Chem 261 Reactions
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
nucleophile
electron rich, donates a pair of electrons, Lewis base
electrophile
electron poor, accepts a pair of electrons, Lewis acid
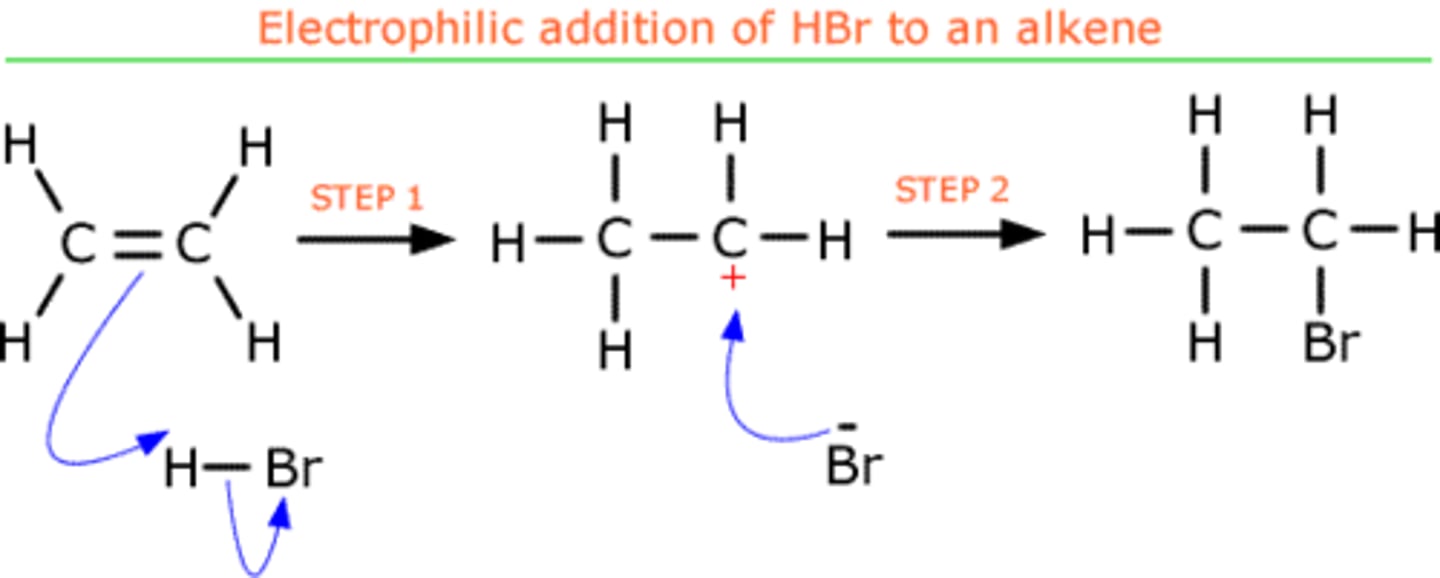
Addition of HX to Alkenes
type of electrophilic addition reaction
regioselective
follows Markovnikov's rule- 3 prime carbon would receive the halogen over the 2 prime (and 3 > 2 > 1)
regioselective
one product is formed exclusively
Markovnikov's Rule
in an electrophilic addition reaction, the nucleophile is added to the most substituted carbon and the electrophile is added to the least substituted carbon
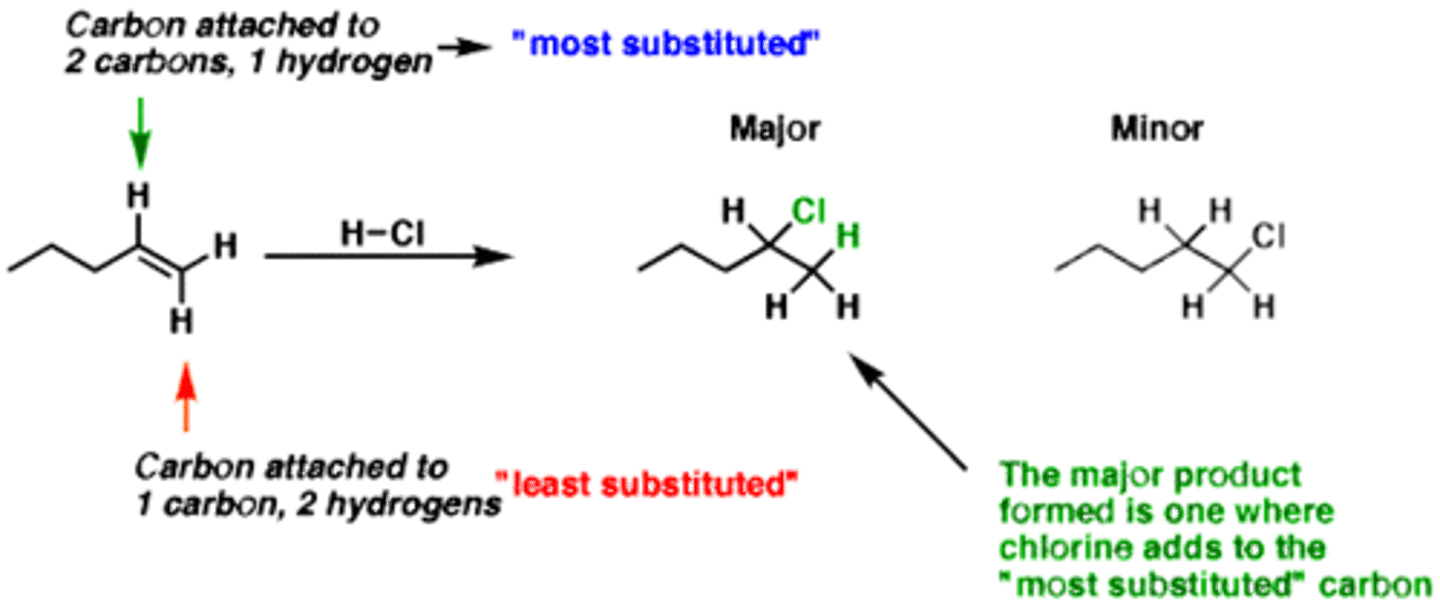
regiospecific
one of more and less of another
two structural isomer products; one made more than the other
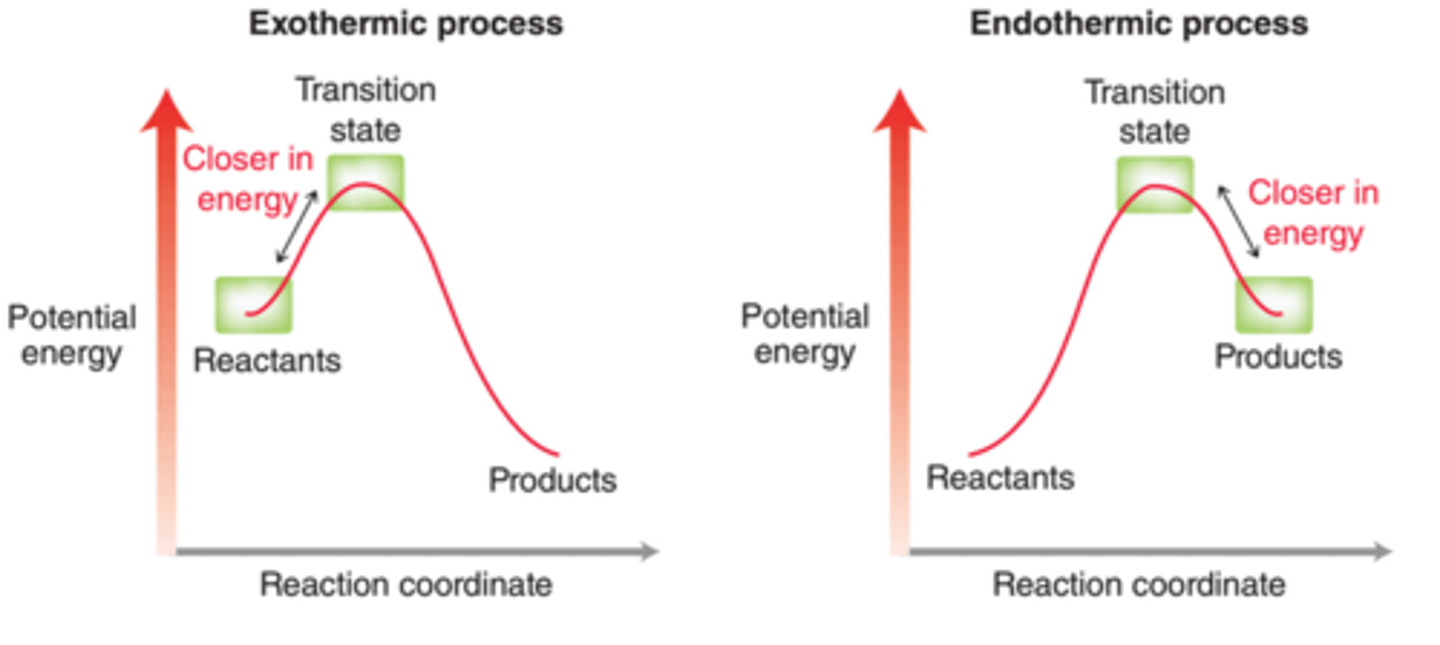
Hammond postulate
the transition state is more similar in structure to the species to which it is more similar in energy
exo= more similar to reactants
endo= more similar to products
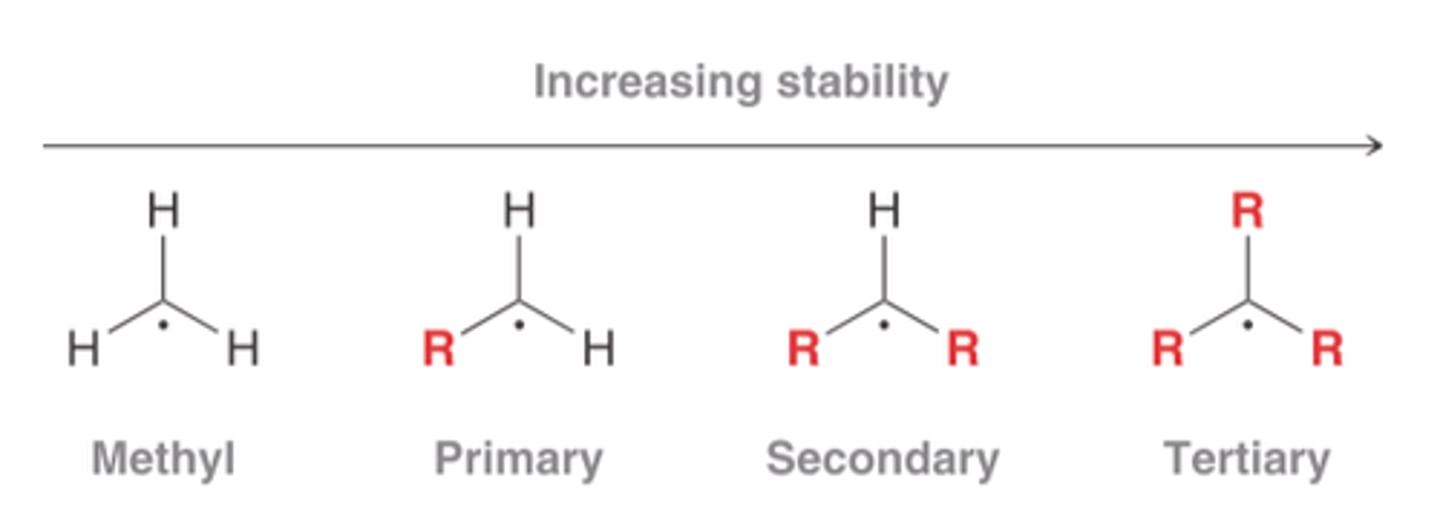
carbocation stability
highly reactive intermediates; cannot be observed directly in the reaction mixture as they react as soon as they are formed
the higher the hydride affinity, the least stable the carbocation has and the more energy the molecule has
methyl is the least stable, then phenyl... multiple rings are more stable due to resonance
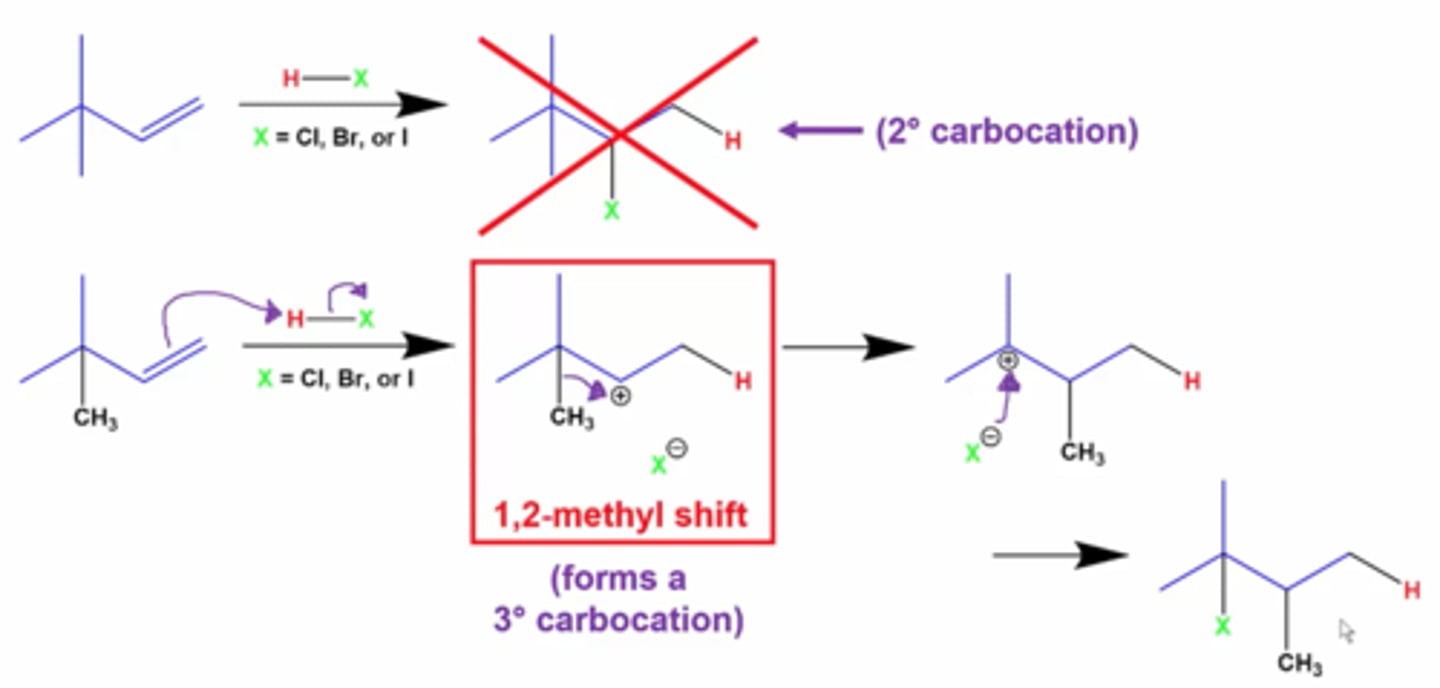
1,2 methyl shift
Rearrangement of a less stable carbocation to a more stable carbocation by the shift of a methyl group from one carbon atom to an adjacent carbon atom.
