XIII.2 - Glycolysis
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
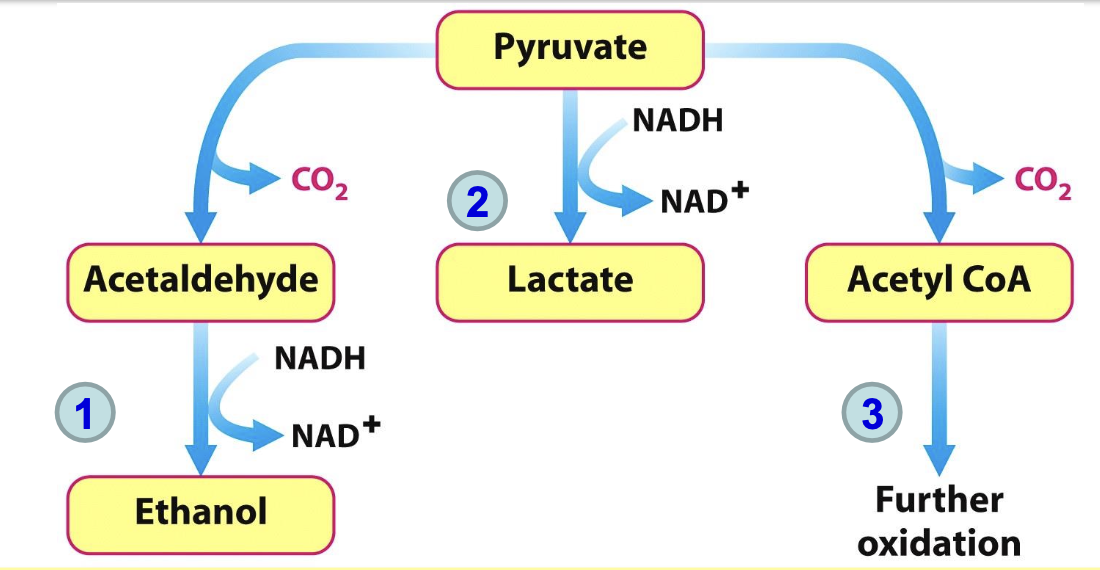
NAD⁺ regeneration from pyruvate metabolism
NAD⁺ must be regenerated for glycolysis to continue (step 6, GAPDH) as it is reduced to NADH in the oxidation of GAP. This can occur through:
fermentation of pyruvate → ethanol (anaerobie)
fermentation of pyruvate → lactic acid (anaerobie)
the citric acid cycle/ electron transport chain in aerobic respiration.
Ethanol fermentation
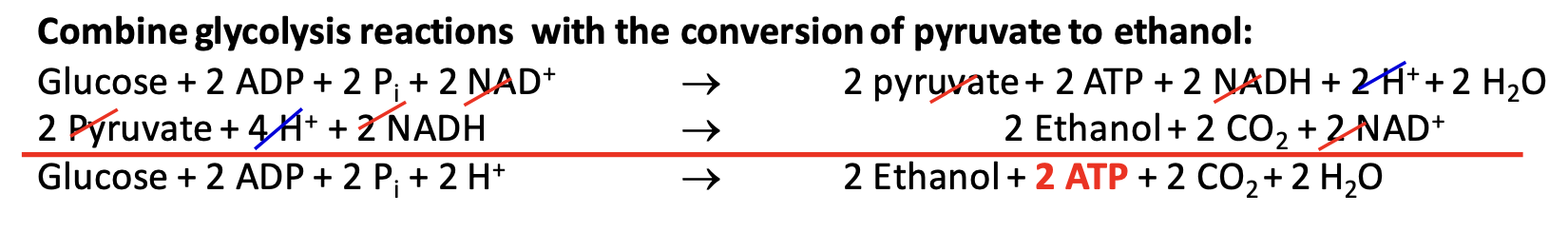
In yeast and some microorganisms, pyruvate is converted to ethanol in a two-step anaerobic process. This includes the decarboxylation of pyruvate to acetaldehyde and its subsequent reduction to ethanol, regenerating NAD⁺ for glycolysis. Only supplies 2 ATP molecules per glucose
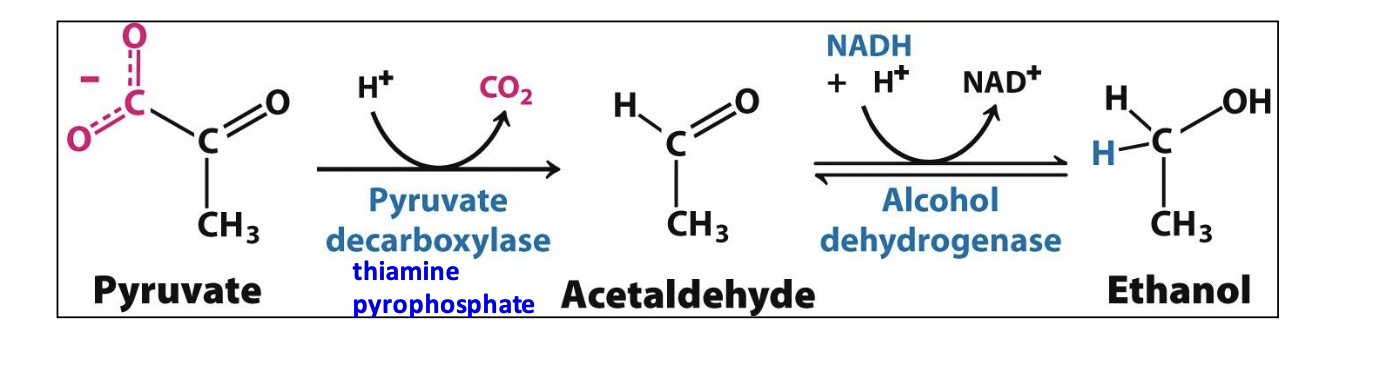
