higher chemistry - alcohols and carboxylic acids - units 2.2 + 2.3
1/7
Earn XP
Description and Tags
unit 2 - nature's chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
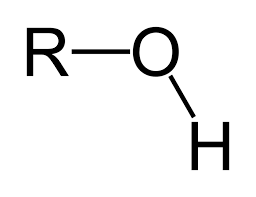
what is the functional group?
hydroxyl group
link between molecular size and boiling point of alcohols
as molecular size increases, boiling point increases due to increasing london dispersion forces, so more energy is required to break them
link between the number of hydroxyl groups in a molecule and it’s viscosity
as the number of hydroxyl groups in a molecule increases, viscosity increases due to more hydrogen bonds, making the molecules more difficult to move over eachother quickly
link between molecular size and solbulity of alcohols in water
as molecular size increases, solubility decreases as the carbon chain part of the molecule is non polar and insoluble in water
link between moelcular size and boiling point of carboxylic acid
as the molecular size increases, boiling point increases as intermolecular forces increase and a hydrogen bond which require more heat energy to overcome
why is the number of electrons relevant when comparing boiling points of different compounds
molecules with the same amount of electrons will have LDFS but if there are big differences, hydrogen bonding is present
why are smaller carboxylic acids soluble in water
they’re polar and water is a polar solvent, like dissolves like
why are larger carboxlyic acids insoluble in water
as the hydrocarbon chain increases, polarity of the molecule decreases, making it non polar and insoluble in water