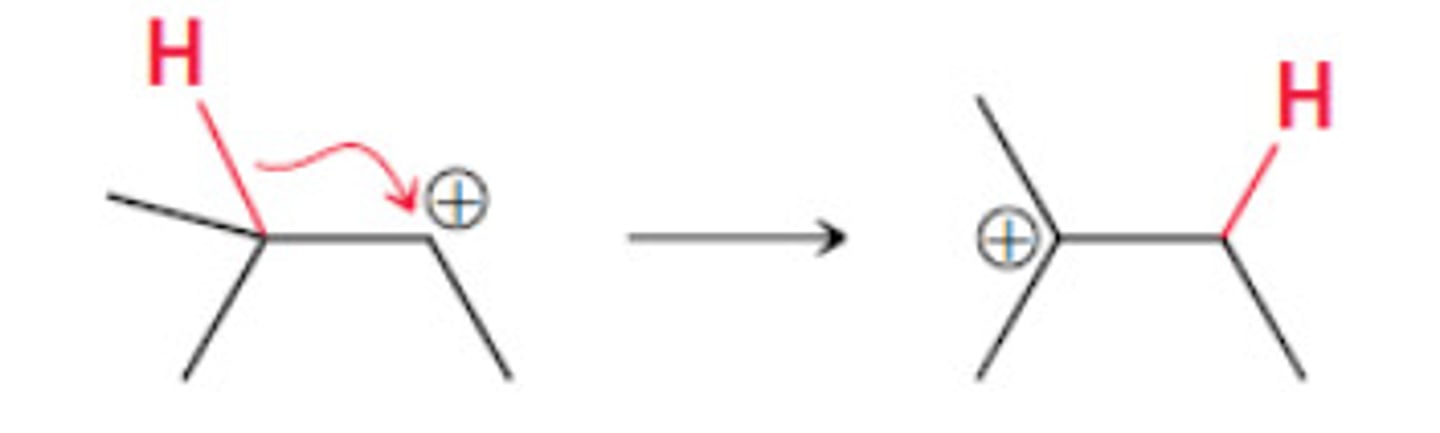
hydride shift
When a hydrogen moves from one carbocation to another for stability (normally not more than 1 carbon away)
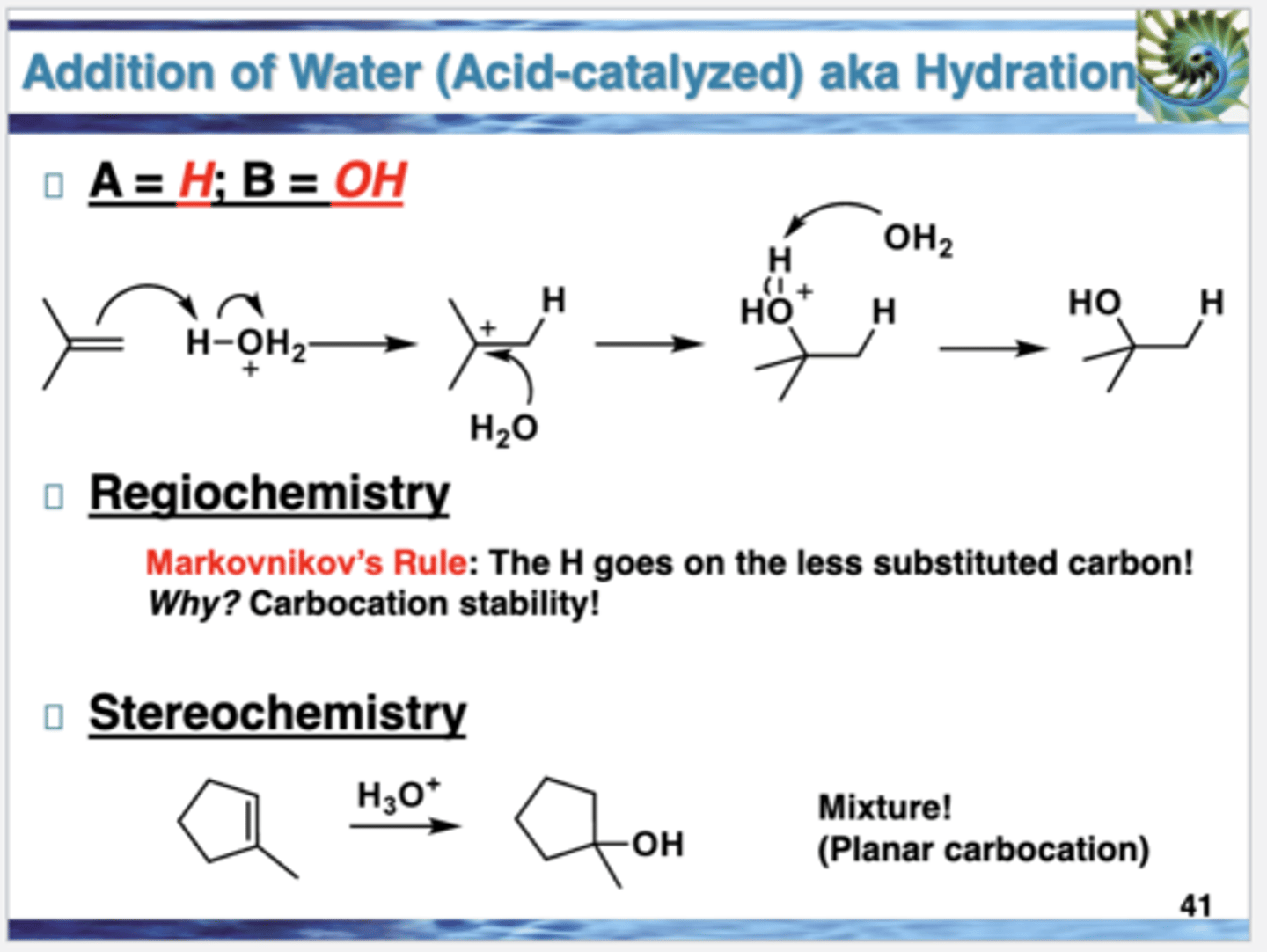
Addition of Water to Alkenes
also known as acid-catalyzed hydration of alkenes
reversible reaction, whether the alkene or the alcohol predominates at equilibrium depends on the reaction conditions
low temperatures and high concentrations of water favor the alcohol; higher temperatures and removal of water favors the alkene
properties of carbocations
they can trap nucleophiles
they can rearrange
they can trap alkene nucleophiles
they can lose a proton to form alkenes
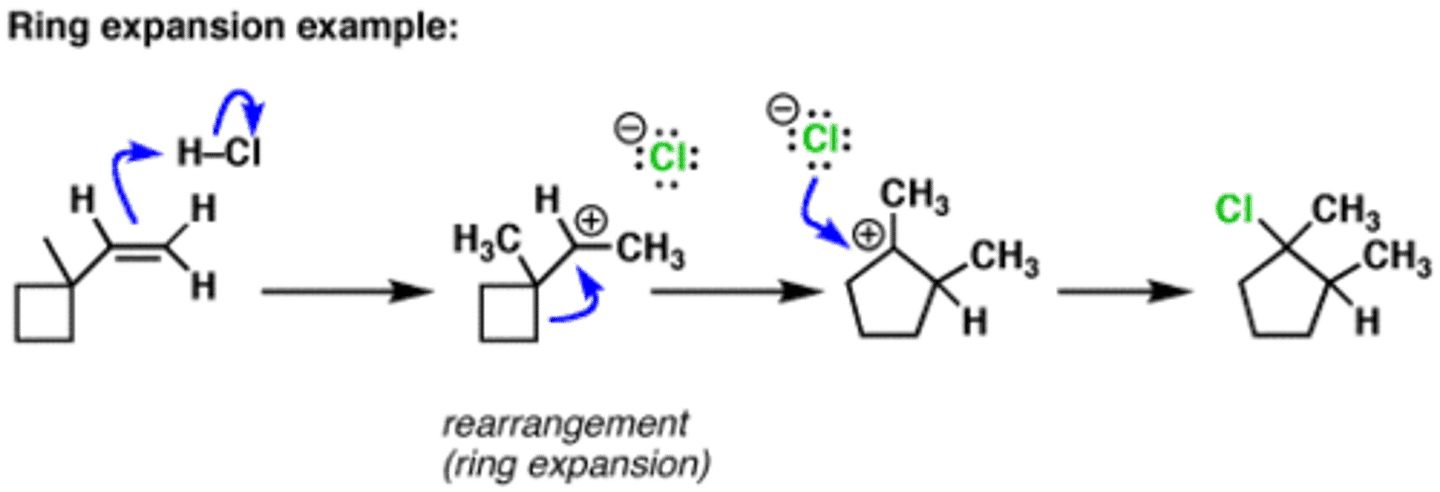
ring expansion
a ring having greater strain expands to form a larger ring, allowing for the resulting carbocation to have less angle strain; energetically downhill
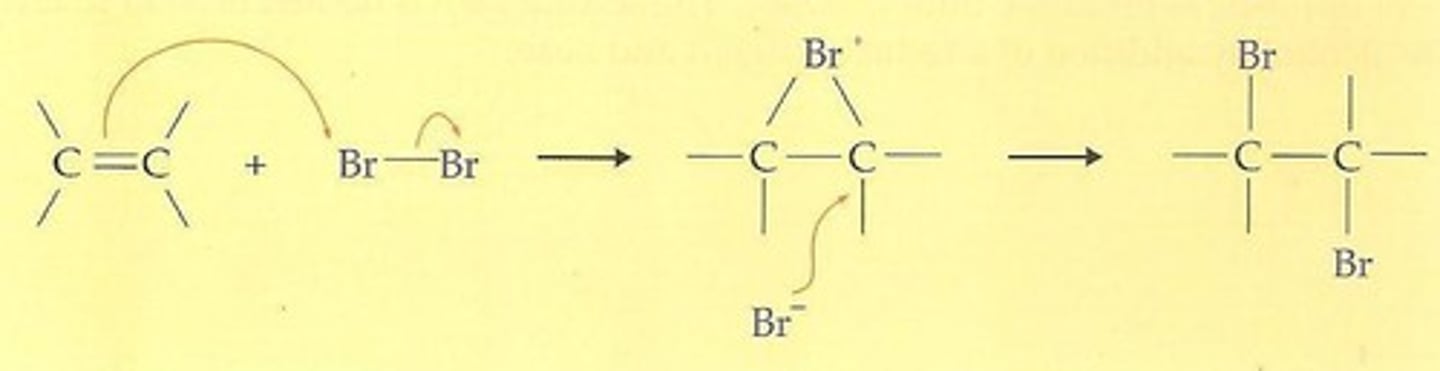
Halogenation of Alkenes
Br2 or Cl2
nucleophilic opening of a bridged ion by backside attack of Br- or Cl- at carbon, overall anti addition to double bond
stereospecific
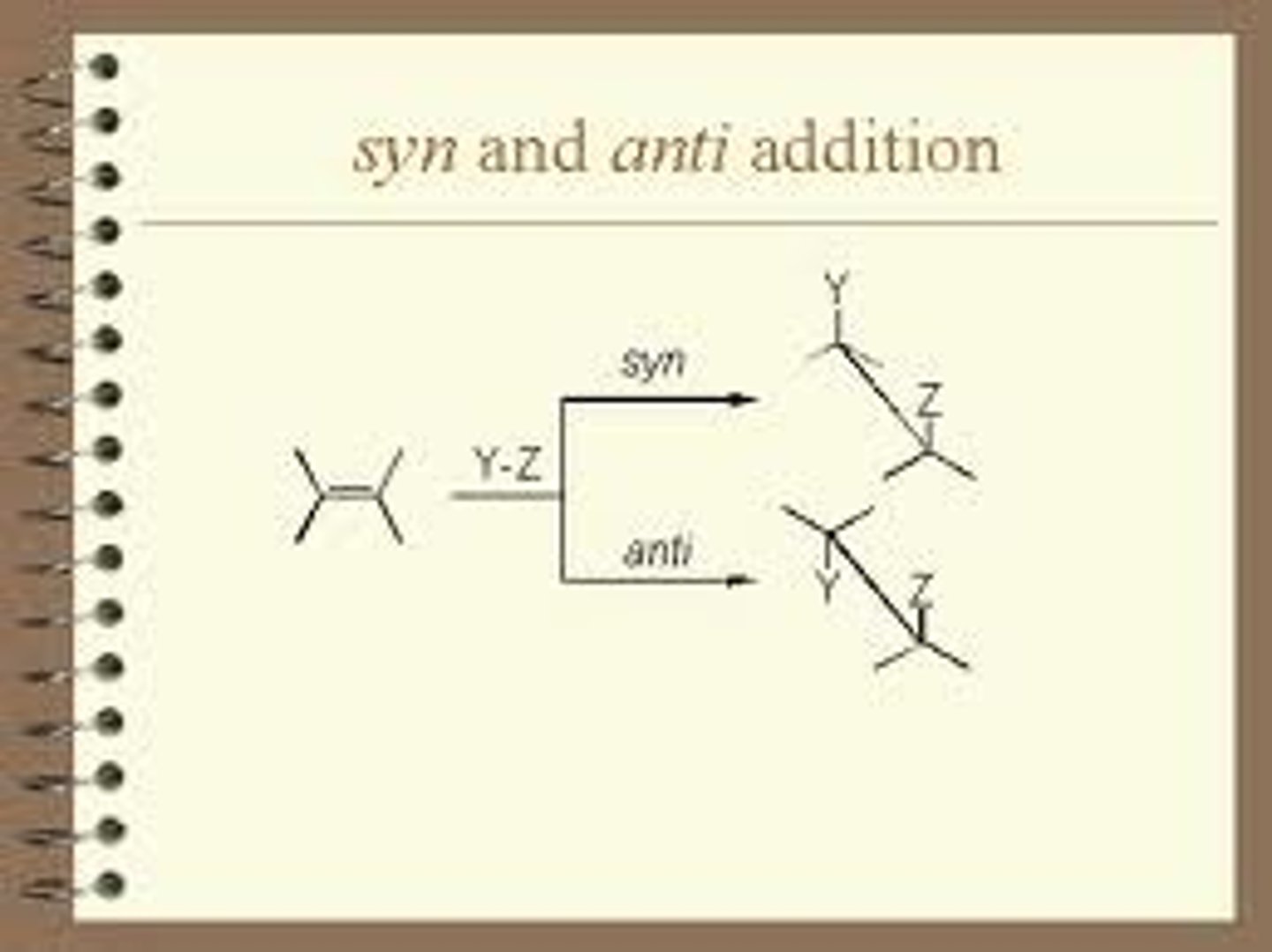
stereospecific
single reactant forms an unequal mixture of stereoisomers; due to 3D mechanism of reaction
usually involves the formation of a bridge
anti addition
An addition reaction in which two substituents are added to opposite sides (or faces) of a double bond or triple bond.