Alcohol dehydrogenase’s active site has a Zn²⁺ bound to 2 Cys + 1 His, which facilitates transfer of H⁻ from NADH allowing for reduction into ethanol
Lactic acid formation
In muscle cells and some microorganisms, pyruvate is reduced to lactate (anaerobie), regenerating NAD⁺ needed for glycolysis to continue. This process provides quick energy but produces only 2 ATP per glucose.
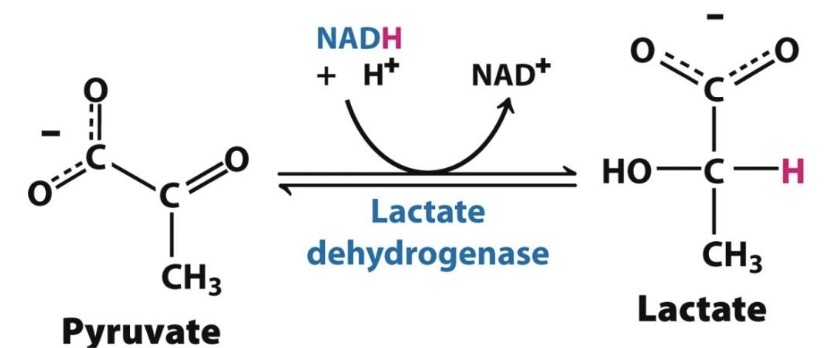
Typically takes place under conditions where [O₂] is limiting and muscles are heavily contracting
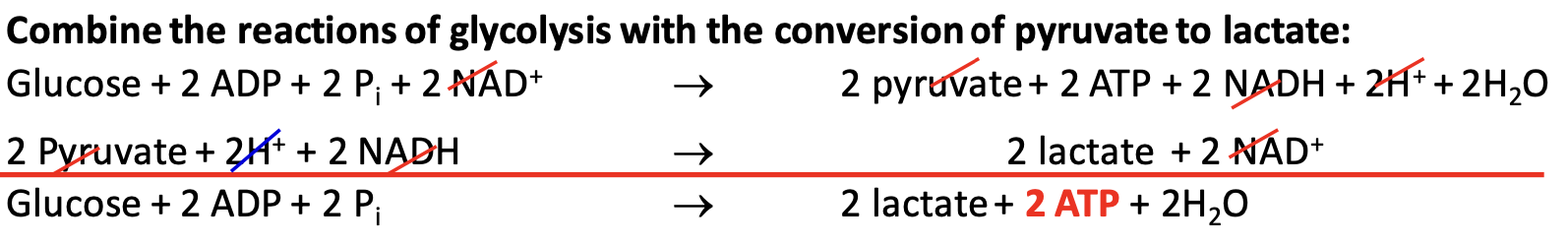
Fate of pyruvate in aerobic conditions
More energy can be extracted than what is obtained thru fermentation. Entering citric acid cycle, a pyruvate dehydrogenase complex in mito. catalyzes conversion of pyruvate to acetyl CoA. NAD⁺ is generated when NADH transfers e⁻ to O₂ through transport chain.
