Bio Unit 2 test
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
What are Phospholipids made of [5]
Polar head [1] composed of a glycerol and phosphate molecule
Two non-polar tails [1] composed of fatty acids (hydrocarbon chains) [1]
are amphipathic [1] (hydrophobic and phillic)
![<p><span style="color: rgb(255, 70, 70);">Polar head [1]</span> composed of a <span style="color: rgb(255, 70, 70);">glycerol and phosphate molecule</span></p><p><span style="color: rgb(255, 70, 70);">Two non-polar tails [1]</span> composed of <span style="color: rgb(255, 70, 70);">fatty acids (hydrocarbon chains) [1]</span></p><p><span style="color: rgb(255, 70, 70);">are amphipathic [1]</span> (hydrophobic and phillic)</p>](https://knowt-user-attachments.s3.amazonaws.com/c7ddfa58-bb90-4e9a-af7f-1453562a081c.png)
Lipid bilayer structure [3]
annotate image [5]
B2.1.1—Lipid bilayers as the basis of cell membranes
Hydrophobic tails point inwards
Hydrophobic interactions between tails hold bilayer together [1]
Naturally forms a continuous sheet in water [1]
Hydrophilic heads attracted to water in cytoplasm or extracellular fluid [1]
Photo annotation [5]
![<p>Hydrophobic tails point inwards</p><ul><li><p><strong>Hydrophobic interactions between tails hold bilayer together [1]</strong></p></li></ul><ul><li><p><strong>Naturally forms a continuous sheet in water [1]</strong></p></li><li><p><strong>Hydrophilic heads attracted to water in cytoplasm or extracellular fluid [1]</strong></p></li></ul><p>Photo annotation [5]</p>](https://knowt-user-attachments.s3.amazonaws.com/387c8cd9-67a5-4155-bab3-7f8941aae686.png)
Lipid bilayer as barriers [4]
1 example for each permeability level [4]
B2.1.2—Lipid bilayers as barriers
Hydrophobic hydrocarbon chains have low permeability to:
Large molecules [1]
Hydrophilic particles [1]
Ions [0.5]
Polar molecules [0.5]
⇒ Membrane functions as an effective barrier between aqueous (extra-intracellular fluid) solutions[1]
Students should understand that the hydrophobic hydrocarbon chains that form the core of a membrane have low permeability to
large molecules and hydrophilic particles, including ions and polar molecules, so membranes function as effective barriers between
aqueous solutions.
![<p><strong>Hydrophobic hydrocarbon</strong> chains have <strong>low permeability</strong> to:</p><ul><li><p>Large molecules [1]</p></li><li><p>Hydrophilic particles [1]</p><ul><li><p>Ions [0.5]</p></li><li><p>Polar molecules [0.5]</p></li></ul></li></ul><p><strong>⇒ Membrane functions as an effective barrier between aqueous</strong> (extra-intracellular fluid) <strong>solutions[1]</strong></p><p></p><p>Students should understand that the hydrophobic hydrocarbon chains that form the core of a membrane have low permeability to </p><p>large molecules and hydrophilic particles, including ions and polar molecules, so membranes function as effective barriers between </p><p>aqueous solutions.</p>](https://knowt-user-attachments.s3.amazonaws.com/a5c95a6c-47a4-43ce-aae1-e8253da7682a.png)
What is simple diffusion in the case of lipid bilayers [3]
B2.1.3—Simple diffusion across membranes
Passive transport. Movement of small uncharged molecules [1]
moves from High concentration to low until equalibrium [1]
They ‘squeeze between’ the polar phospholipid heads then move through the membrane to the other side [1]
Use movement of oxygen and carbon dioxide molecules between phospholipids as an example of simple diffusion across membranes.
![<p>Passive transport. Movement of <strong>small uncharged molecules</strong> [1]</p><p><strong>moves from High concentration to low until equalibrium</strong> [1]<br>They ‘squeeze between’ the polar phospholipid heads then move through the membrane to the other side [1]</p><p></p><p>Use movement of oxygen and carbon dioxide molecules between phospholipids as an example of simple diffusion across membranes.</p>](https://knowt-user-attachments.s3.amazonaws.com/340be6e0-4bd8-4cfa-9c0e-d6f366475fde.png)
What is faciliitated diffusion in the case of lipid bilayers [1]
Role of channel proteins [2]
B2.1.6—Channel proteins for facilitated diffusion
Passive transport using a membrane protein [0.5]
Movement of large polar molecules (ex sodium ions) from high to low concentration [0.5]
open → allows specific ions to diffuse through (ex sodium channel)
close → stops diffusion
[1]
⇒ allow for selective permeability of membranes [1] (control how many go in and out)
Students should understand how the structure of channel proteins makes membranes selectively permeable by allowing specific
ions to diffuse through when channels are open but not when they are closed
![<p>Passive transport using a <strong>membrane protein [0.5]</strong></p><ul><li><p>Movement of <strong>large polar molecules</strong> (ex sodium ions) from <strong>high to low concentration [0.5]</strong></p></li></ul><div data-type="horizontalRule"><hr></div><p>open → <strong>allows specific ions to diffuse through</strong> (ex sodium channel)</p><p>close → <strong>stops diffusion</strong></p><p><strong>[1]</strong></p><p>⇒ allow for<strong> selective permeability of membranes [1] </strong>(control how many go in and out)</p><p>Students should understand how the structure of channel proteins makes membranes selectively permeable by allowing specific </p><p>ions to diffuse through when channels are open but not when they are closed</p>](https://knowt-user-attachments.s3.amazonaws.com/27dcad07-7457-428f-ac74-b27a1c35b261.png)
EXTRA > Good to know
what is a carrier protein?