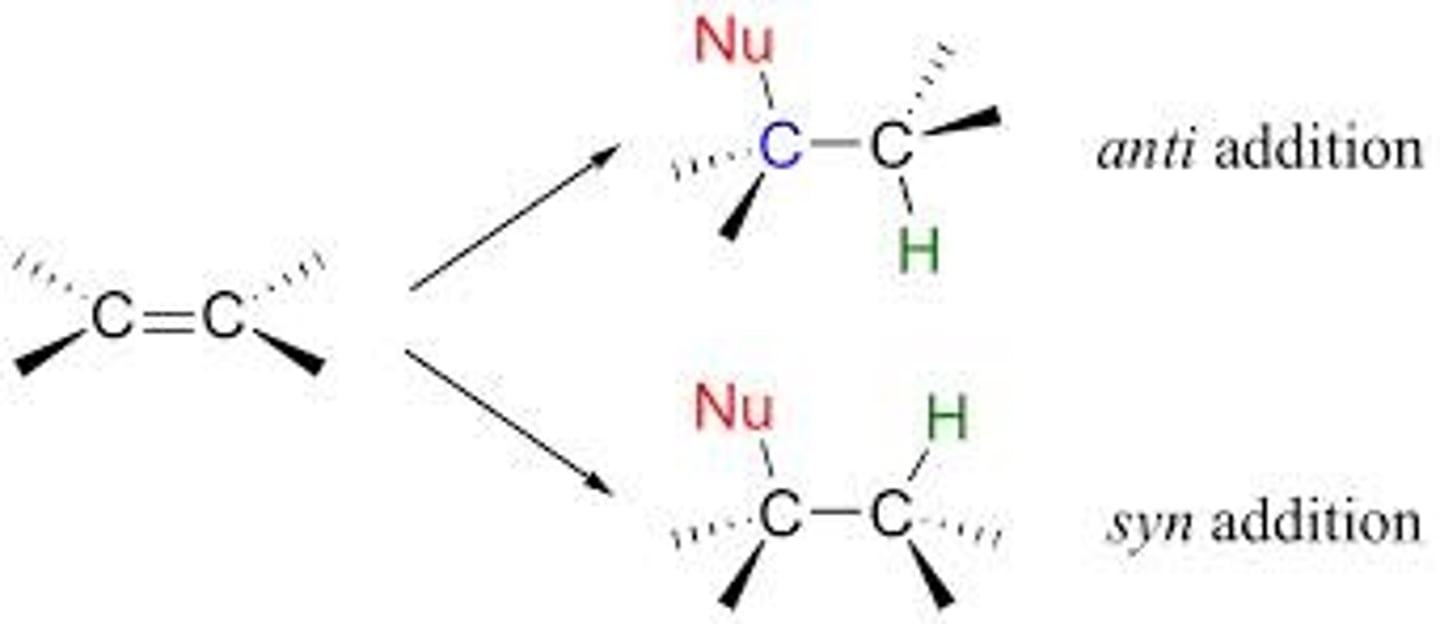
syn addition
addition of constituents to an alkene on the same side of the bond
deprotonation
The removal of a hydrogen cation (H+) from a molecule
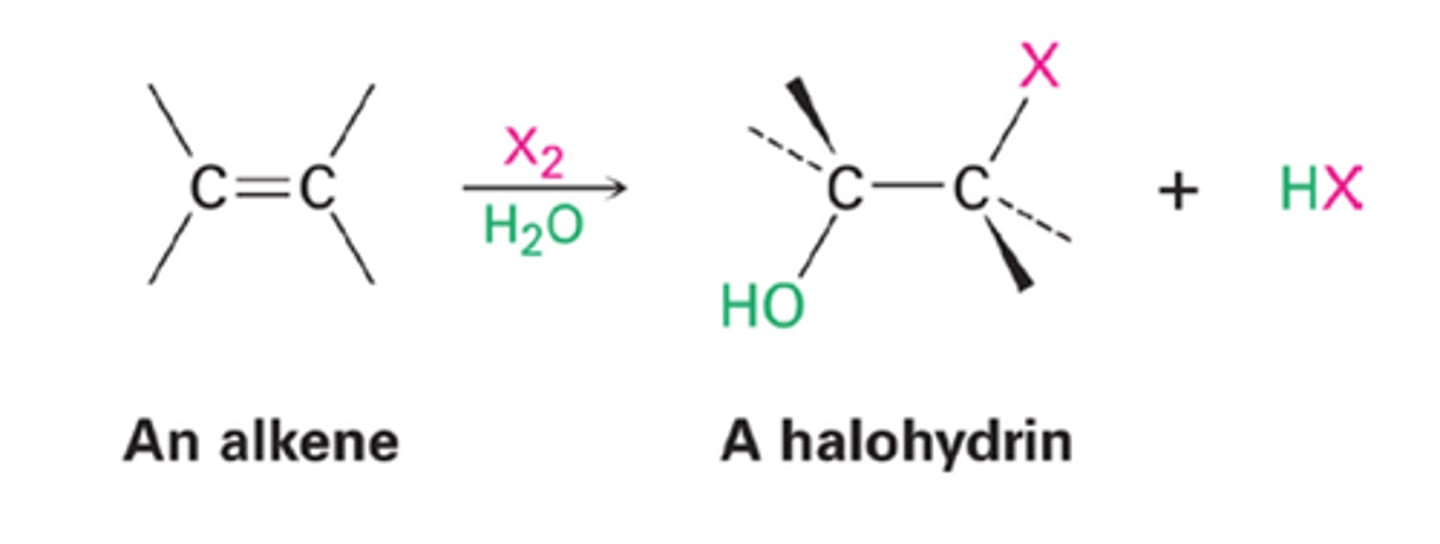
halohydrin (mixed additions to alkenes)
-Addition of X2
-Reagents: Br2 or Cl2 in H2O
-Bromonium or chloronium ion intercepted by H2O
-Markovnikov addition of H2O
-Anti addition stereochemical preference
-Z conformation makes SS and RR pair of enantiomers
mixed reagents:
RS-Cl = anti addition, stereospecific, makes only two stereoisomers (bridge)
O=N-Cl = no bridge, makes 4 stereoisomers
I-Cl = anti addition, stereospecific, makes only two stereoisomers (bridge)
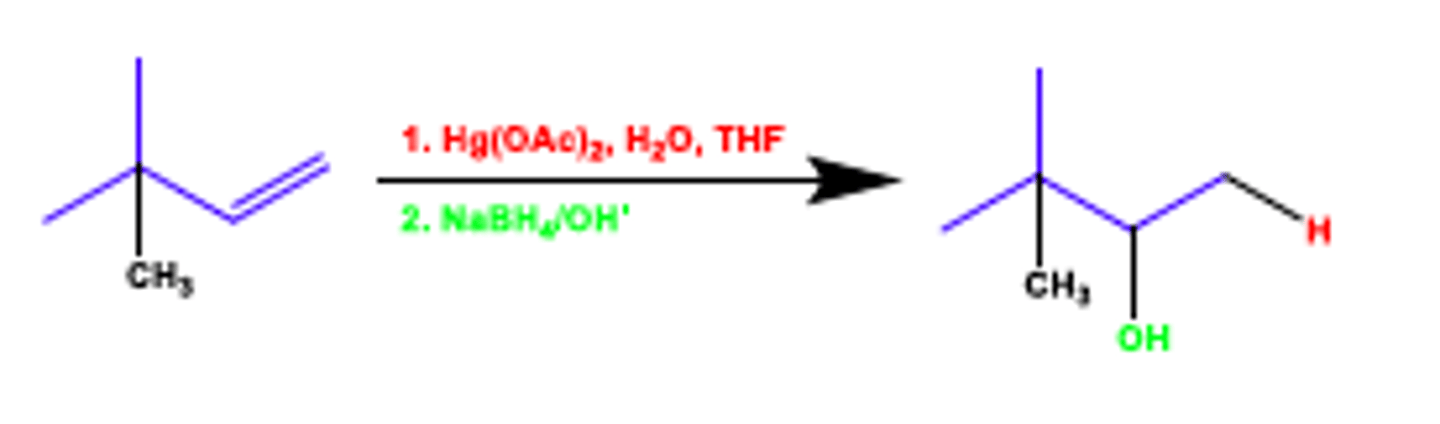
oxymercuration (addition of mercury acetate and water to alkenes to make alcohols or ethers (E+ = +HgOCOCH3))
markovnikov addition, no rearrangements, high yield
variation to make markovnikov ethers: use alcohol as solvent
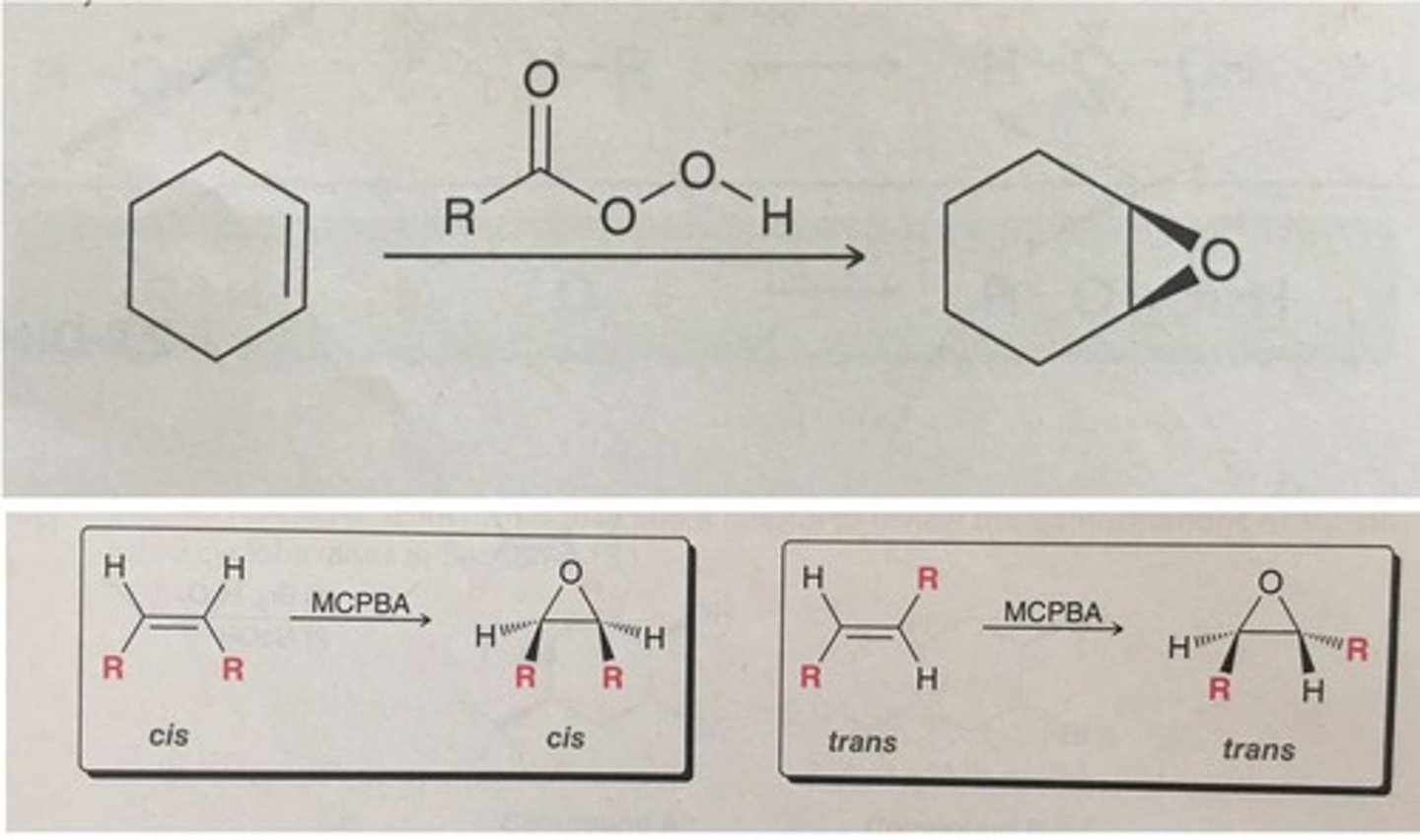
preparation of epoxide
-epoxides are highly reactive, strained ring, polar bond
-syn addition
-concerted (all happening at same time)
-stereospecific
