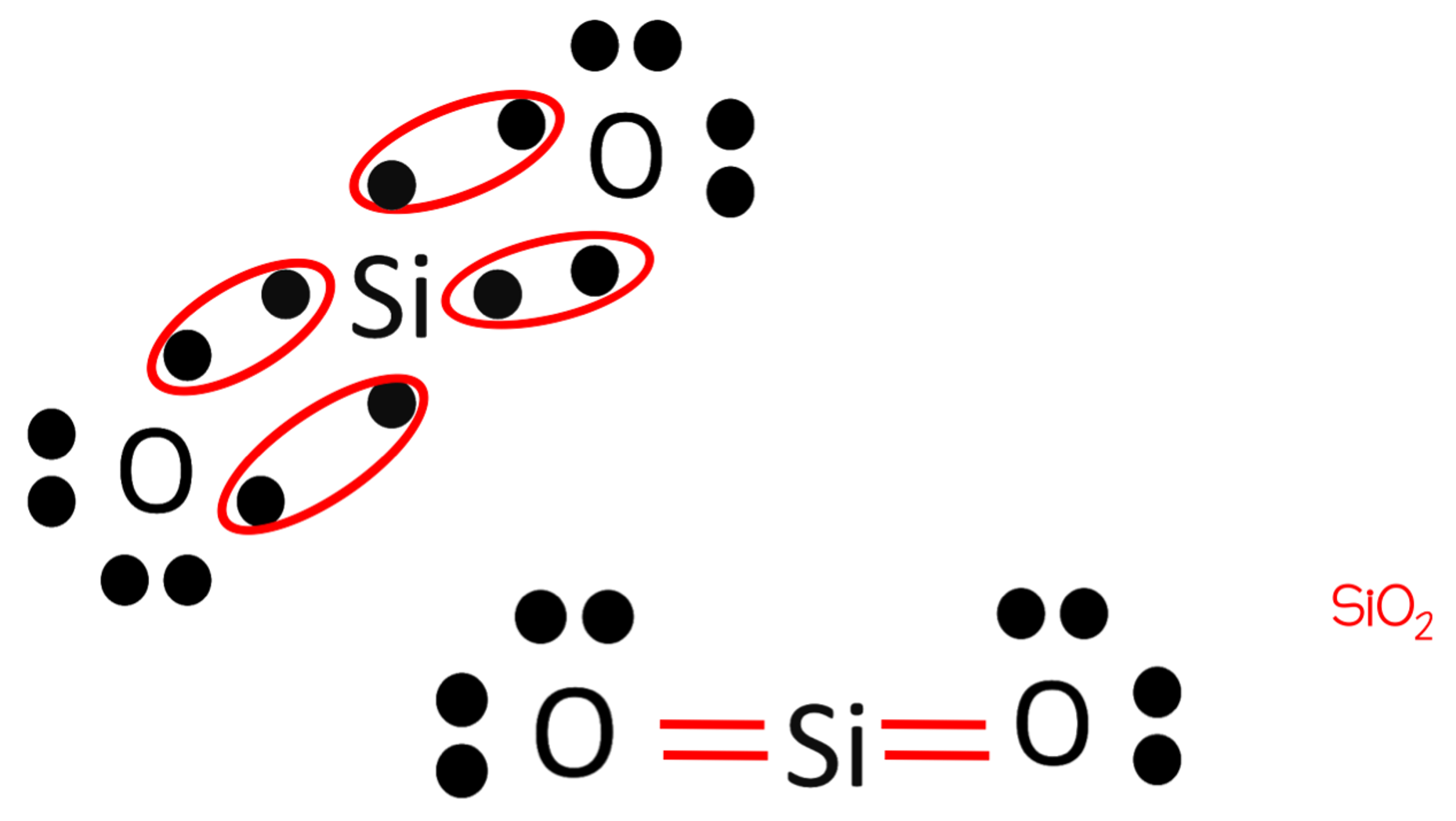
Draw the electron dot structure for the bond that would form between silicon and oxygen.