Carrier proteins bind to solutes then change conformation to transport them to the other side [1]
![<p>Carrier proteins <strong>bind to solutes then change conformation to transport them to the other side </strong>[1]</p>](https://knowt-user-attachments.s3.amazonaws.com/22697762-1c57-4552-ba87-41842826a604.png)
What are Integral and Peripheral proteins [3]
B2.1.4—Integral and peripheral proteins in membranes
Integral proteins are embedded into one or both lipid layers [1] → Are amphipathic and can thus extend into the bilayer [1]
Peripheral proteins are attached to only one surface (inner or outer) of the bilayer [1]
Emphasize that membrane proteins have diverse structures, locations and functions. Integral proteins are embedded in one or both
of the lipid layers of a membrane. Peripheral proteins are attached to one or other surface of the bilayer
Functions of membrane proteins [2]
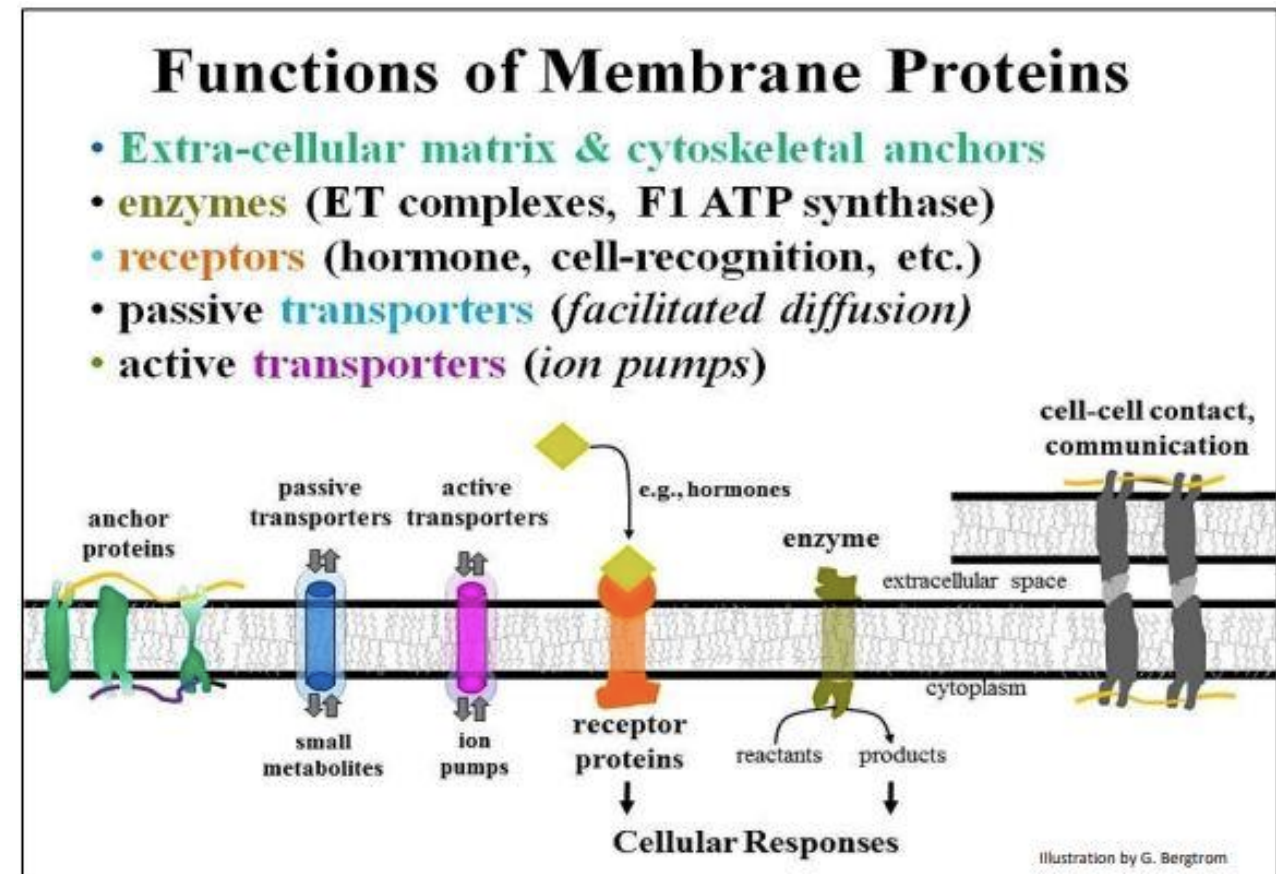
What is osmosis? [2]
what are aquaphorins and it’s role? [3]
B2.1.5—Movement of water molecules across membranes by osmosis and the role of aquaporins
Include an explanation in terms of random movement of particles, impermeability of membranes to solutes and differences in solute concentration [3 main points to hit]
Movement of water molecules across a semipermeable membrane from low solute concentration to high [2] (water concentration)
An integral protein that rapidly transports water molecules [1]
Bc Water is polar and cannot pass through the bilayer Aquaporin is lined hydrophilic side chains [1]
allows water to move in and out of cell via osmosis [1]
(Water can move bidirectionally depending on the concentration gradient)
Membrane impermeable to solutes
Water moves until both sides have equal solute concentration
Random movement of water molecules but no net movement of wate [3]