-Z makes meso compounds
-E makes SS or RR enantiomers
concerted
all of the steps in the mechanism happen at the same time
Cyclization of 1,2 halohydrins (another method for preparing epoxides)
-adding Cl2 or Br2 in water
-then adding OH- to form the epoxide
-intramolecular reaction
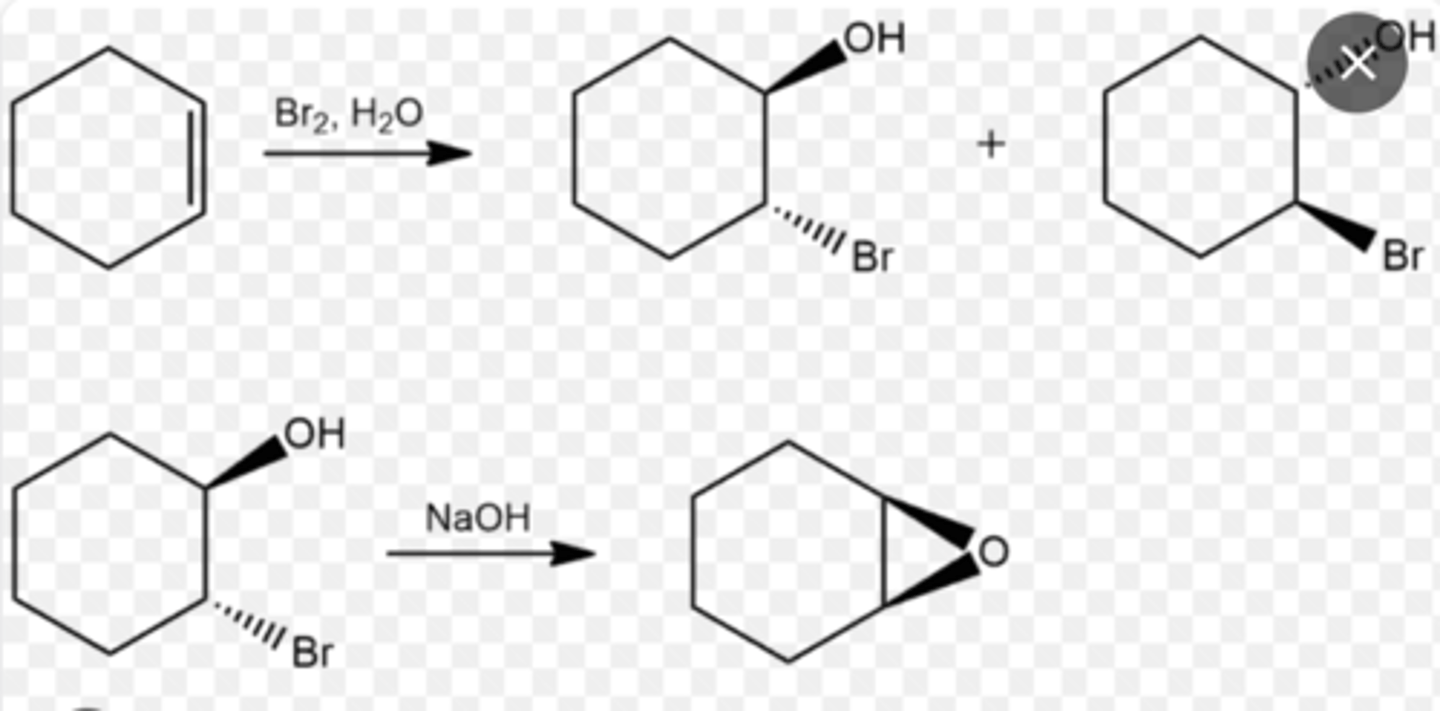
ozonolysis of alkenes (aka oxidative cleavage)
-ozone (O3) placed in a dry ice bath at -78 C (solid carbon dioxide in acetone)
-H2O/Zn or (CH3)2S added in step 2
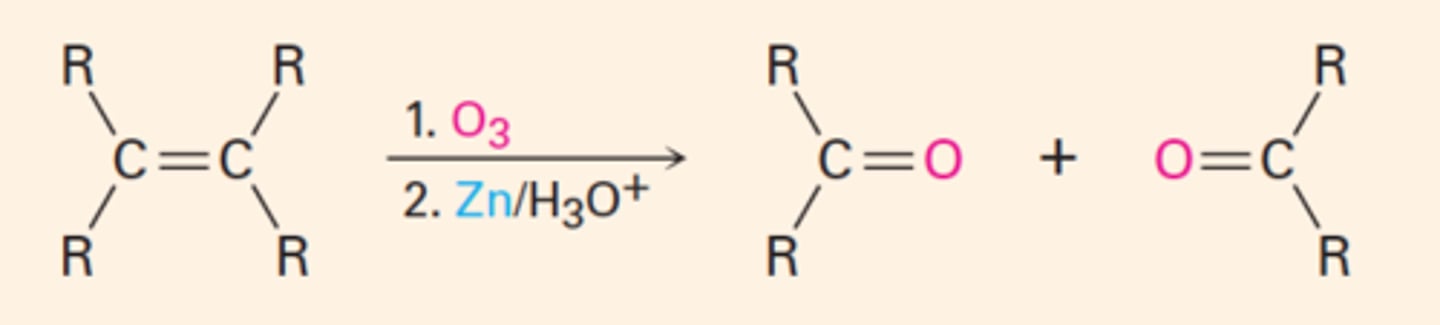
hydroboration
-anti-markonikov (no rearrangement as no carbocation is involved)
-syn addition
-BH3 (borane) reagent used in electrophilic addition reactions
-regioselective in opposite way
-different from bridge attacks, which are anti
-Z makes SS and RR pair of enantiomers
-E makes RS +SR; stereospecific
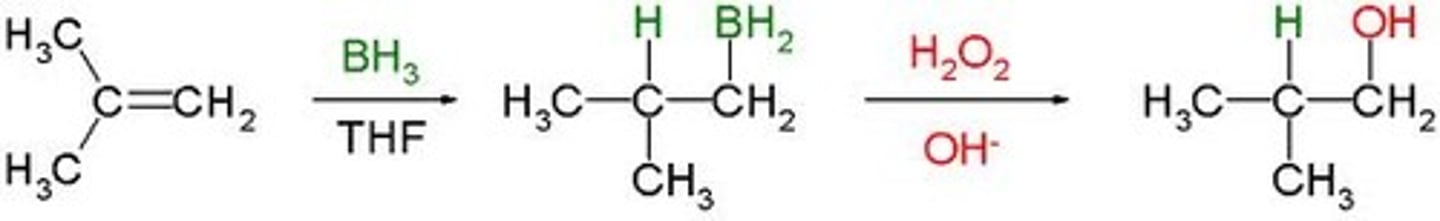
anti-Markovnikov
electrophile adds to the less substituted carbon, usually happens with a peroxide
dihydroxylation of alkenes
-syn OR anti addition, stereospecific
-adding a catalytic amount (very little!) of osmium tetroxide (OsO4) and (CH3)3(COOH) and NaOH as an oxidizing agent
