Buffers
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is a buffer solution?
resists changes in pH (keep the pH almost constant) when small amounts of acids or alkalis are added
A buffer can consists of ____
weak acid - conjugate base
or
weak base - conjugate acid
Ethanoic acid & sodium ethanoate as a buffer
Ethanoic acid is a weak acid and partially ionises in solution to form a relatively low concentration of ethanoate ions

Sodium ethanoate is a salt which fully ionises in solution

There are reserve supplies of the acid (CH3COOH) and its conjugate base (CH3COO-)
The buffer solution contains relatively high concentrations of CH3COOH (due to partial ionisation of ethanoic acid) and CH3COO- (due to full ionisation of sodium ethanoate)
In the buffer solution, the ethanoic acid is in equilibrium with hydrogen and ethanoate ions

When H+ ions are added:
The equilibrium position shifts to the left as H+ ions react with CH3COO- ions to form more CH3COOH until equilibrium is re-established
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much as it reacts with the added H+ ions
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much as CH3COOH is formed from the reaction of CH3COO- with H+
As a result, the pH remains reasonably constant

When OH- ions are added:
The OH- reacts with H+ to form water
OH- (aq) + H+ (aq) → H2O (l)
The H+ concentration decreases
The equilibrium position shifts to the right and more CH3COOH molecules ionise to form more H+and CH3COO- until equilibrium is re-established
CH3COOH (aq) → H+ (aq) + CH3COO- (aq)
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much when CH3COOH dissociates to form more H+ ions
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much
As a result, the pH remains reasonably constant

The pH of a buffer solution can be calculated using:
The Ka of the weak acid
The equilibrium concentration of the weak acid and its conjugate base (salt)
To determine the pH, the concentration of hydrogen ions is needed which can be found using the equilibrium expression

Since -log10 [H+] = pH, the expression can also be rewritten as:


Controlling the pH of blood
In humans, HCO3- ions act as a buffer to keep the blood pH between 7.35 and 7.45
Body cells produce CO2 during aerobic respiration
This CO2 will combine with water in blood to form a solution containing H+ ions
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This equilibrium between CO2 and HCO3- is extremely important
If the concentration of H+ ions is not regulated, the blood pH would drop and cause ‘acidosis’
Acidosis refers to a condition in which there is too much acid in the body fluids such as blood
This could cause body malfunctioning and eventually lead to coma
If there is an increase in H+ ions
The equilibrium position shifts to the left until equilibrium is restored
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This reduces the concentration of H+ and keeps the pH of the blood constant
If there is a decrease in H+ ions
The equilibrium position shifts to the right until equilibrium is restored
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This increases the concentration of H+ and keeps the pH of the blood constant
what is a buffer a mix of?
weak acid (HA) and one its salts (A-)
what the two ways of making buffers?
ethanoic acid
soidum ethanoate / NaOH
RULE FOR BUFFERS
(H+)≄ (A-)
EQUATION for weak acid + conjugate base:
CH3COOH → ← CH3COO- + H+
CH3COONa —> CH3COO- + NA+
equation of weak acid + strong base:
CH3COOH + NaOH —> CH3COO-NA+ + H20
use ice
buffers work best when
PHs equal to value of weak acids pKa. This is when fraction of weak acids conc divided by salt conc is 1
pH titration curve:
Calibrate pH metre
Measure acid: stir acid with magnetic stirrer
Fill burette with base and record inital
Add base slowly 1cm of base to acid and record new Ph
Continue adding base
Add base until pH stops changing
Plot curve of Ph against volume of base added
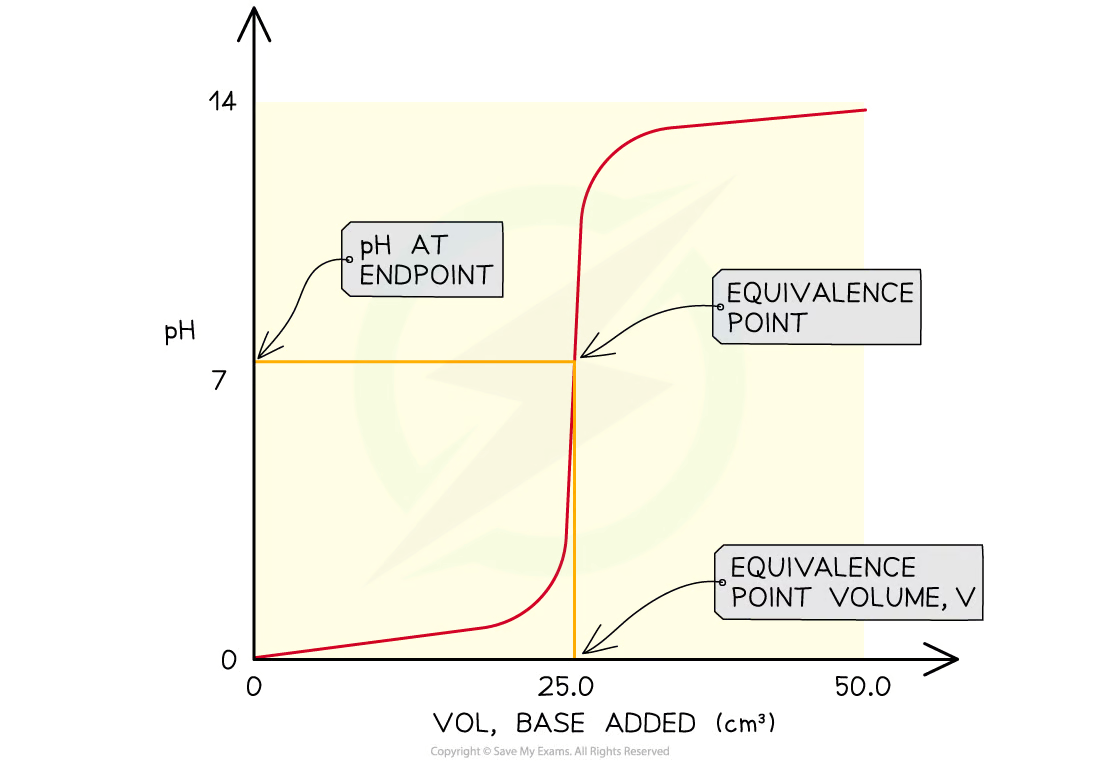
From the curves you can:
Determine the pH of the acid by looking where the curve starts on the y-axis
Find the pH at the equivalence point
Find volume of base at the equivalence point
Obtain the range of pH at the vertical section of the curve
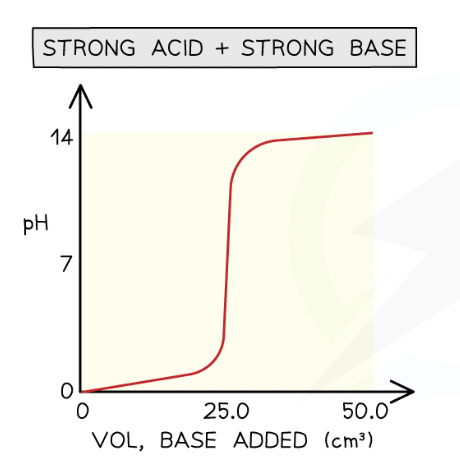
strong acid + strong base graph”
Initial pH change is small
pH rises faster as more base is added
sharp rise in pH at equivalence point (equivalence point)
solution is alkaline after steep rise (strong base)
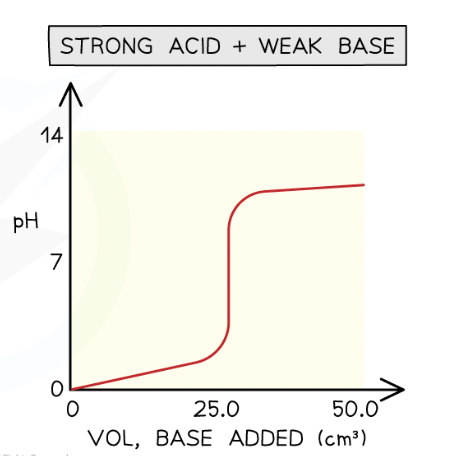
strong acid - weak base:
intial pH rise is small
steep rise in pH during neutralise
equivalence below 7 pH
solution is alkaline after steep ris
steep tise im pH seen at end (weak base)
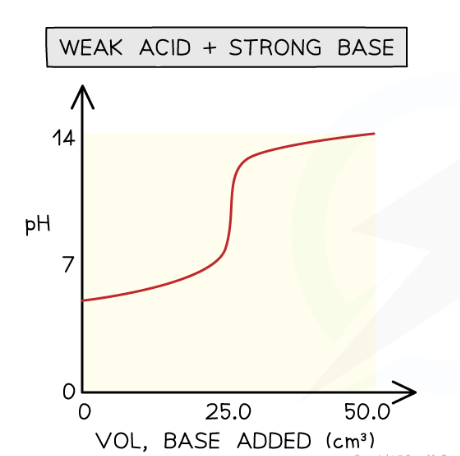
weak acid- strong base:
starting pH is higher
intially pH rises fast (creates buffer)
steep rise in pH during neutralisatoon
equivlaence point above pH 7
solution is alkaline after steep rise (strong base)
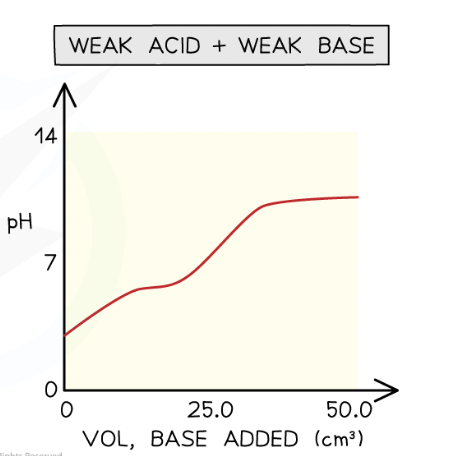
weak acid- weak base:
no clear equivalence point
how do indicator work?
weak acids and the undissociated and dissociated form in equilibrium as a conjugate acid base pair
two forms of indicator molecule are different colours or one is coloured and one is colourless
equation of indicator
HIn (aq) + H2O (l) ⇌ H3O+ (aq) + In- (aq)
colour 1 colour 2
HIn and its conjugate base In- are different colours
The colour of the solution depends on the relative concentrations of the two species
If the solution is acidic, the above equilibrium will be shifted to the left and more HIn will be present
Colour 1 will thus dominate
If the solution is alkaline, the above equilibrium will shift to the right and more In- will be present
Colour 2 will thus dominate
The colour of the indicator thus depends on the pH of the solution
The colour will not change suddenly at a certain pH, but will change gradually over a pH range
The colour of the indicator depends on the ratio of [HIn] to [In-]
Ka of the indicator