![<p>Movement of water molecules <strong>across a semipermeable membrane</strong> <strong>from low solute concentration to high [2] </strong>(water concentration)</p><div data-type="horizontalRule"><hr></div><p>An integral protein that rapidly transports water molecules [1]</p><p>Bc Water is polar and cannot pass through the bilayer Aquaporin is lined <strong>hydrophilic side chains </strong>[1]</p><p>allows water to move in and out of cell via osmosis [1]</p><p>(Water can move bidirectionally depending on the concentration gradient)</p><div data-type="horizontalRule"><hr></div><p>Membrane impermeable to solutes</p><p>Water moves until both sides have equal solute concentration</p><p>Random movement of water molecules but no net movement of wate [3]</p>](https://knowt-user-attachments.s3.amazonaws.com/ad3fbede-c245-4578-aed6-4c74f3cbcd8b.png)
What are pump proteins? [2]
Compare and contrast between Facilitated diffusion and active transport and simple diffusion [2]
B2.1.7—Pump proteins for active transport
Pump proteins use energy [1] (from ATP) to move specific particles against their concentration gradient [1]
Facilitated diffusion and active tran
sport allow membrane selective permeability [1]
Simple diffusion is not selective (depends only on particle size and hydrophilic/hydrophobic properties) [1]
Students should appreciate that pumps use energy from adenosine triphosphate (ATP) to transfer specific particles across
membranes and therefore that they can move particles against a concentration gradient
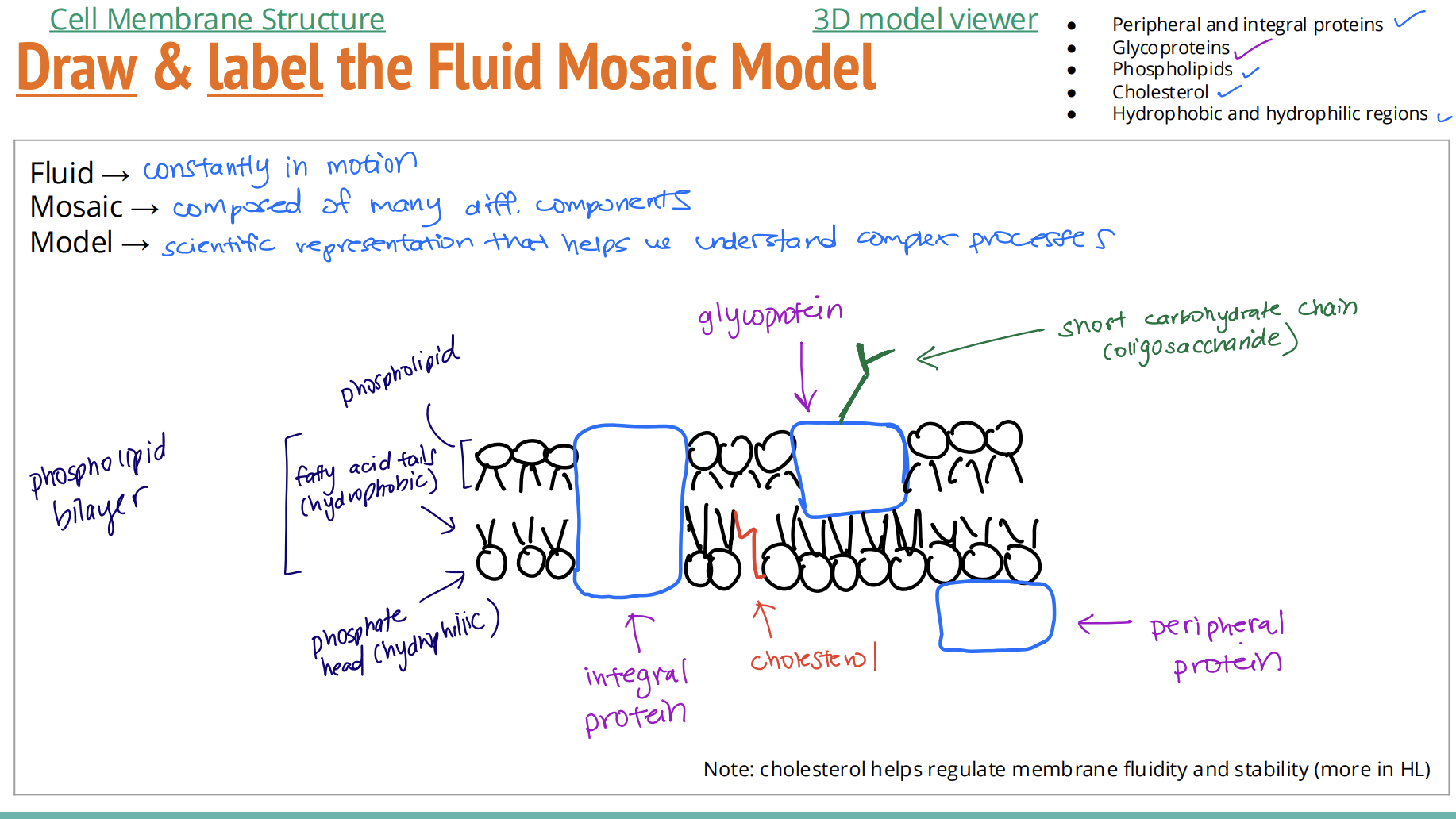
Draw & label the Fluid Mosaic Model [9]
B2.1.10—Fluid mosaic model of membrane structure
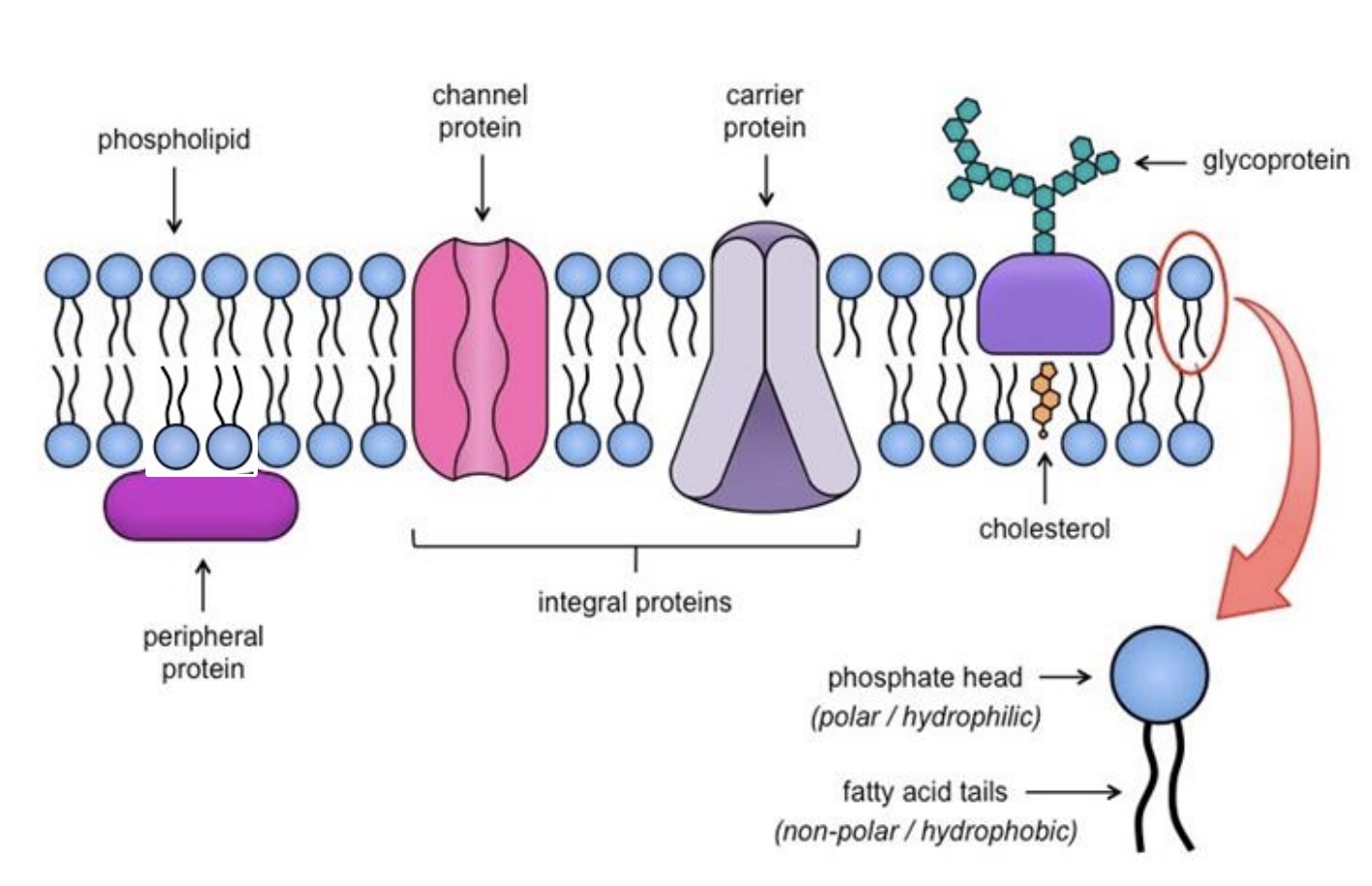
Students should be able to draw a two-dimensional representation of the model and include peripheral and integral proteins,
glycoproteins, phospholipids and cholesterol. They should also be able to indicate hydrophobic and hydrophilic regions
what are attatched to Glycoproteins and Glycolipids and what are their roles [3]