-Z makes meso compounds
-E makes RR and SS enantiomers
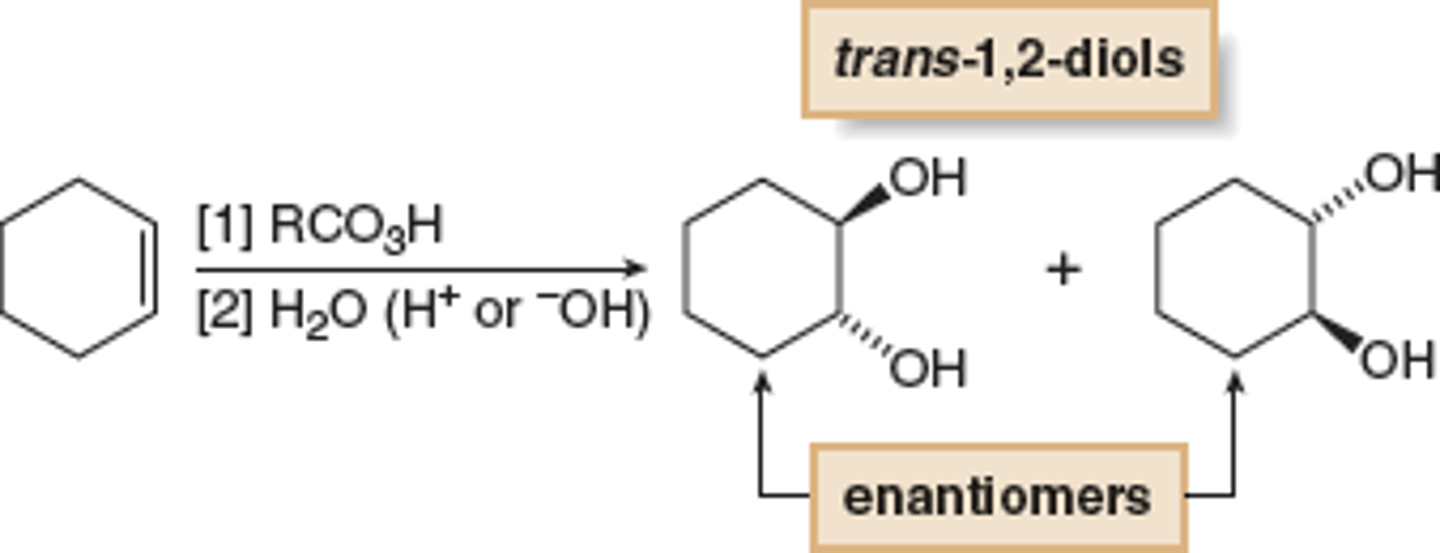
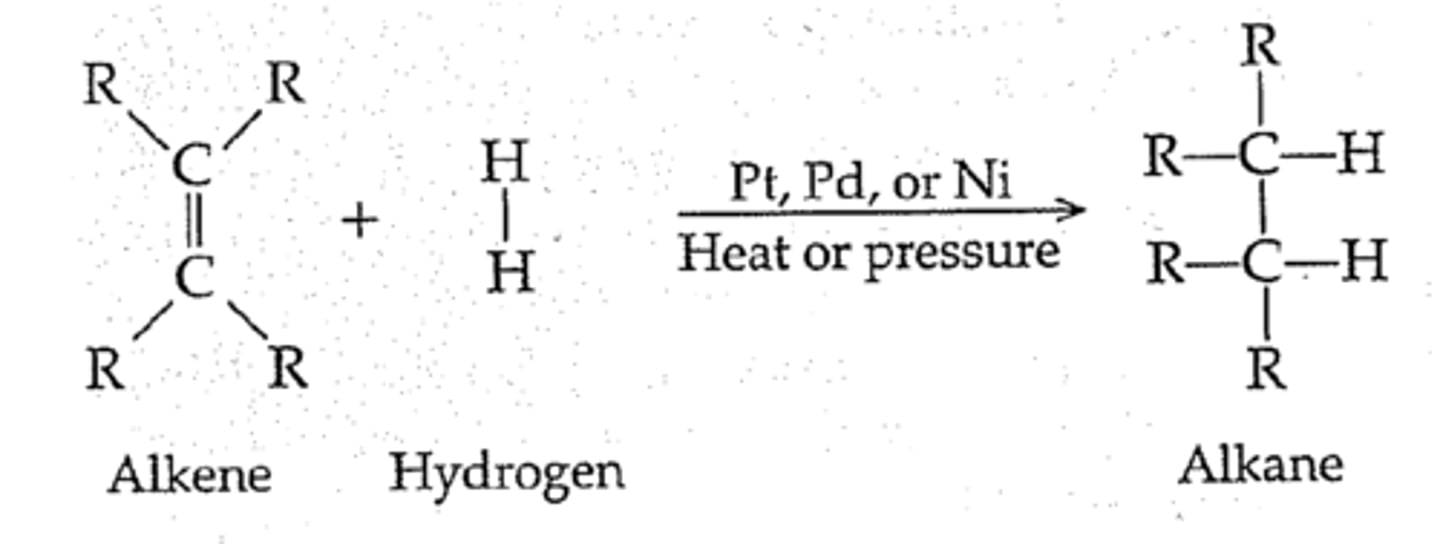
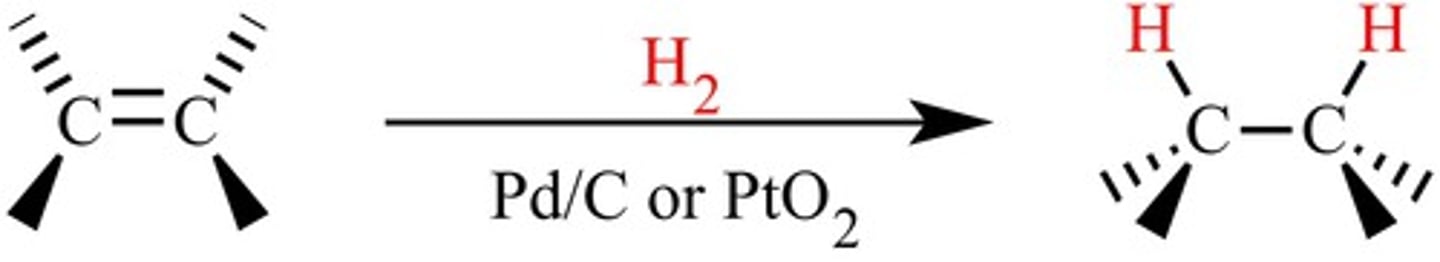
hydrogenation of alkenes
-Reagents: H2 over metal catalyst (Pd, Pt, Ni)
-Syn addition; can be stereospecific
heat of hydrogenation of an alkene
energy difference between the starting alkene and the product alkane; calculated by measuring the amt of heat released in a hydrogenation reaction
least heat given off = most stable
stability of alkenes in relation to heat of hydrogenation
1. increasing alkyl substitution stabilizes an alkene
2. conjugated dienes are more stable than non-conjugated dienes
3. trans alkenes are more stable than cis alkenes (steric repulsions in cis)
reaction of alkynes with H-X
Markovnikov addition
stereoselective
Z form often predominates
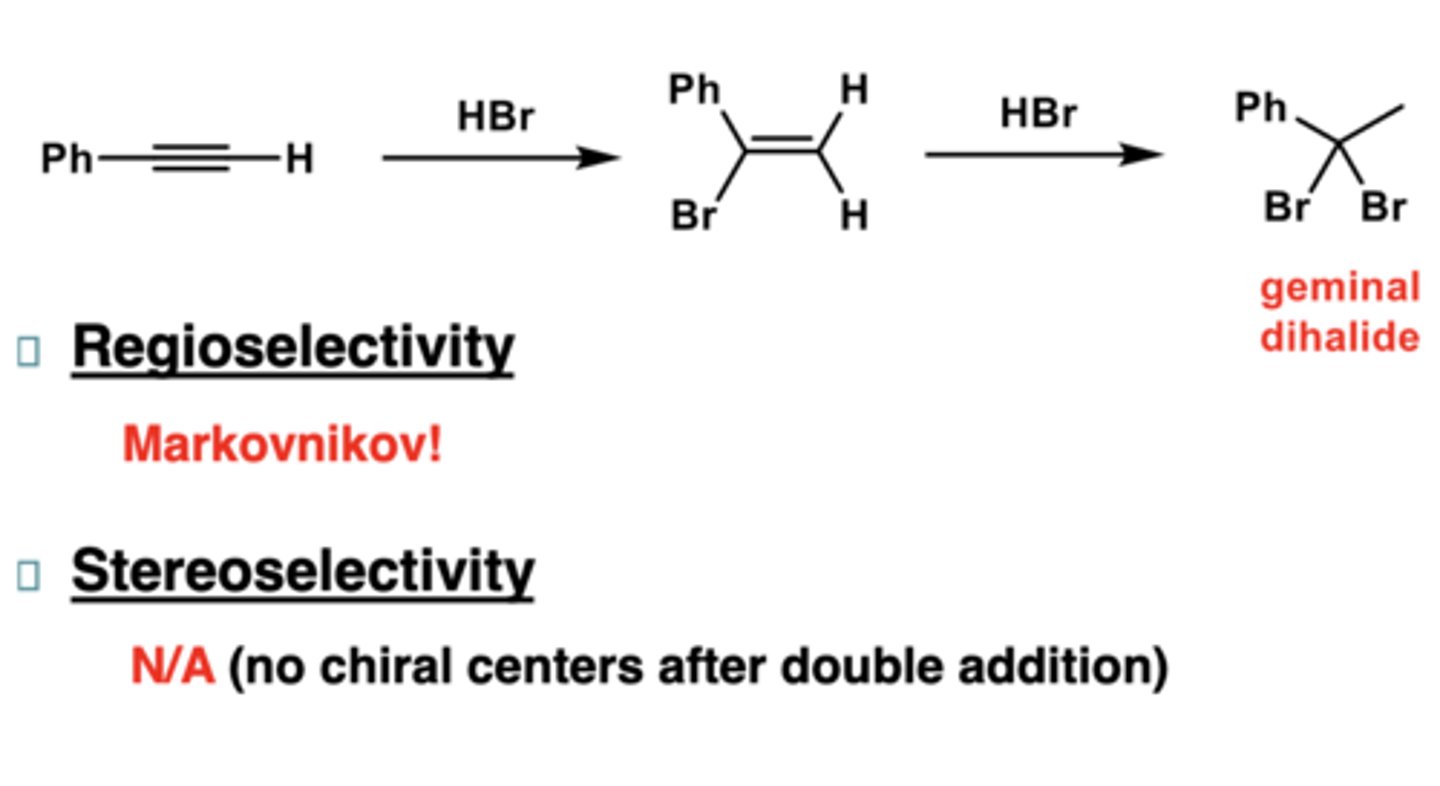
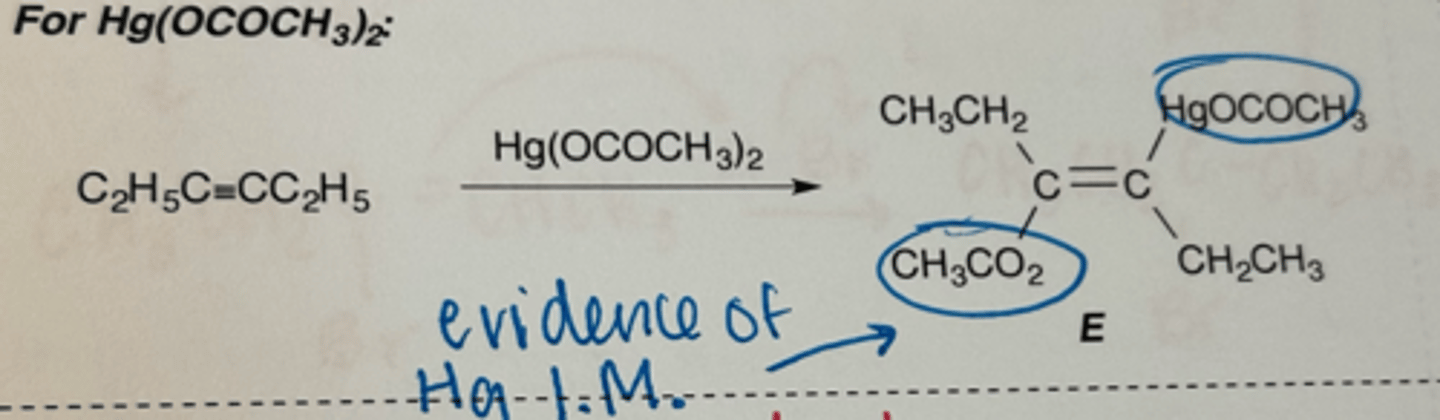
reaction of alkynes with Hg(OCOCH3)2
markovnikov addition
stereoselective
reaction of alkynes with Cl2/Br2
choice of intermediate depends on the structure
1. alkyl groups tend to favor the BRIDGED ion