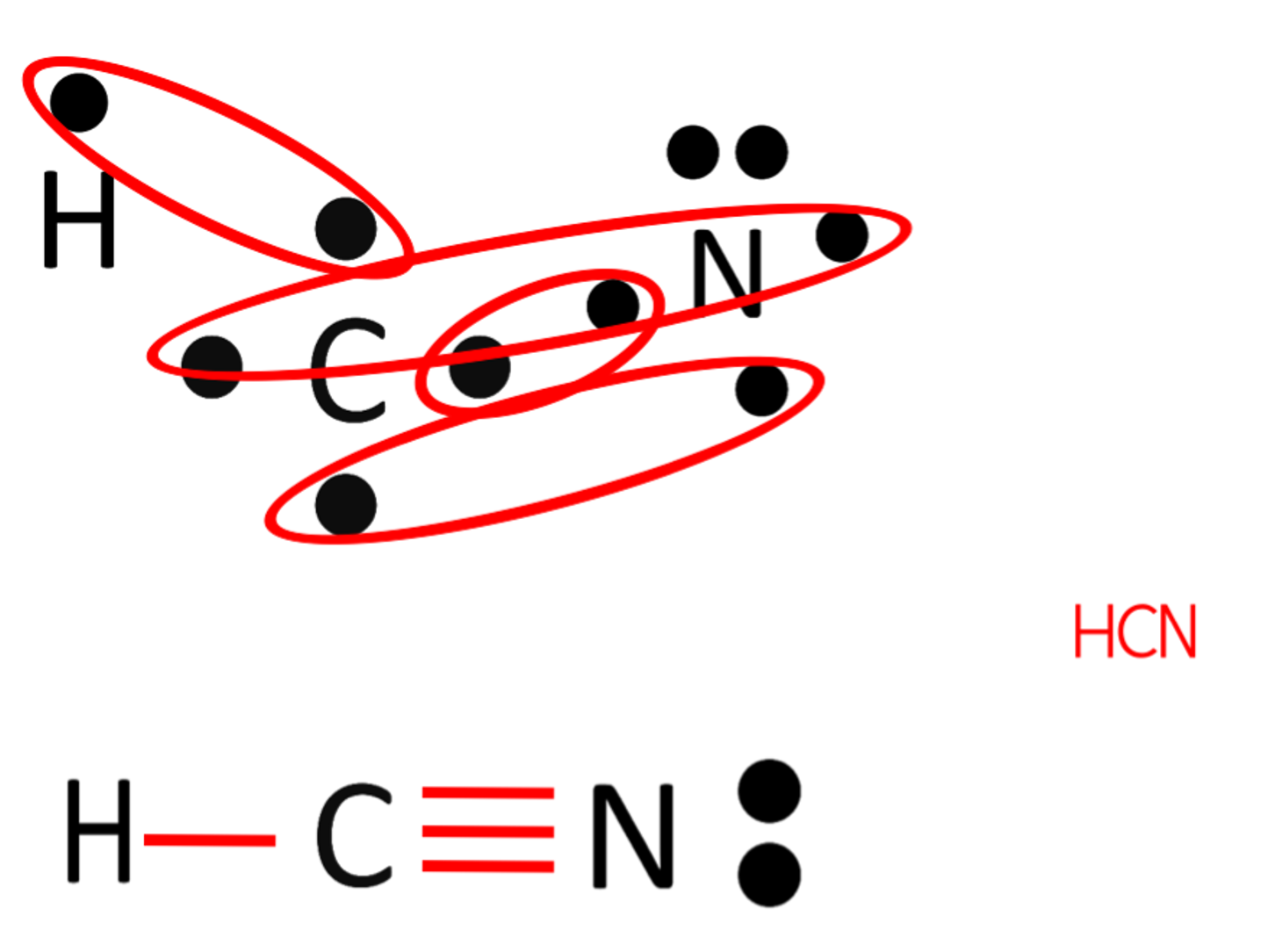
Draw the electron dot structure for HCN.
Ammonium
NH4+1.
Nitrate
NO3−1
Hydroxide
OH-1
Phosphate
PO4-3 |
Sulfate
SO4-2
Acetate
C2H3O2−1
Chlorate
ClO3 −1 |