Glycoproteins: short carbohydrate chain (oligosaccharide) attached to proteins [1]
Glycolipids: carbohydrate group attached to lipids [1]
Responsible for cell adhesion and cell recognition [1]
Describe process of solvation [5]
2.3.1—Solvation with water as the solvent
Hydrogen bonds form between polar solute and polar water molecules [1]
● Slightly positively charged H atom of water attracted to negative ions [1]
● Slightly negatively charged O atom of water attracted to positive ion [1]
Water molecule surround charged ions using “hydration shells” [1]
Solute particles separated and surrounded by water [1] → solvation
Include hydrogen bond formation between solute and water molecules, and attractions between both positively and negatively charged ions and
polar water molecules.
What is Tonicity [2]
What are the different types of tonicity [6]
D2.3.2—Water movement from less concentrated to more concentrated solutions
D2.3.3—Water movement by osmosis into or out of cells
the ability of an extracellular solution to make water move in or out of cell [2]
HYPERTONIC: High solute concentration outside cell than inside cell → water moves out [1]
(cell will shrink/shrivel) [1]
HYPOTONIC: Low solute concentration outside cell than inside cell → water moves in [1]
(cell will swell) [1]
ISOTONIC: Equal solute concentration outside cell than inside cell → water moves in and out in equilibrum [1]
(cell will remain the same) [1]
Students should express the direction of movement in terms of solute concentration, not water concentration. Students should use the terms “hypertonic”, “hypotonic” and “isotonic” to compare concentration of solutions
Students should be able to predict the direction of net movement of water if the environment of a cell is hypotonic or hypertonic. They should
understand that in an isotonic environment there is dynamic equilibrium rather than no movement of water.