2. groups such as phenyl, which stabilize the free carbocation, tend to proceed via the VINYL CARBOCATION
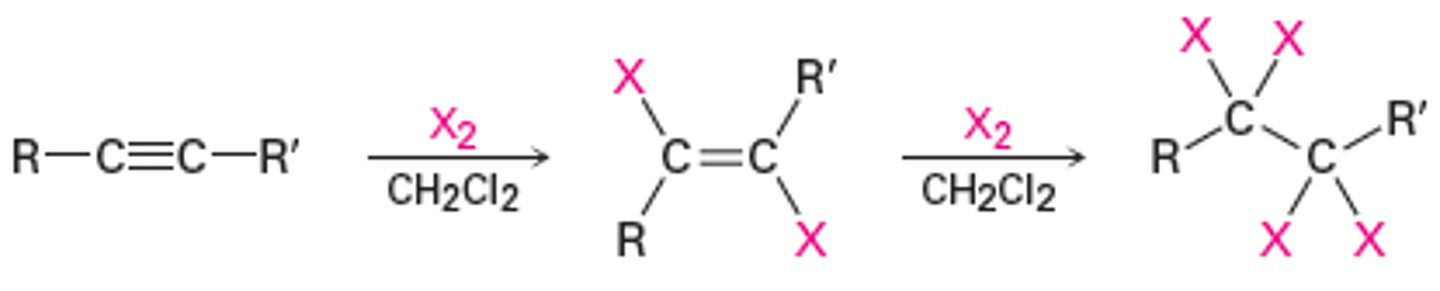
addition of HCl or HBr to alkynes
terminal alkynes proceed with Markovnikov addition, Z products
internal alkynes proceed via a bridged intermediate, making E products
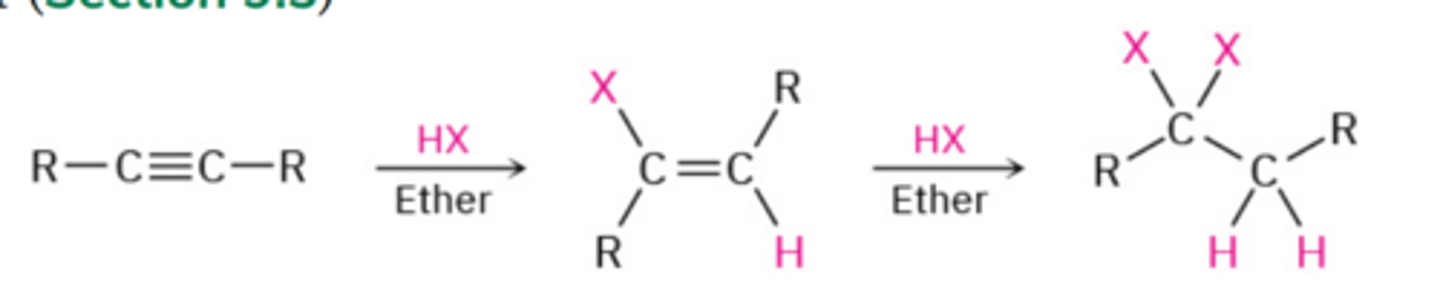
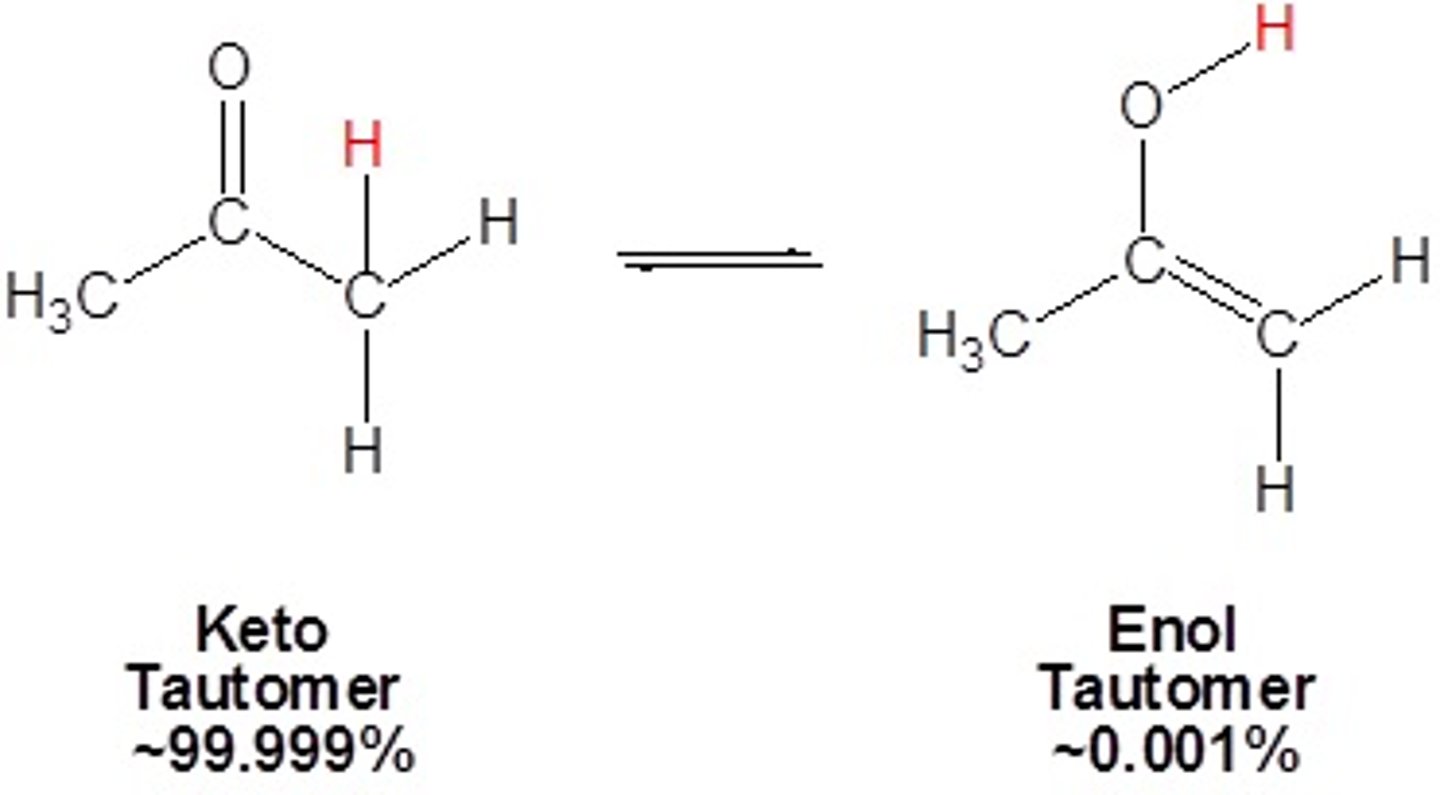
tautomerization
The rearrangement of bonds within a compound, usually by moving a hydrogen and forming a double bond
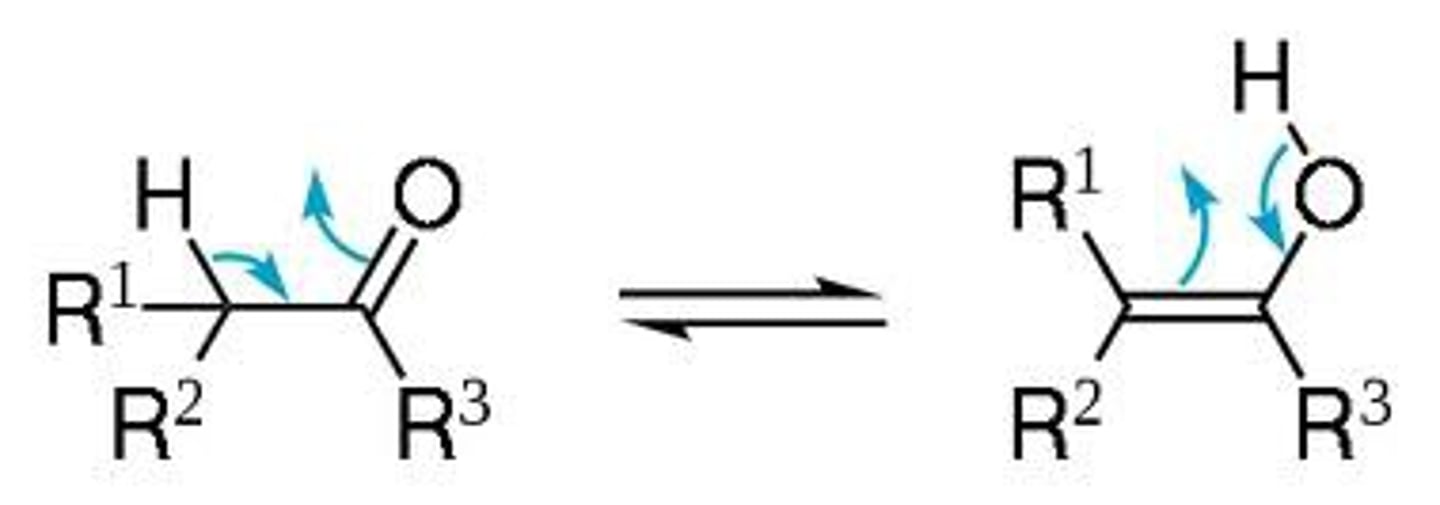
enol
The resonance form of a carbonyl that has a carbon-carbon double bond (ene) and an alcohol (-ol)
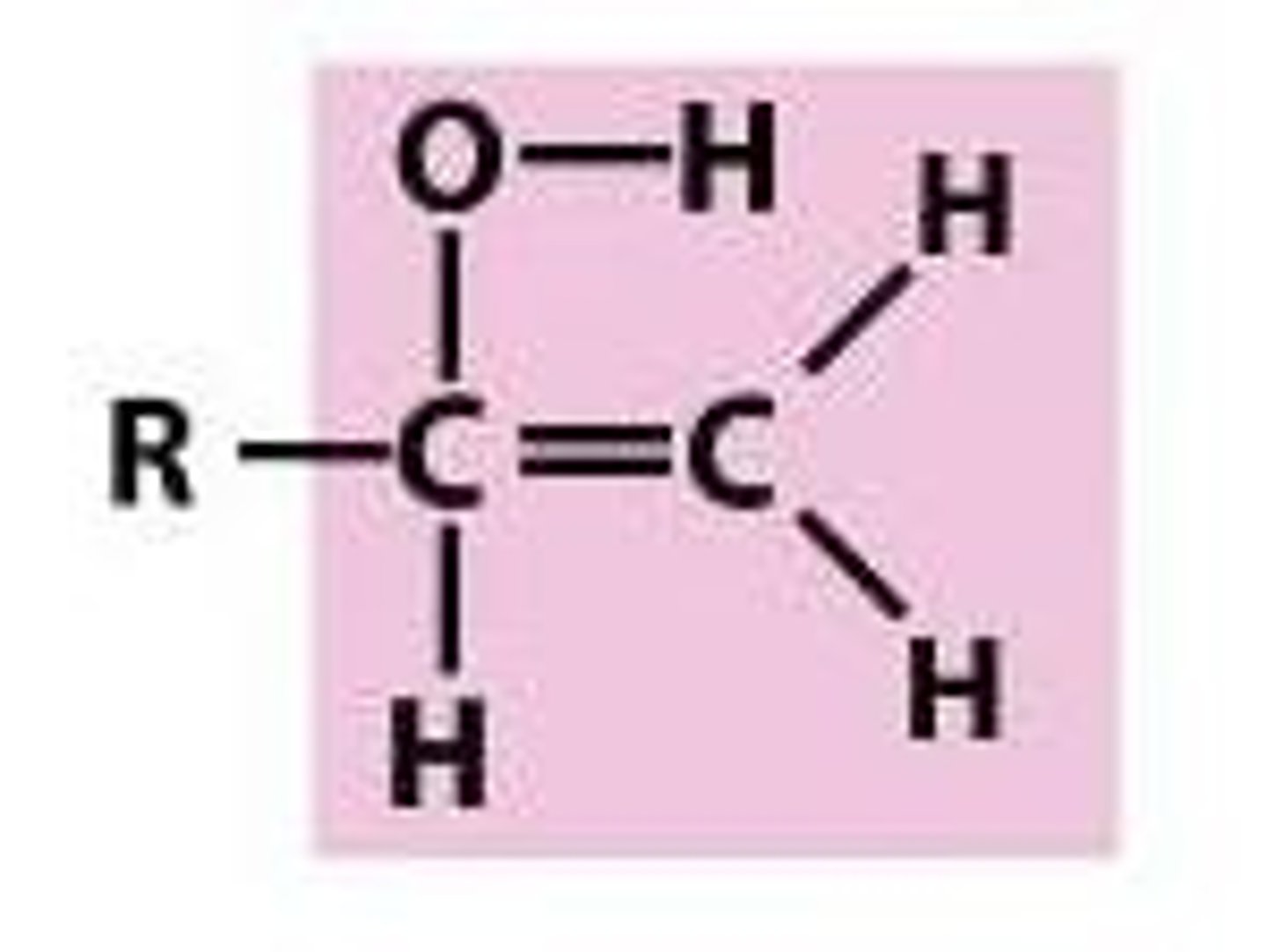
keto
C=O form, more stable than enol
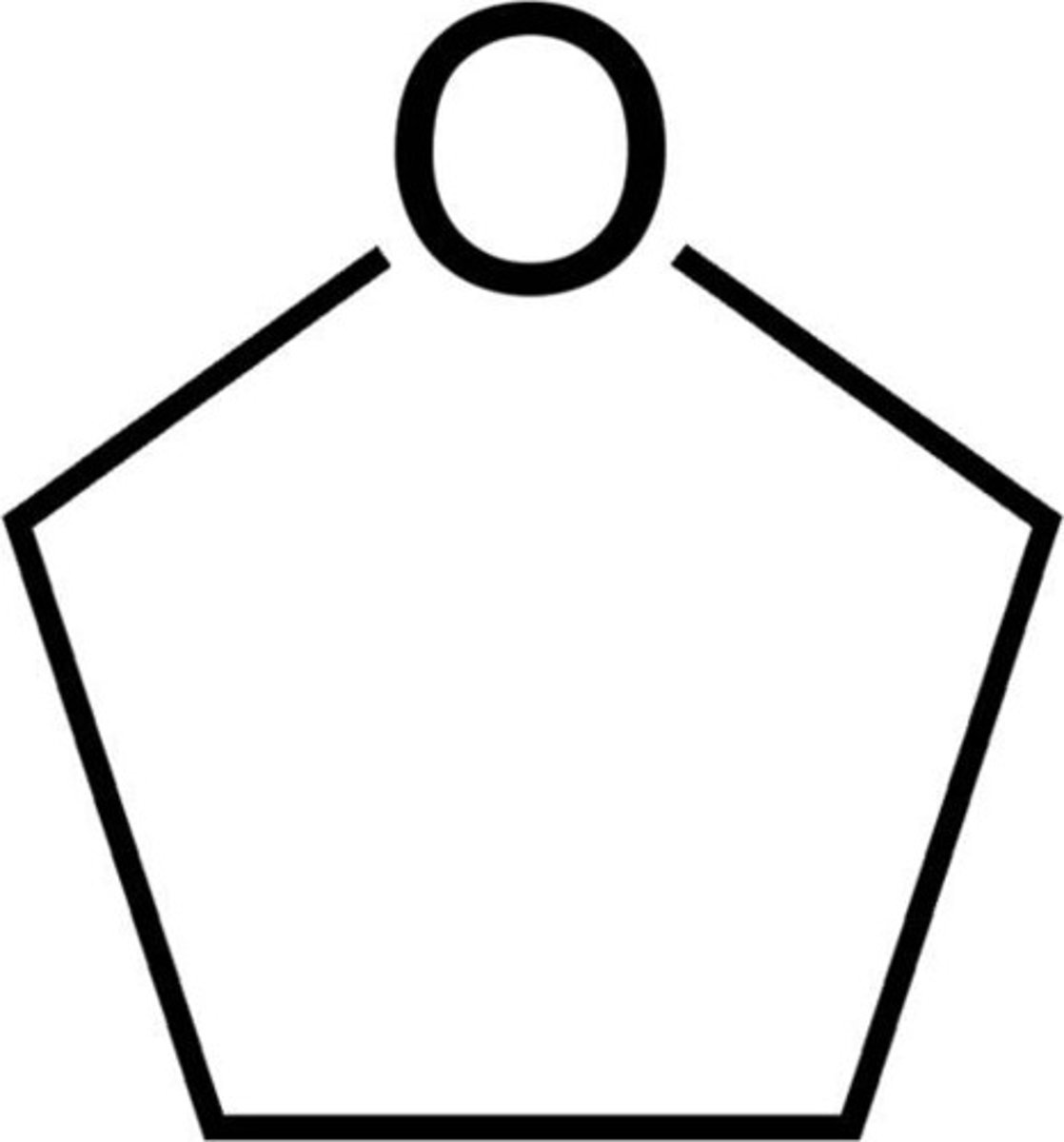
THF
tetrahydrofuran, inert solvent, slightly polar
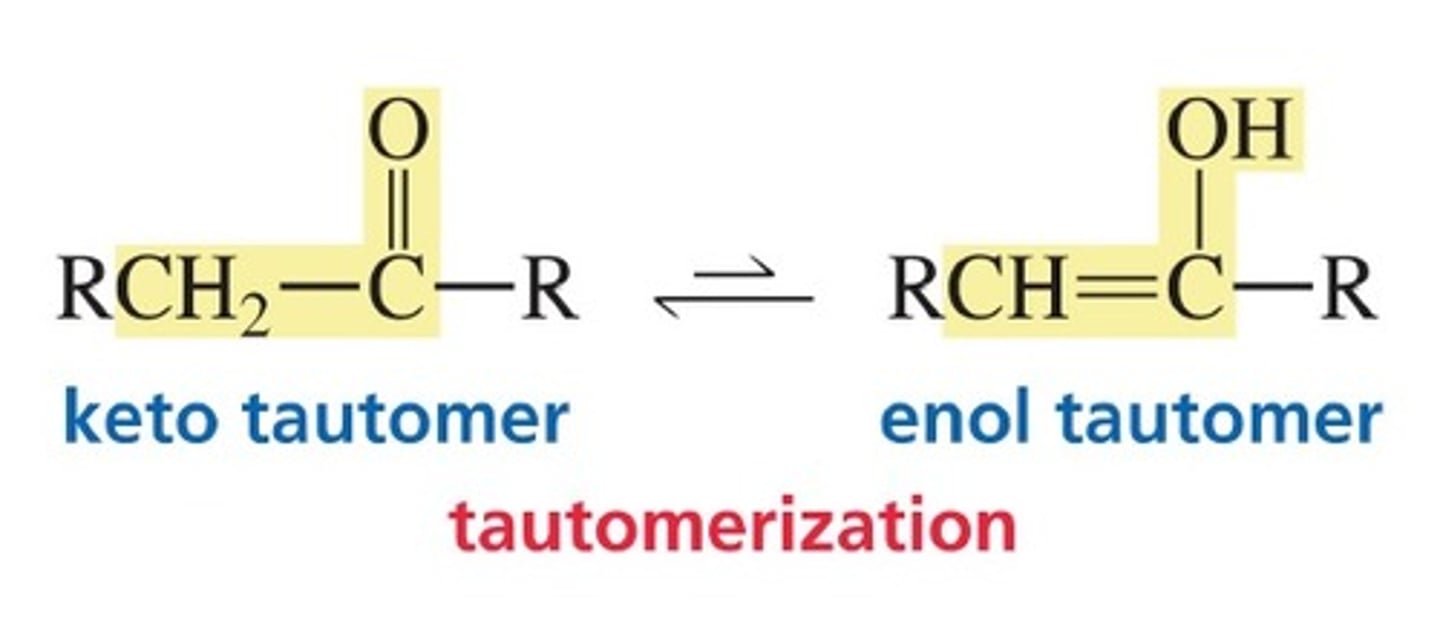
tautomers
- Two isomers, which differ in the placement of a proton and the double bond
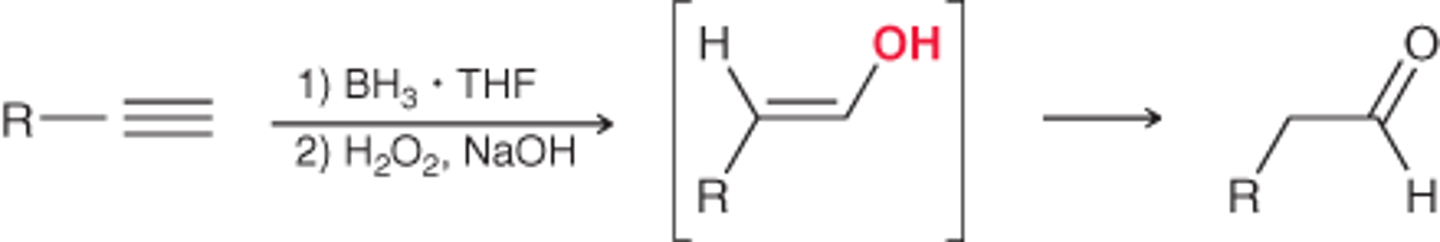
hydroboration of an alkyne
proceeds via tautomerization
keto favored at equilibrium over enol (two structural isomers)
catalytic hydrogenation