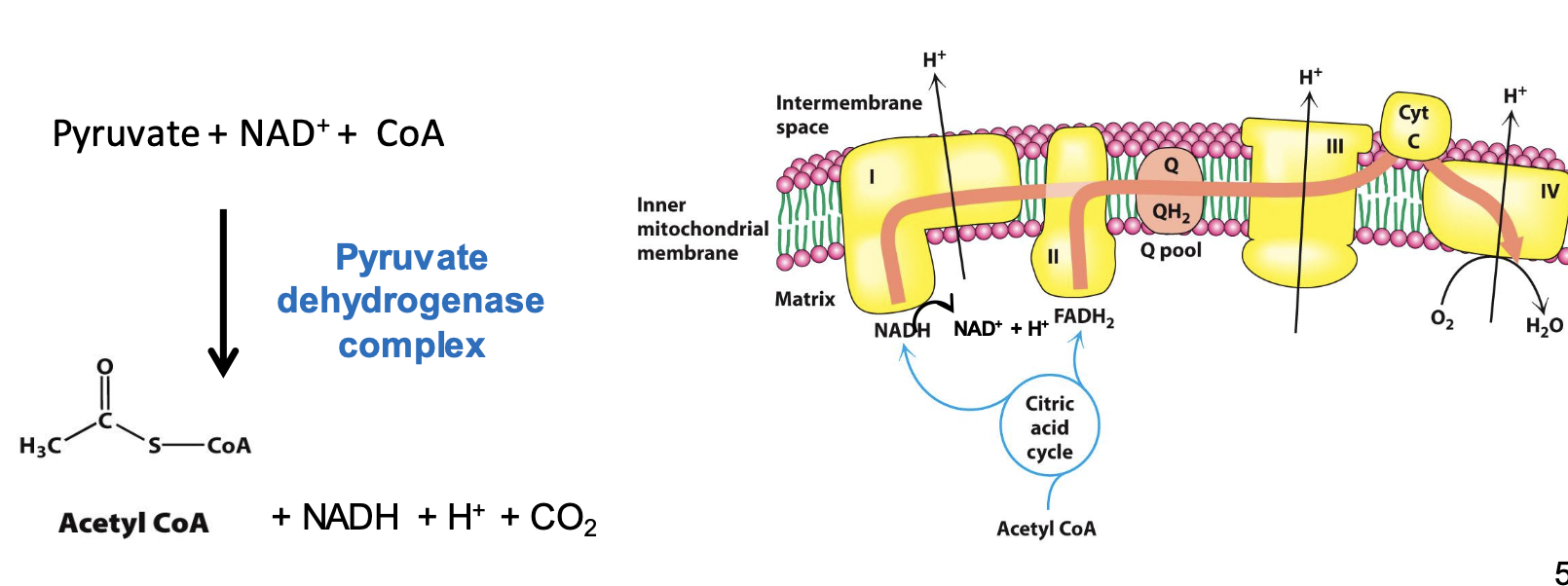
Regulation of glycolysis
→ 3 enzymes catalyze irreversible rxs: hexokinase (1), phosphofructokinase (3), & pyruvate kinase (10)
→ controlled by: reversible bonding of allosteric effectors, covalent modificatº, controlling [enzyme]
Glycolysis regulation in muscle
Glycolysis in skeletal muscle provides ATP for muscle contraction. Primary control is the ratio of ATP to AMP. As [ATP]/[AMP] decreases, enzyme activity increases
→ low [ATP] (signalled by high [AMP]) increases PFK’s affinity for F-6P. AMP binds regulatory site reversing ATP’s inhibitory action = more active R form (left shift)
→ high [ATP] inhibits enzyme affinity for F-6P as it binds to AMP site
![<p>Glycolysis in skeletal muscle provides ATP for muscle contraction. Primary control is the ratio of ATP to AMP. As [ATP]/[AMP] decreases, enzyme activity increases</p><p>→ low [ATP] (signalled by high [AMP]) increases PFK’s affinity for F-6P. AMP binds regulatory site reversing ATP’s inhibitory action = <strong>more active R form (left shift)</strong></p><p>→ high [ATP] inhibits enzyme affinity for F-6P as it binds to AMP site</p>](https://knowt-user-attachments.s3.amazonaws.com/0e336f6e-5a8c-4e1e-9204-928c8b5e6991.png)
PFK regulation in muscle by pH
A decrease in pH also increases the inhibitory effect on ATP
→ low pH can arise from muscle temporarily functioning w/out enough O₂ = lack of lactic acid
→ inhibiting PFK helps stop tissue damage due to excess acid
Regulation of hexokinase in muscles
Hexokinase is inhibited by its product, G-6P, through negative feedback. This inhibition occurs when downstream steps, like PFK, slow down, leading to G-6P accumulation and signaling that energy demands are low. Inhibition of PFK inhibits hexokinase
Regulation of pyruvate kinase in muscles
Pyruvate kinase is allosterically inhibited by ATP when energy is abundant and activated by F-1,6-BP (feed-forward activation) to keep glycolysis in sync with upstream reactions during active energy demand.