![<p>the ability of an <strong>extracellular solution</strong> to make <strong>water move in or out of cell [2]</strong></p><div data-type="horizontalRule"><hr></div><p><u>HYPERTONIC:</u> <strong>High</strong> solute concentration outside cell than inside cell → water moves <strong>out [1]</strong></p><ul><li><p><strong>(</strong>cell will <strong>shrink/shrivel) [1]</strong></p></li></ul><p><u>HYPOTONIC:</u> <strong>Low</strong> solute concentration outside cell than inside cell → water moves <strong>in [1]</strong></p><ul><li><p><strong>(</strong>cell will <strong>swell) [1]</strong></p></li></ul><p><u>ISOTONIC:</u> <strong>Equal</strong> solute concentration outside cell than inside cell → water moves <strong>in and out in equilibrum [1]</strong></p><ul><li><p><strong>(</strong>cell will<strong> remain the same) [1]</strong></p></li></ul><div data-type="horizontalRule"><hr></div><p>Students should express the direction of movement in terms of solute concentration, not water concentration. Students should use the terms “hypertonic”, “hypotonic” and “isotonic” to compare concentration of solutions</p><div data-type="horizontalRule"><hr></div><p>Students should be able to predict the direction of net movement of water if the environment of a cell is hypotonic or hypertonic. They should </p><p>understand that in an isotonic environment there is dynamic equilibrium rather than no movement of water.</p>](https://knowt-user-attachments.s3.amazonaws.com/edaac788-2c44-4a8e-9359-c4cc4a3809df.png)
How can animal cells be affected by different tonicity environments and why? [6]
What to organisims in hypotonic enviroments do to live? [2]
D2.3.5—Effects of water movement on cells that lack a cell wal
Hypotonic: Can burst (Lysis)
Hypertonic: can shrink and crenate
Isotonic: No change
[3]
Animal cells lack a cell wall [1]
Isotonic tissue fluid in multicellular organisms [1] needs to be maintained to prevent harm to cells and maintain proper function [1]
have contractile vacuoles to constantly expel water to prevent bursting
(contain store expel water when the vacuole contracts) [2]
Include swelling and bursting in a hypotonic medium, and shrinkage and crenation in a hypertonic medium. Also include the need for removal of
water by contractile vacuoles in freshwater unicellular organisms and the need to maintain isotonic tissue fluid in multicellular organisms to prevent
harmful changes
What happens to plant cells in hypertonic and hypotonic environments? [6]
D2.3.6—Effects of water movement on cells with a cell wall
Hypotonic: Causes increase in internal pressure called turgor pressure against the rigid cell walls [2]
Cell walls prevent bursting and allow plant cells to maintain a “turgid” shape
Hypertonic
Plasmolysis: cell membranes shrink away from cell walls [2]
Lose turgor pressure and cell shrinks [1]
Include the development of turgor pressure in a hypotonic medium and plasmolysis in a hypertonic medium
![<p>Hypotonic: Causes increase in internal pressure called <strong>turgor pressure</strong> against the <strong>rigid cell walls [2]</strong></p><ul><li><p>Cell walls prevent bursting and allow plant cells to maintain a <strong>“turgid”</strong> shape</p></li></ul><p>Hypertonic</p><p><strong>Plasmolysis</strong>: cell membranes shrink away from cell walls [2]</p><p><strong>Lose turgor pressure</strong> and cell shrinks [1]</p><div data-type="horizontalRule"><hr></div><p>Include the development of turgor pressure in a hypotonic medium and plasmolysis in a hypertonic medium</p>](https://knowt-user-attachments.s3.amazonaws.com/d5589a9d-c896-4f5a-aead-ae6487284aea.png)
Medical applications of isotonic solutions [9]
D2.3.7—Medical applications of isotonic solution
Organ transplantation [1]
Organ needs to be bathed in a fluid that is isotonic to the cytoplasm of organ’s cells [1]
Prevent loss or gain of water → reduce risk of damage for a successful transplant [1]
Intravenous (IV) fluids [1]
Replace lost fluids, administer drugs, blood transfusion [3]