reducing an alkene by adding molecular hydrogen to double bond with aid of metal catalyst. e.g. pt, pd, ni. takes place on surface of metal so it does syn addition, gets us to a single bond
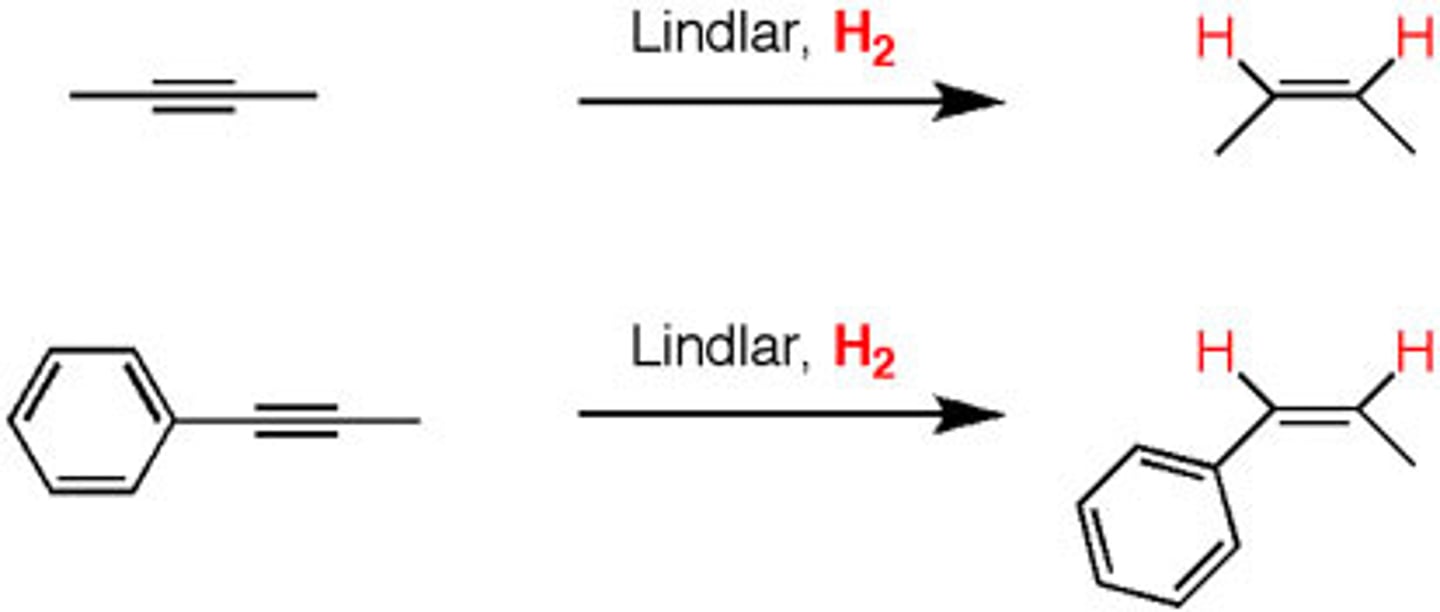
lindlar pd
"poisoned" Pd catalyst, allows for an INTERNAL triple bond to be converted into a cis alkene
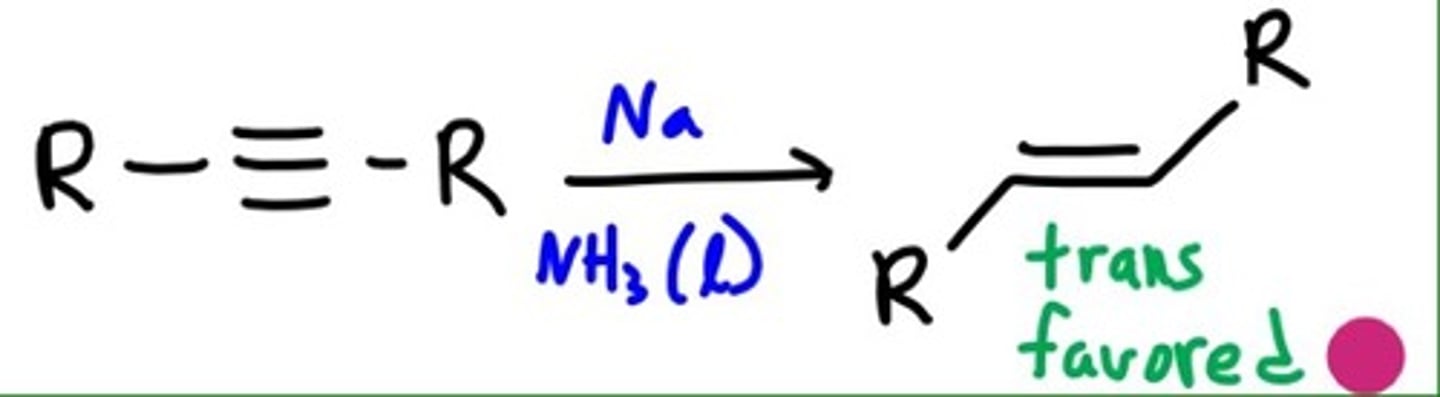
dissolving metal reduction
converts an alkyne into a trans alkene, anti addition
Li, Na, or K plus NH3 at -78 degrees Celsius
reactions with acetylide ion
The conjugate base of acetylene or any terminal alkyne
Useful as a strong base and is a good nucleophile
used for chain elongation in synthesis
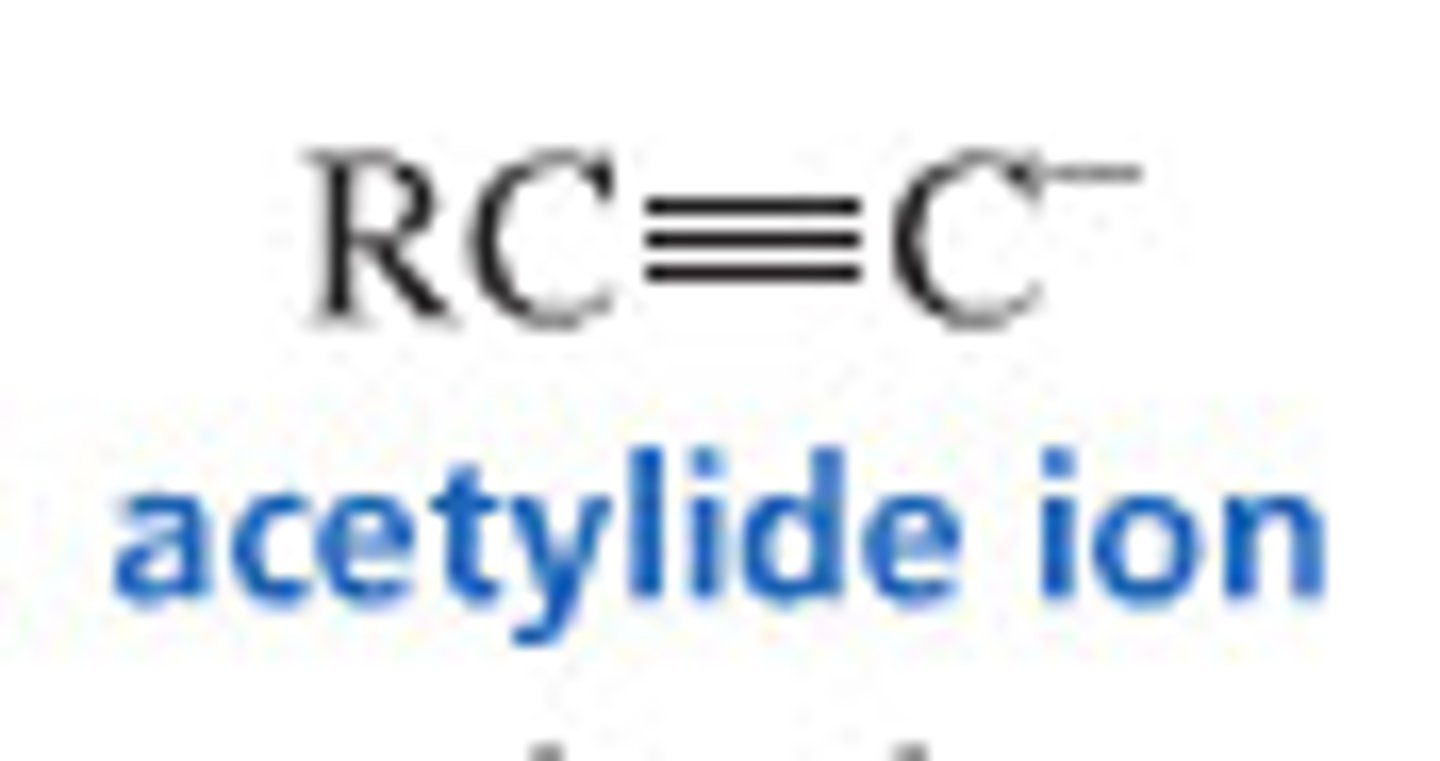
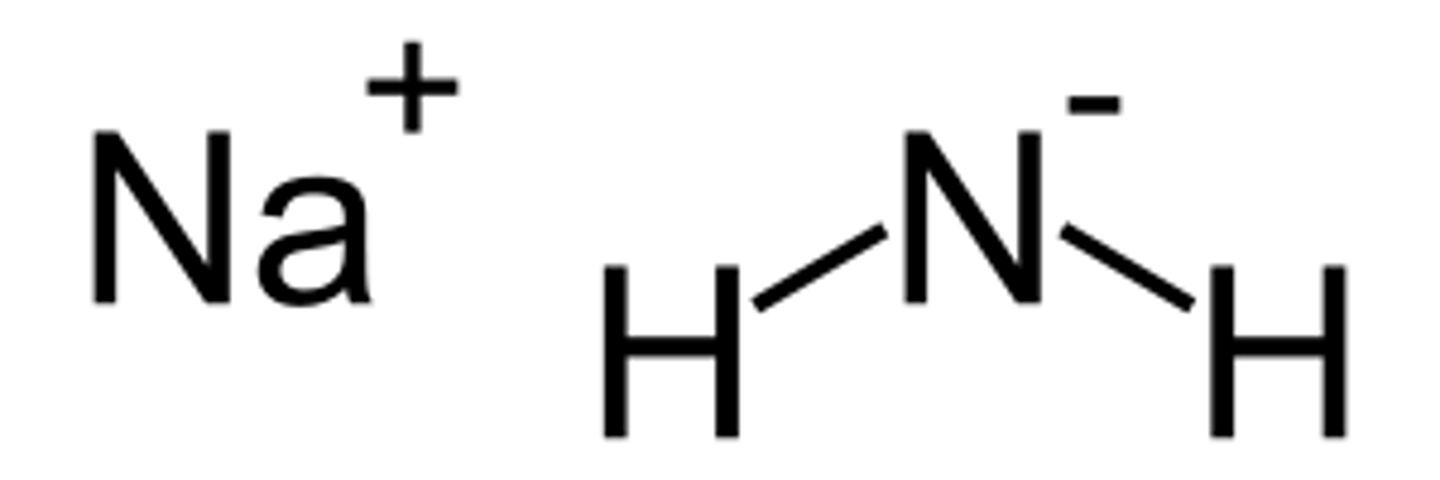
sodamide
very strong base, CB of NH3
used for chain elongation (1st step)
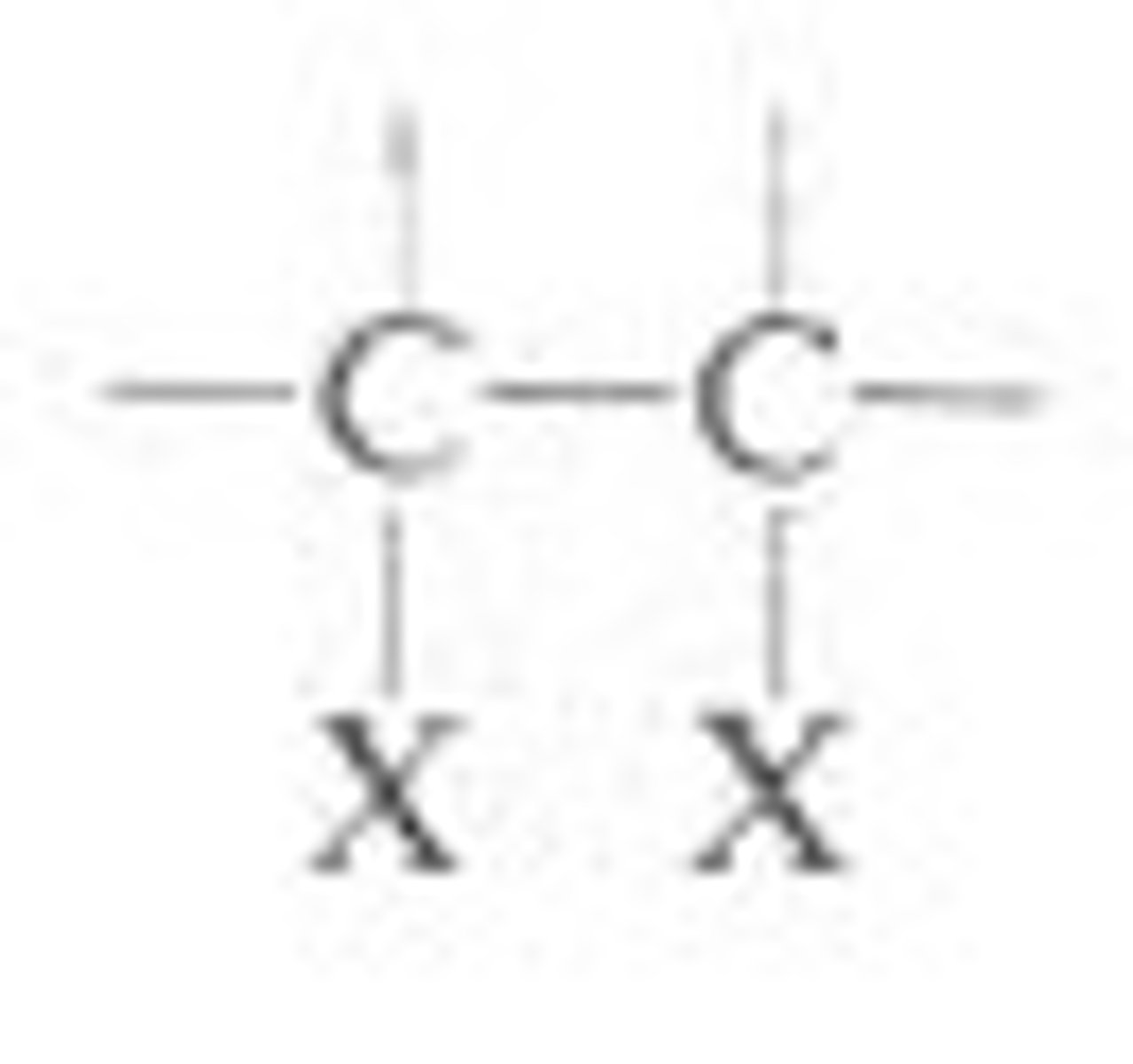
vicinal dihalide
a dihalide with the halogens on adjacent carbon atoms
used for chain elongation (first step)
retrosynthetic analysis
designing a synthesis by working backward from product to reactant
localized electrons
cannot show moving by resonance
delocalized electrons
show resonance structures