Glycolysis Regulation in Muscle (Exercise vs. Rest)
During exercise - decrease [ATP]/[AMP] due to contraction activates PFK = glycolysis. More F-1,6-BP produced = pyruvate kinase feed forward stimulation
At rest - high [ATP] inhibits PFK and pyruvate kinase. Hexokinase inhibited by its product G-6P (negative feedback) & indirectly with PFK inhibition by ATP. If Glu not needed, pathway slows
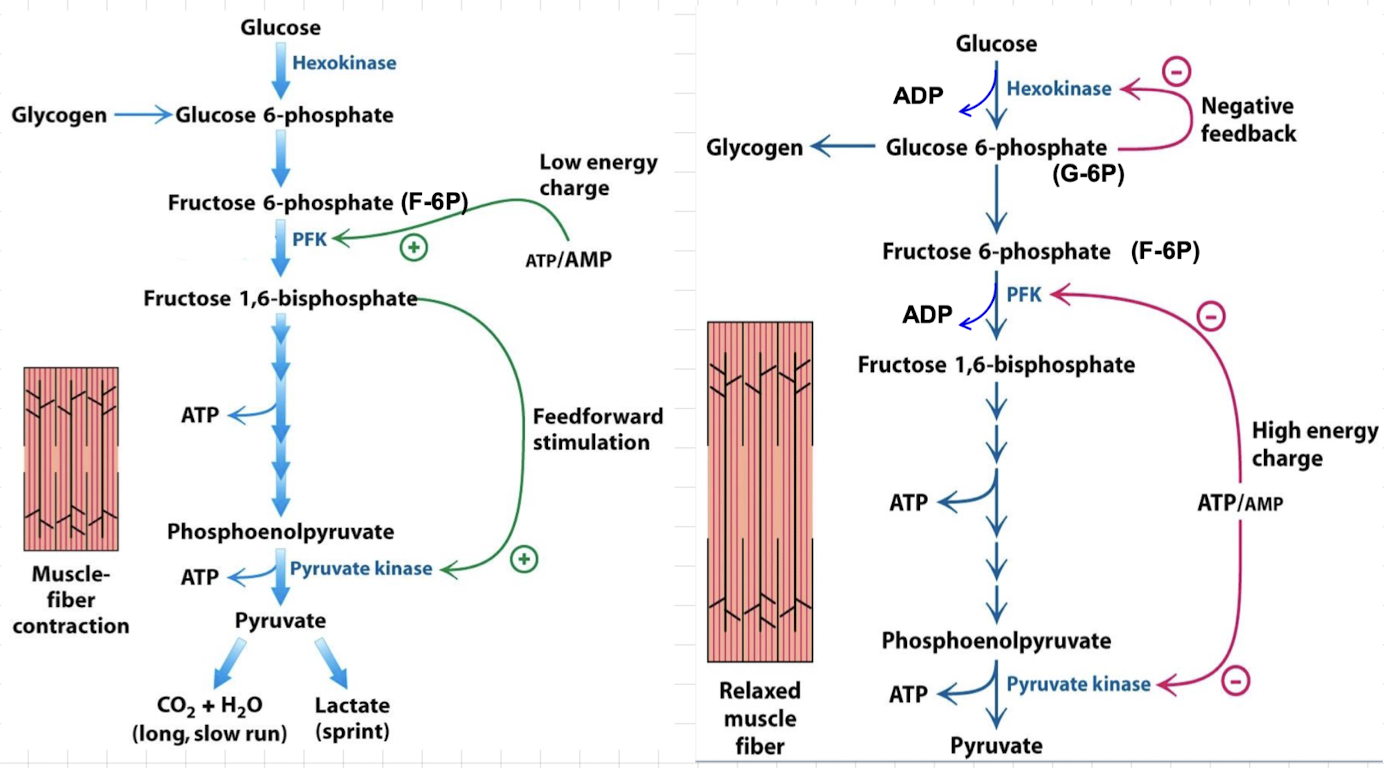
Regulation of glycolysis in the liver
→ maintains blood-glucose (stores Glc when abundant, releases when low)
→ Uses Glc to generate reducing power (NADPH)
→ Uses Glc to synthesize biochemical intermediate