More rapid and direct absorption into circulatory system if fluid is isotonic to blood/tissue fluid [2]
Include intravenous fluids given as part of medical treatment and bathing of organs ready for transplantation as examples
Why do cells need energy? [9]
C1.2.2—Life processes within cells that ATP supplies with energy
Metabolism [1]: synthesis of macromolecules (nucleic acids, proteins,) [1]
Active transport [1]: moving substances in and out of cells [1]
Whole cell movement [1]: muscle contraction [1]
Cell component movement [1]: chromosomes during cell division [1]
Energy is needed to sustain fundamental functions, maintain homeostasis, and sustain life processes.[1]
Include active transport across membranes, synthesis of macromolecules (anabolism), movement of the whole cell or cell components such as
chromosomes
How do cells get energy? [3]
from carbon compounds [1]
carbohydrates and lipids mainly
glucose and fatty acids [2]
What form of energy do cells use and it’s properties? [4]
draw simple diagram of ATP [3]
C1.2.1—ATP as the molecule that distributes energy within cells
Chemical energy [1] in the form of the nucleotide adenosine triphosphate (ATP) [1]
adenine + ribose + 3 phosphates [1]
Properties: unstable, readily releases energy [1]
Include the full name of ATP (adenosine triphosphate) and that it is a nucleotide. Students should appreciate the properties of ATP that make it suitable for use as the energy currency within cell
![<p><strong><u>Chemical energy [1]</u></strong> in the form of the <strong>nucleotide adenosine triphosphate (ATP) [1]</strong></p><p><strong>adenine + ribose + 3 phosphates</strong> [1]</p><p>Properties: <strong>unstable, readily releases energy</strong> [1]</p><div data-type="horizontalRule"><hr></div><p>Include the full name of ATP (adenosine triphosphate) and that it is a nucleotide. Students should appreciate the properties of ATP that make it suitable for use as the energy currency within cell</p>](https://knowt-user-attachments.s3.amazonaws.com/3c675f9f-6af6-4287-95a8-7793affd817b.png)
How is ATP formed and the definition of cellular respiration? [6]
C1.2.3—Energy transfers during interconversions between ATP and ADP
C1.2.4—Cell respiration as a system for producing ATP within the cell using energy released from carbon compound
Cellular Respiration is a system in cells for producing ATP,[1] controlled by enzymes [1]
hydrolysis of ATP releases energy for cell activities [1] and releases ADP [1] ATP → ADP + Pi (Pi = phosphate when not bonded to an organic compound.) Energy is released for these processes when ATP is broken down
Glucose & fatty acids are broken down to release energy, energy from food (glucose, fatty acids/carbon compounds) [1] used in the phosphorylation (adding a phosphate group) of ADP to ATP [1]
Students should know that energy is released by hydrolysis of ATP (adenosine triphosphate) to ADP (adenosine diphosphate) and phosphate,
but energy is required to synthesize ATP from ADP and phosphate. Students are not required to know the quantity of energy in kilojoules, but
students should appreciate that it is sufficient for many tasks in the cell
Students should appreciate that glucose and fatty acids are the principal substrates for cell respiration but that a wide range of
carbon/organic compounds can be used. Students should be able to distinguish between the processes of cell respiration and ga s exchange
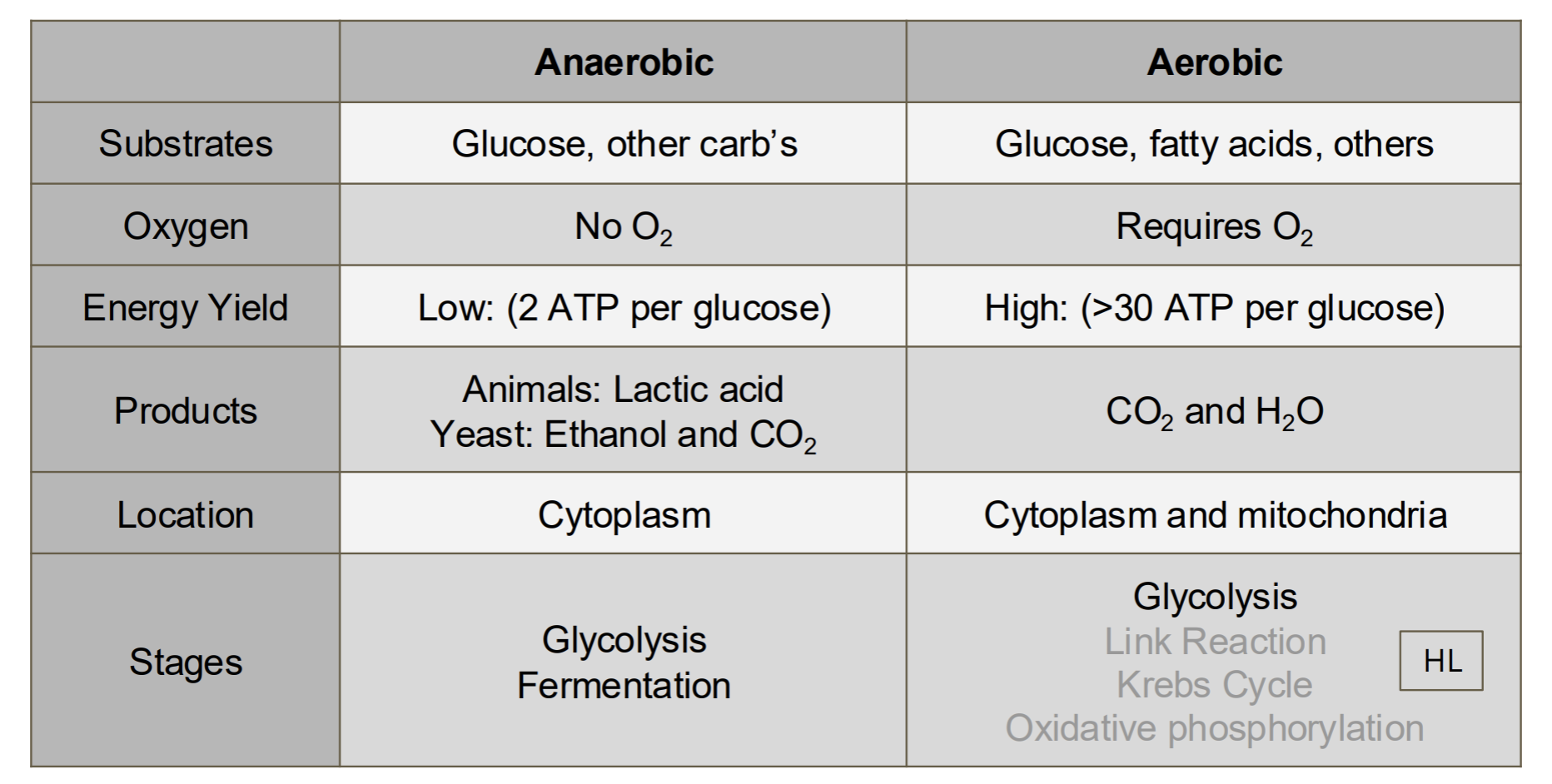
How do cells turn organic compounds into energy [7]
C1.2.5—Differences between anaerobic and aerobic cell respiration in humans
Aerobic respiration [1]
glucose + oxygen → carbon dioxide + water [1]
ADP + P → ATP (high yield) [1]
mitochondria [1]
Anaerobic respiration [1]
glucose → lactate [1]
ADP + P → ATP (low yield) [ ]
cytoplasm [1]
Include which respiratory substrates can be used, whether oxygen is required, relative yields of ATP, types of waste product and where the
reactions occur in a cell. Students should be able to write simple word equations for both types of respiration, with glucose as the substrate.