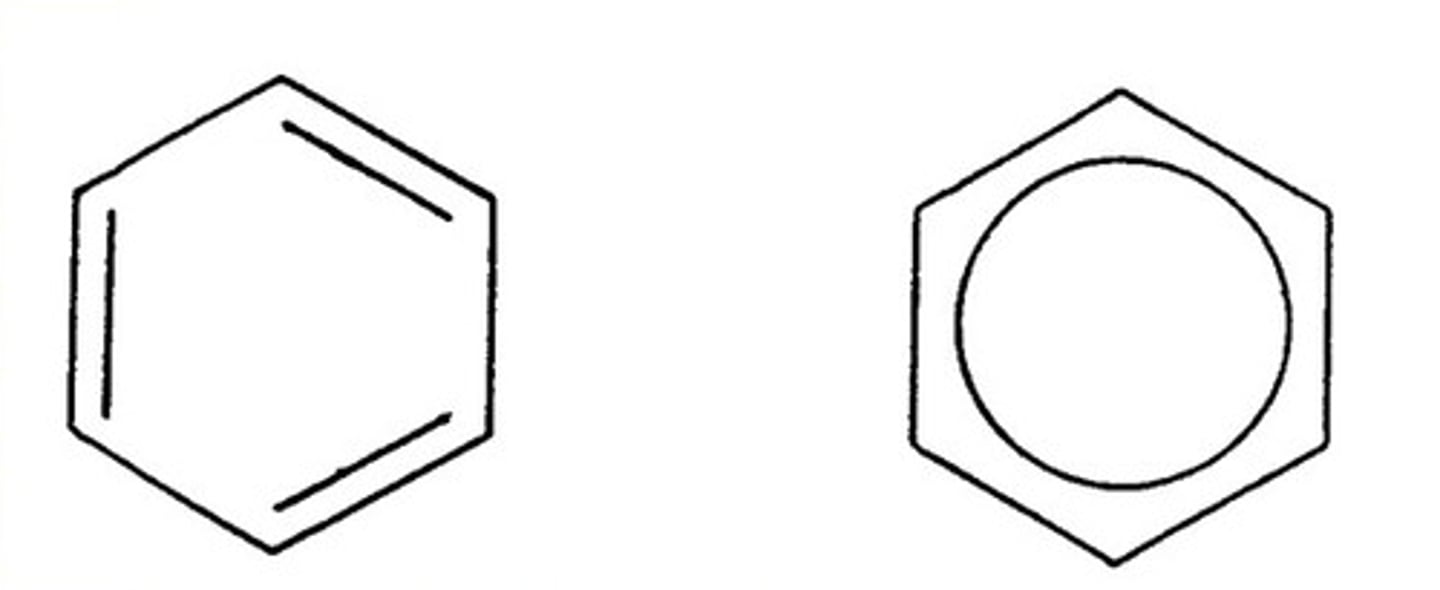
delocalization energy
the extra stability that a molecule gains from having delocalized electrons, also called resonance energy (the higher the resonance energy, the more stable a compound is)
requirements for aromaticity
1. molecule must be cyclic
2. every atom in the ring must have a p-orbital
3. for the pi cloud to form, each p-orbital must overlap with the p-orbitals on either side of it = PLANAR
4. the pi cloud must have an odd number of pairs of pi electrons (4n+2); 2,6,10,14,18...
antiaromatic
fits all criteria for aromaticity except for the proper number of pi electrons
reactions with conjugated dienes
1. kinetic product: predominates when temperature is too low (1,2-addition product)
2. thermodynamic product: predominates when temperature is high (1,4-addition product), most stable because of the more substituted double bond
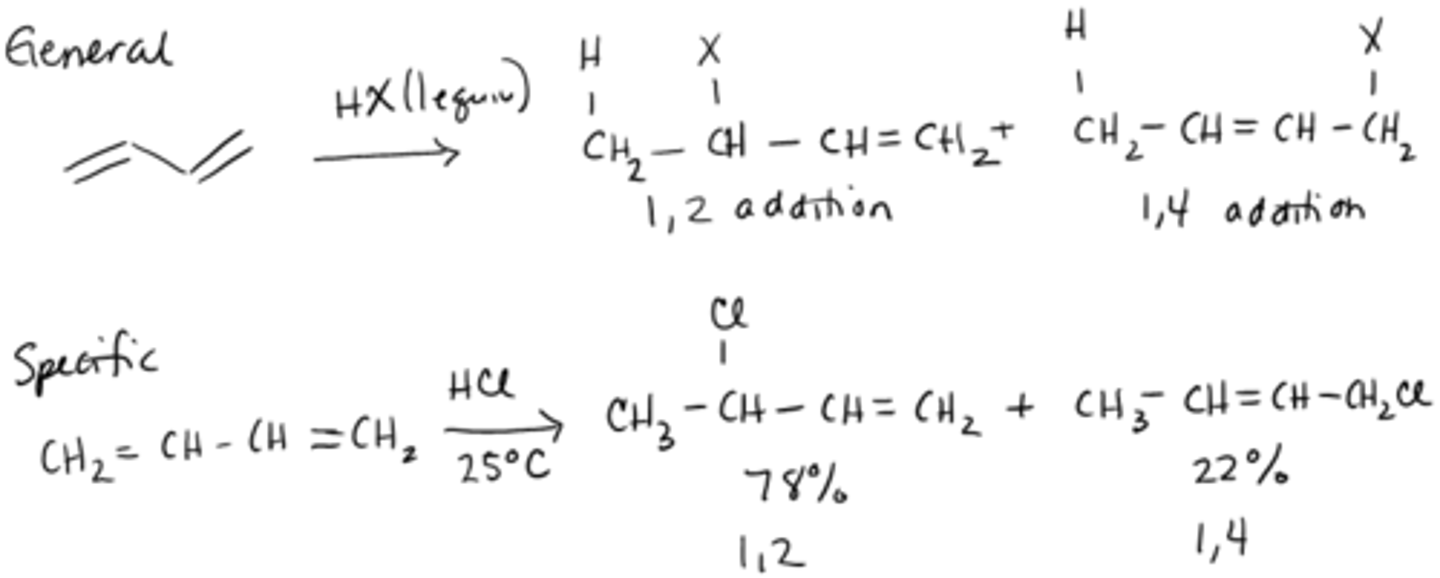
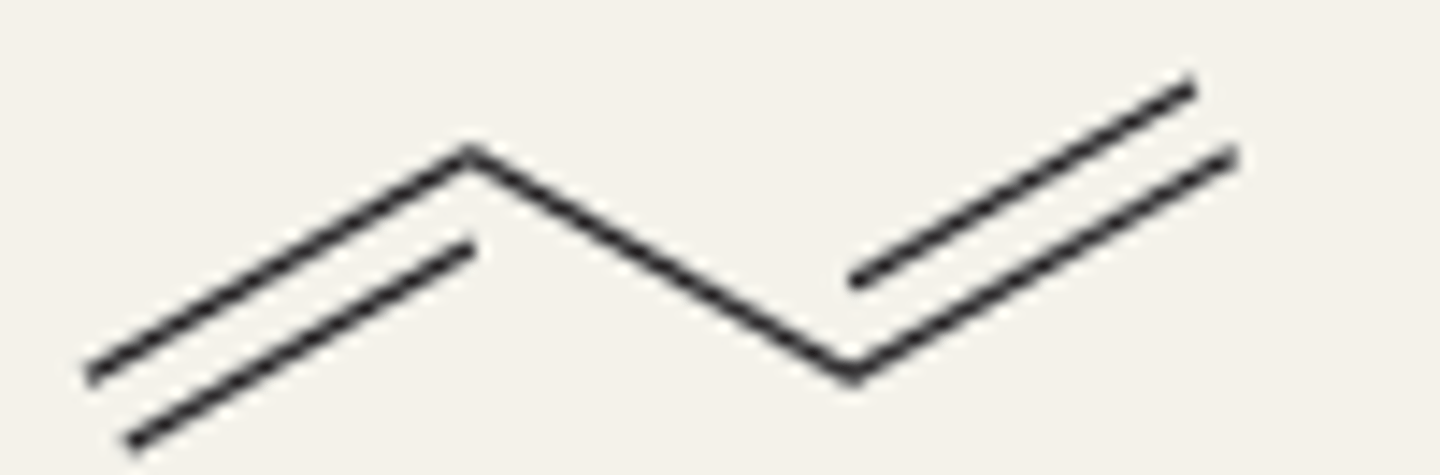
conjugated dienes
two double bonds separated by a single bond
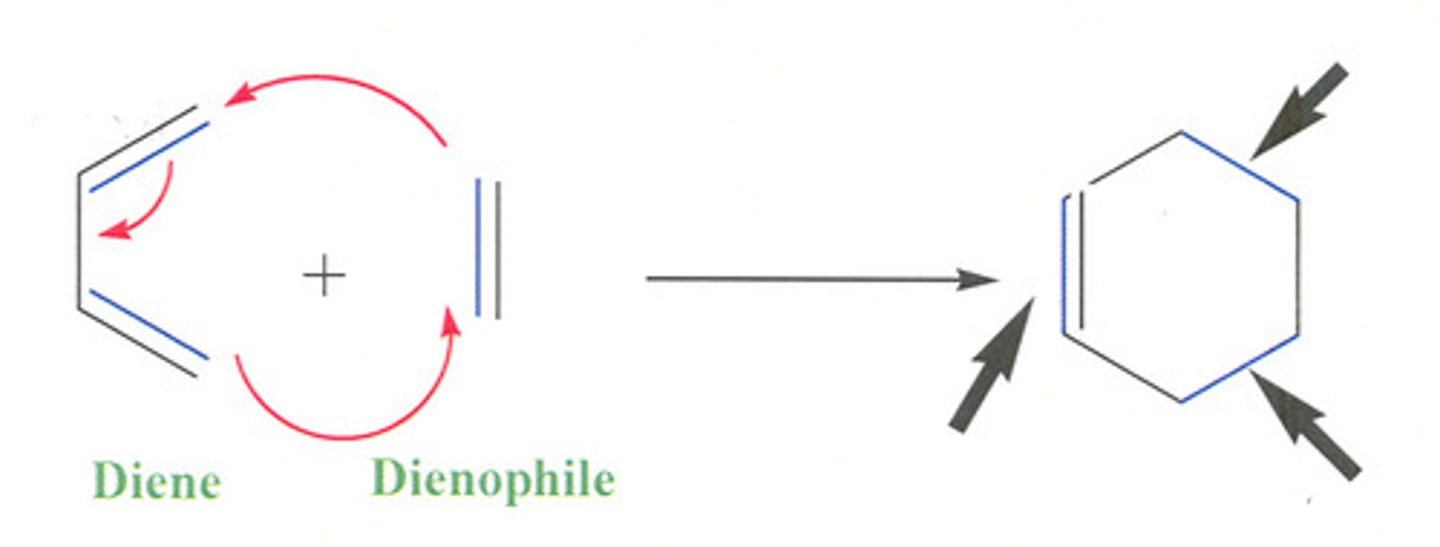
Diels-Alder reaction
pericyclic (ring-forming), concerted, only s-cis reacts