PFK inhibition in Liver
Unimportant metabolic signals: high [ATP] inhibits enzyme, low [ATP] stimulates it/ pH can regulate PFK, lactate isn’t produced in the liver
☆ citrate, a building block for biosynthesis, inhibits liver PFK by enhancing ATP inhibition (when abundant)
![<p>Unimportant metabolic signals: high [ATP] inhibits enzyme, low [ATP] stimulates it/ pH can regulate PFK, lactate isn’t produced in the liver</p><p>☆ <strong>citrate</strong>, a building block for biosynthesis, inhibits liver PFK by enhancing ATP inhibition (when abundant)</p>](https://knowt-user-attachments.s3.amazonaws.com/8d7a26a9-c4d3-47ee-88c2-8f7146b1d975.png)
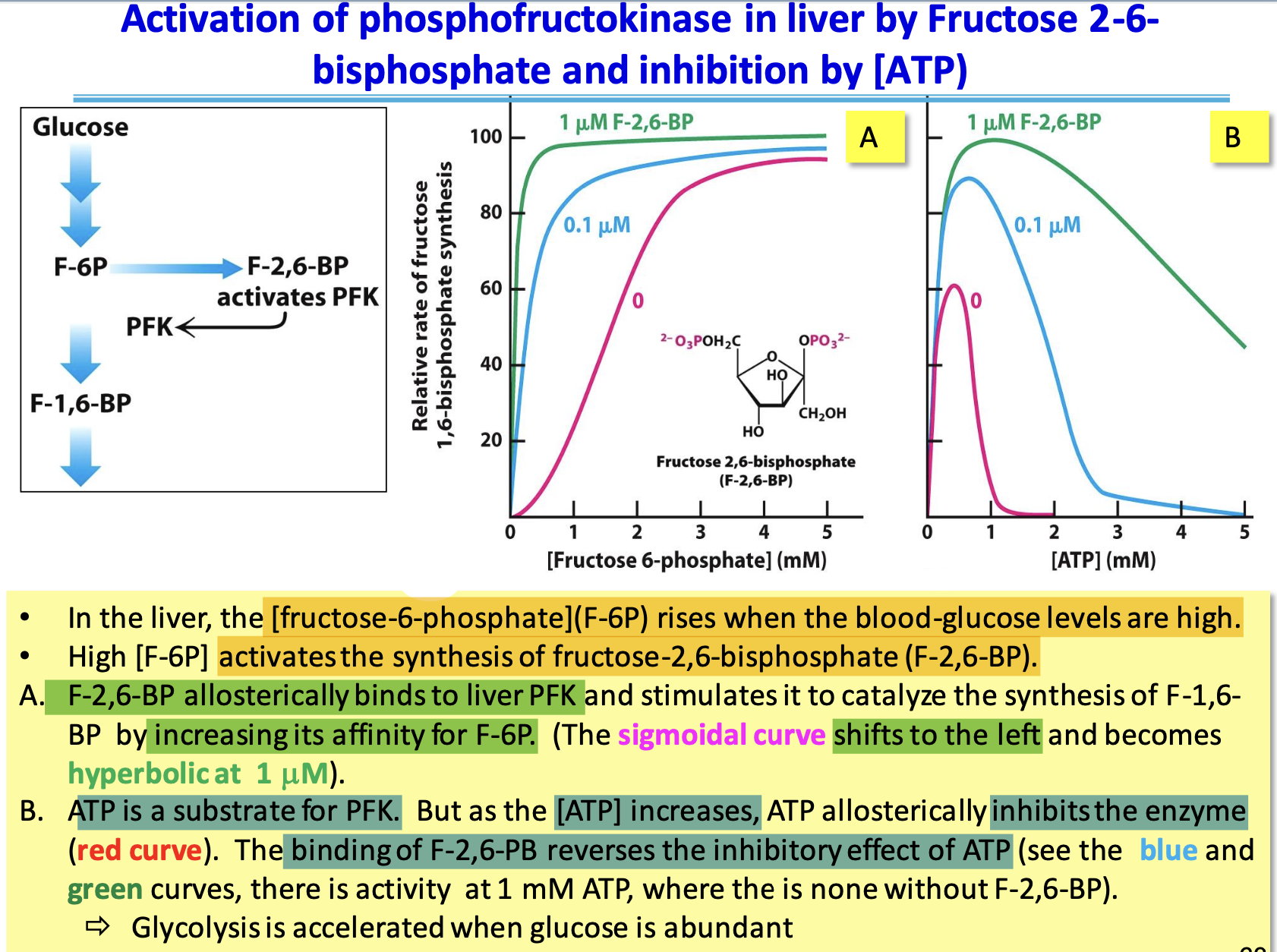
Activation of liver PFK
F-2,6-BP, produced when blood glucose is high, activates PFK and stimulates glycolysis
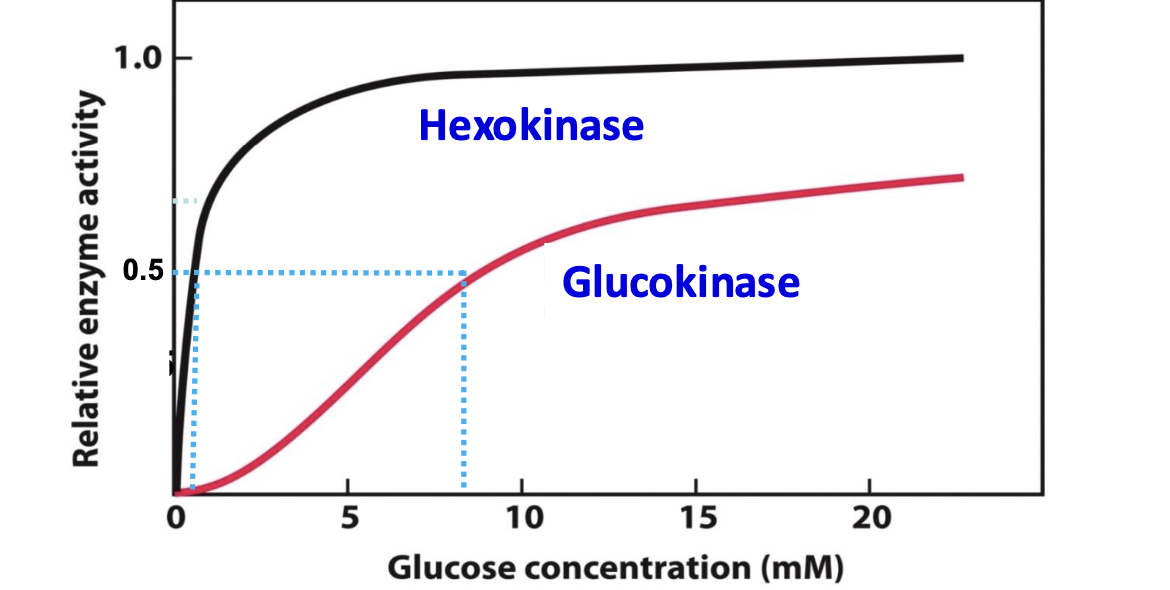
Glucokinase
Provide G-6P for the synthesis of glycogen/formation of fatty acid
Glucokinase has a high KM for glucose (low affinity), meaning it is only active at high glucose concentrations, such as after a meal. This feature ensures glucose uptake and storage in the liver when glucose is abundant without competing with tissues like the brain and muscles for glucose under low conditions.