Students should appreciate that mitochondria are required for aerobic, but not anaerobic, respiration
![<p>Compare and contrast aerobic and anaerobic respiration. [12]</p>](https://knowt-user-attachments.s3.amazonaws.com/abbfbc3d-fb74-4280-9265-76eb44e4c0ac.png)
Compare and contrast aerobic and anaerobic respiration. [12]
Rate Calculation [1]
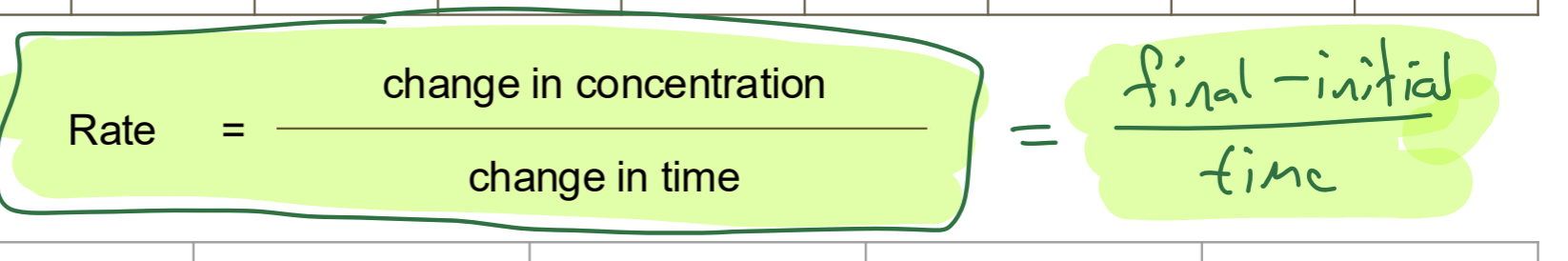
List and outline all processes in life [8]
A2.2.7—Processes of life in unicellular organisms
Metabolism: chemical reactions which occur in a cell [1]
Response to stimuli: reacting to changes in the external environment [1]
Homeostasis: the maintenance of constant internal conditions [1]
Movement: some control over their place and position [1]
Growth: cells can increase in size and/oran increase in the number of cells that make up an organism (in multicellular organisms) [1]
Reproduction: the production of offspring. Can be sexual or asexual [1]
Excretion: the removal of metabolic waste products [1]
Nutrition: the intake or production of nutrients [1]
Include these functions: homeostasis, metabolism, nutrition, movement, excretion, growth, response to stimuli and reproduction
List and outline cell theory [3]
A2.2.1—Cells as the basic structural unit of all living organisms
cell theory states that:
1. All living things are made of individual units called cells [1]
2. Cells are the basic units of life [1]
3. All cells arise from other pre-existing cells (division) [1]
Students should be aware that deductive reason can be used to generate predictions from theories. Based on
cell theory, a newly discovered organism can be predicted to consist of one or more cells
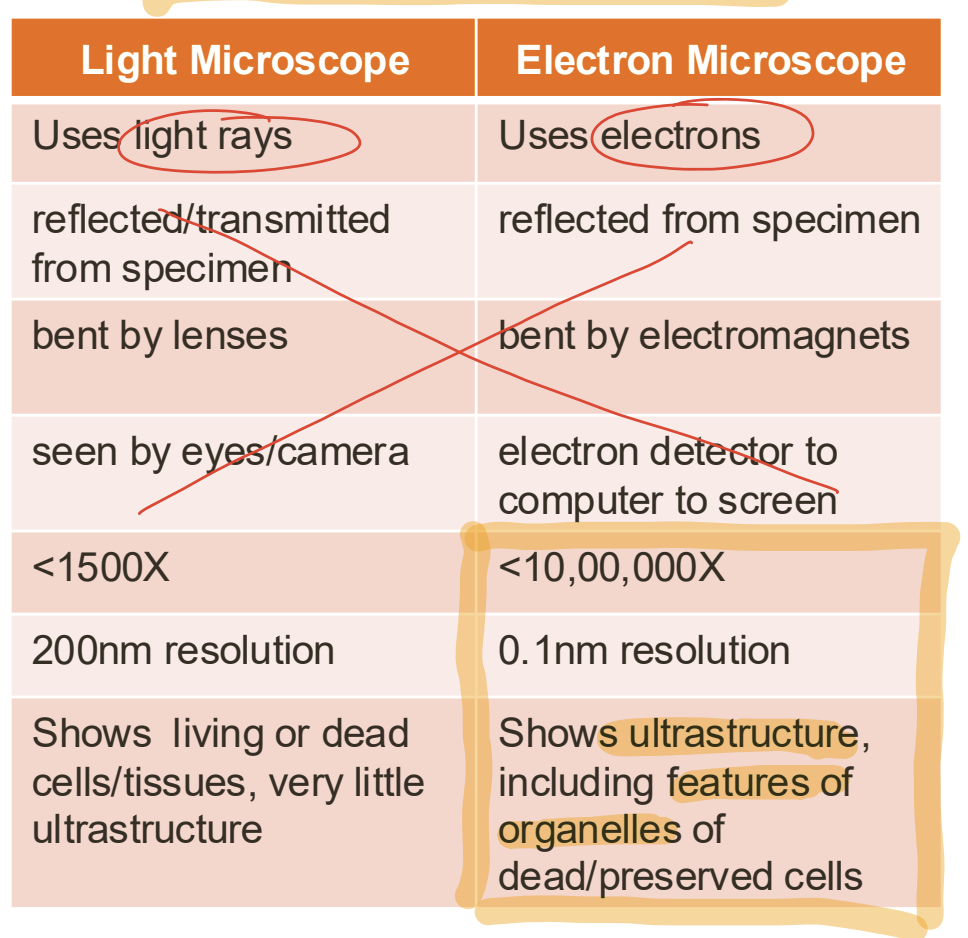
What is magnification and it’s formula [3]
How much larger the object appears compared to its real size [1]
eyepiece lens power x objective lens power = magnification [2]
What is resolution and three example values [4]
Measure of the clarity of the image [1]
● Human eye: 100 μm [1]
● Light microscope: 200 nm [1]
● Electron microscope: 0.01nm [1]
(increasing)
Light microscopes vs electron and benefits on electron [8]
Electron microscopes has higher resolution and magnification
four developments in Microscopy and their advantages [15]
A2.2.3—Developments in microscopy
Freeze fracture microscopy [1]
Samples are frozen and then broken apart [1] using special tools.
The pieces are observed using an electron microscope to see the internal structure [1]
Cryogenic electron microscopy [1]
Samples are frozen to cryogenic temperatures (-180°C or colder) [1]. This makes the molecules more stable [1].
Improves resolution and reduces damage from the electron beam. [1]
Often used in imaging of molecules [1]
Fluorescent stains [1]
Used in light microscopy
Added dye will attach to specific structures [1]
Labelled areas appear at bright spots [1]
e.g. shows molecules too too small to be seen
(neurotransmitters or proteins). [1]
Immunofluorescence [1]
Used in light microscopy
Allows very specific visualization [1]
e.g. fluorescent tags may be attached to antibodies which attach to specific molecules (proteins, carb’s, etc) [1]
include the advantages of electron microscopy, freeze fracture, cryogenic electron microscopy, and the use of
fluorescent stains and immunofluorescence in light microscopy
Structures common to all cells [11]
A2.2.4—Structures common to cells in all living organisms
DNA as their genetic material [1]
able to store and transfer information [1]
codes for a diversity of proteins [1]
passed on to offspring (genetic material)[1]
Cytoplasm composed of mainly water (cytosol) [1]
contains a variety of carbon-based compounds, ions and inorganic compounds [1]
the site where most chemical reactions take place [1]
Plasma membrane composed of lipids that surround the cytoplasm and protects its contents [1]
compartmentalizes cell [1]
selectively permeable [1]
contains proteins for cell-to-cell communication, transport, cell adhesion, cell identity [1]
Typical cells have DNA as genetic material and a cytoplasm composed mainly of water, which is enclosed by a
plasma membrane composed of lipids. Students should understand the reasons for these structures.