endpoint of the reaction is where there is a balance between [HIn] and [In-]. At this point these two concentrations are equal

Taking negative logs of both sides:
pKa = pH
Choosing a suitable indicator
Around the equivalence point of a titration, the pH changes very rapidly
Indicators change colour over a narrow pH range approximately centred around the pKa of the indicator
An indicator will be appropriate for a titration if the pH range of the indicator falls within the rapid pH change for that titration
Strong acid-strong base
In strong acid - strong base titrations, the pH changes from 4 to 10 at the end-point so a suitable indicator must change colour within this range
Methyl red and phenolphthalein are suitable indicators for these titrations
Methyl orange is not ideal but it shows a significant enough colour change at the end point so is widely used
Weak acid-strong base
In weak acid - strong base titrations, the pH changes from 7 to 10 at the end-point so a suitable indicator must change colour within this range
Phenolphthalein is the only suitable indicator for weak acid - strong base titrations that is widely available
Strong acid-weak base
In strong acid - weak base titrations, the pH changes from 4 to 7 at the end-point so a suitable indicator must change colour within this range
Methyl red is the most suitable indicator for these titrations
However methyl orange is often used since it shows a significant enough colour change at the end-point and is more widely available than methyl red
Weak acid-weak base
In weak acid -weak alkali titrations, there is no sudden pH change at the end-point and thus there are no suitable indicators for these titrations
The end-points of these titrations cannot be easily determined
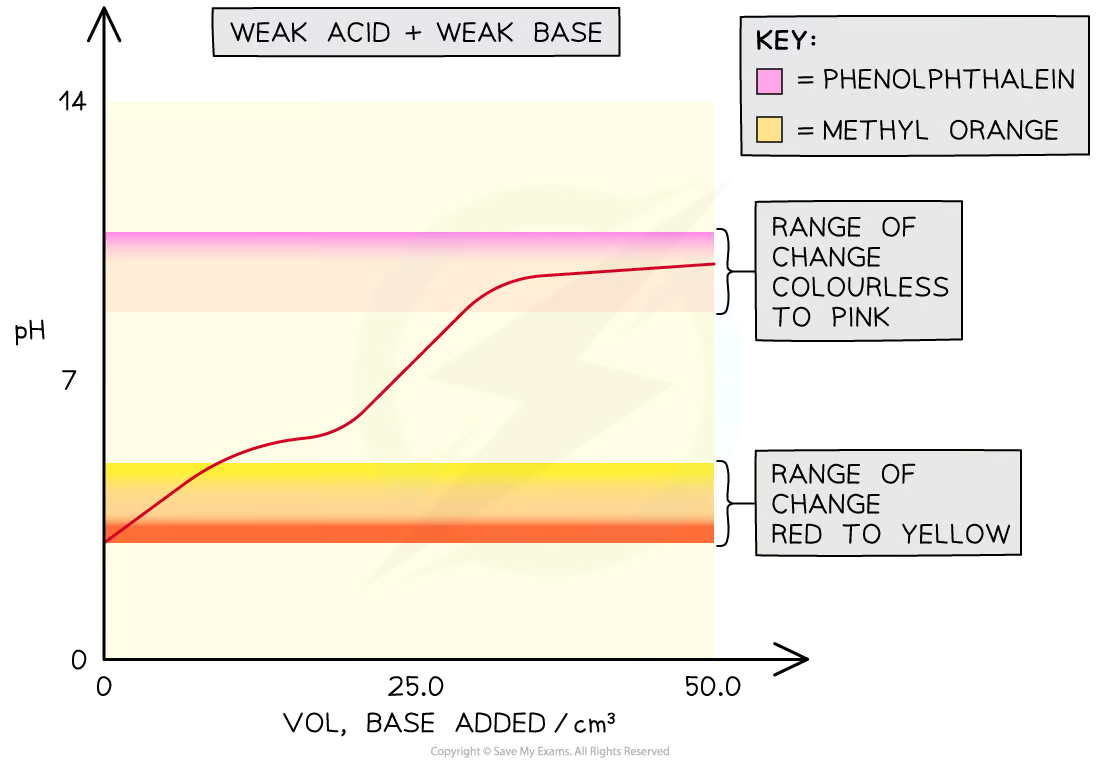
phenolphthalein
pH at equivalence: 7-11
weak acid
strong base
methyl orange
pH equivalence: 3-7
acid strong
weak base
end point (when indicator changes colour) occurs in middle when conc. of two species is equal