Isozymes of pyruvate kinase
L form in the liver
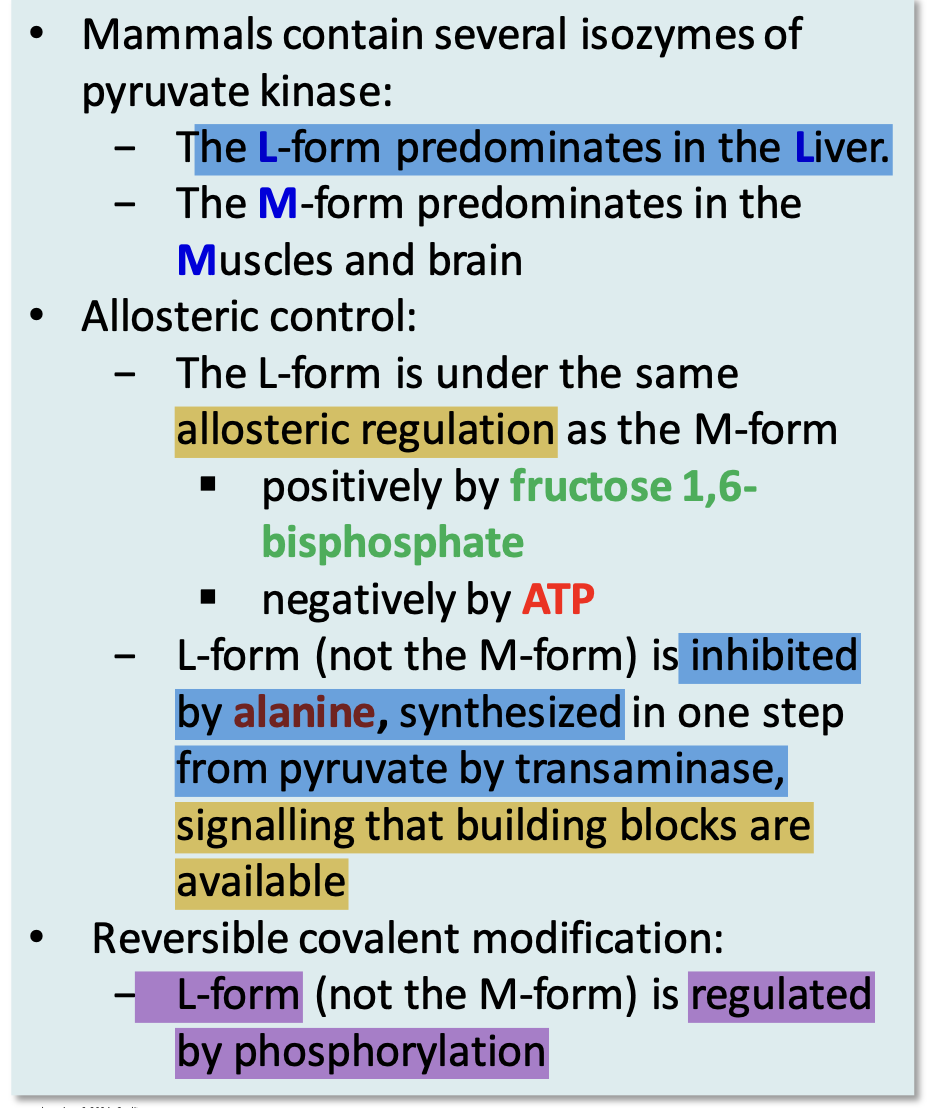
Regulation of pyruvate kinase in the liver
The liver isozyme of pyruvate kinase (L-form) is regulated allosterically by ATP and fructose-1,6-bisphosphate and is also modulated by phosphorylation. When blood glucose is low, phosphorylation decreases liver pyruvate kinase activity, allowing glucose to be conserved for other tissues.
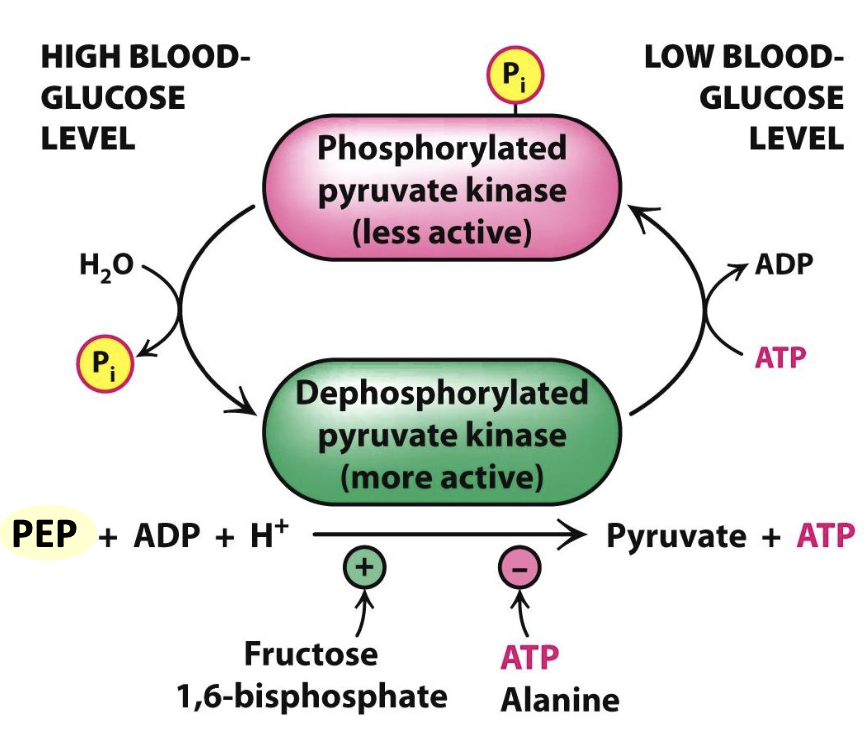
Hexokinase vs. Glucokinase in Liver
The liver contains both hexokinase and an isozyme called glucokinase. Glucokinase has a lower affinity for glucose, activating only when glucose levels are high. This allows the liver to store glucose as glycogen when abundant, while other tissues receive glucose when levels are low.
Entry of Fructose into Glycolysis in the Liver
Fructose enters glycolysis in the liver via the fructose-1-phosphate pathway, bypassing the PFK regulation step. This pathway allows fructose to be metabolized independently of glucose regulation, which can contribute to metabolic imbalances if excessive.
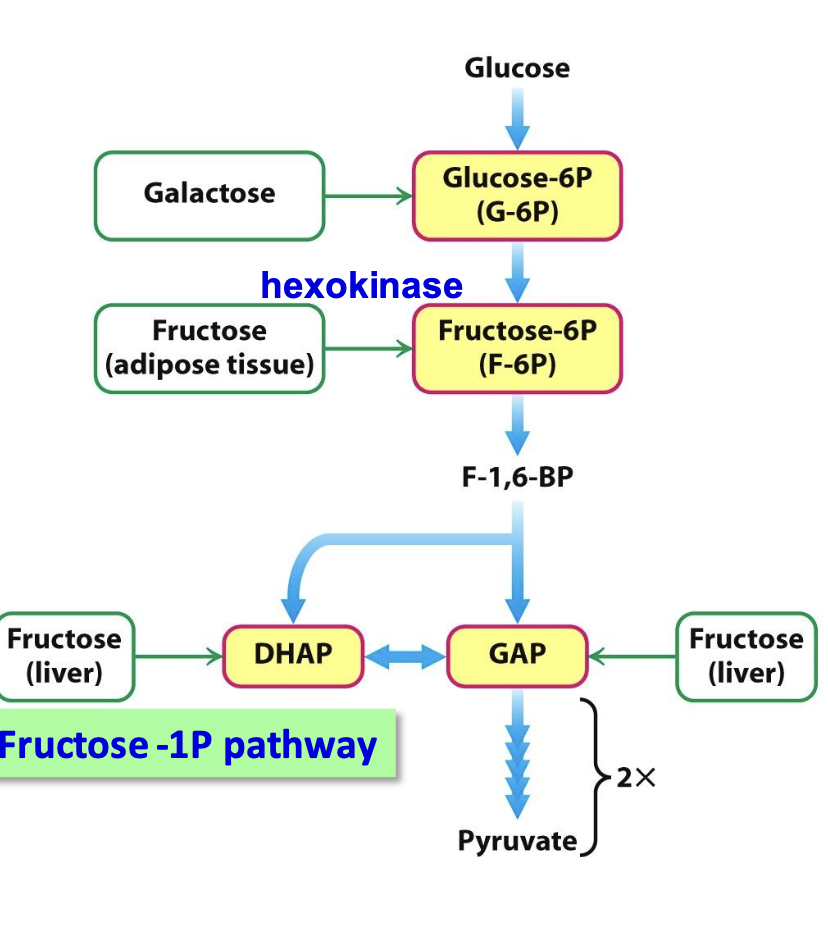
Entry of Galactose into Glycolysis in the Liver
Galactose is converted to glucose-6-phosphate via the galactose-glucose interconversion pathway, allowing it to enter glycolysis at the same stage as glucose. This process involves several steps to convert galactose into a usable glycolytic intermediate.