Eukaryotic Cell Structure of Fungal Cells [5]
Cell wall made of chitin (polysaccharide) [1]
Large vacuoles break down unwanted molecules [1]
Some are unicellular [1]
e.g. yeast [0.5]
Some are multicellular [1]
e.g. mushrooms [0.5]
![<ul><li><p>Cell wall made of <strong>chitin </strong>(polysaccharide) [1]</p></li></ul><ul><li><p>Large vacuoles break down unwanted molecules [1]</p></li><li><p>Some are unicellular [1]</p><ul><li><p><strong>e.g. yeast</strong> [0.5]</p></li></ul></li><li><p>Some are multicellular [1]</p><ul><li><p><strong>e.g. mushrooms </strong>[0.5]</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/6bc9bb86-7ef6-4519-ac2d-cc3337205a03.png)
![<p>Differences in Eukaryotic Cell Structures? [7]</p><p>A2.2.8—Differences in eukaryotic cell structure between animals, fungi and plants</p>](https://knowt-user-attachments.s3.amazonaws.com/1555ce18-999a-4690-ba17-7bf32ce38f05.png)
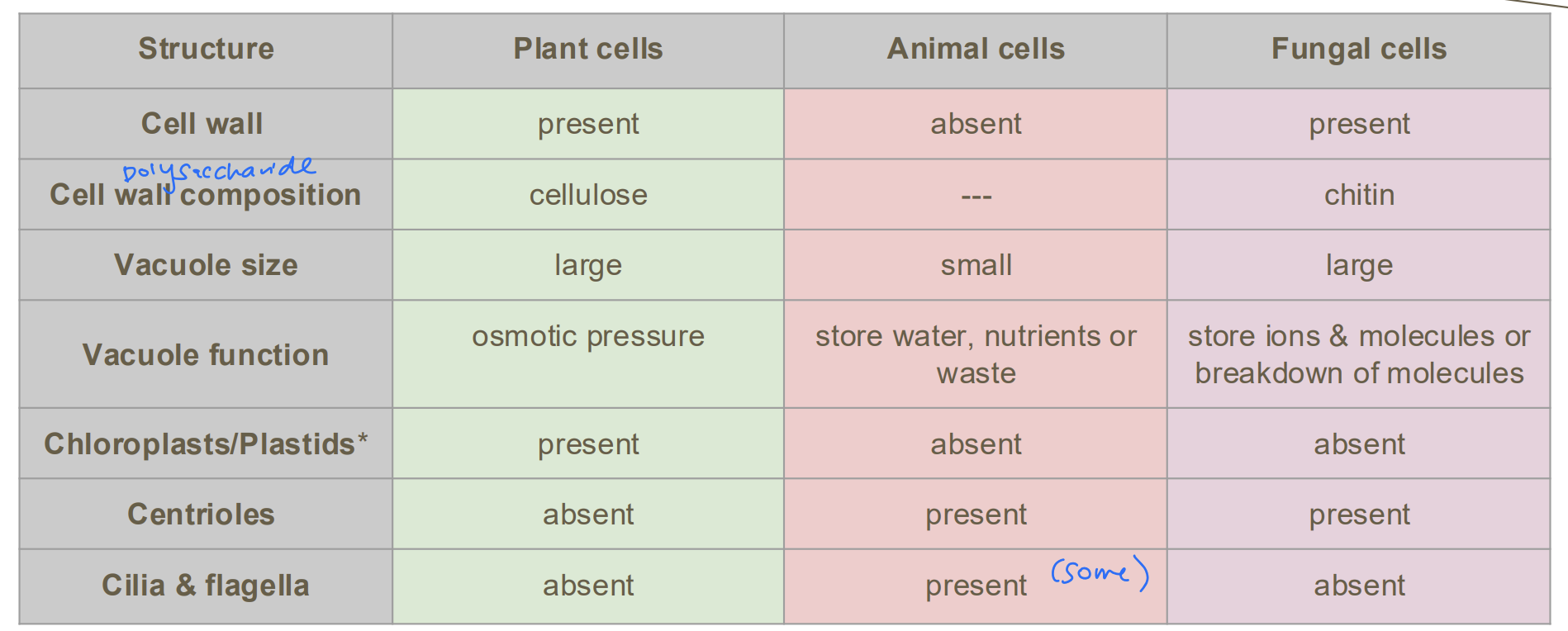
Differences in Eukaryotic Cell Structures? [7]
A2.2.8—Differences in eukaryotic cell structure between animals, fungi and plants
Include presence and composition of cell walls, differences in size and function of vacuoles, presence of
chloroplasts and other plastids, and presence of centrioles, cilia and flagella
Examples of Atypical Cell Structure in Eukaryotes [8]
A2.2.9—Atypical cell structure in eukaryotes
Know these examples:
Skeletal muscle: multinucleated [1](multiple nuclei) Formed from
many muscle cells fused together. [1]
Aseptate fungal hyphae: multinucleated [1], do not have normal separations to make them single units [1]
Red blood cells: anucleate [1] mature rbc do not have to increase SA to carry o2 [1]
Phloem sieve tube elements: anucleate [1] nuclei are broken down to provide more space for nutrient transport [1]
Use numbers of nuclei to illustrate one type of atypical cell structure in aseptate fungal hyphae,
skeletal muscle, red blood cells and phloem sieve tube elements.
what lis light energy transformed in in phtosynthesis? [1]
What is the product of photosynthesis and celluar respiration? [2]
C1.3.1—Transformation of light energy to chemical energy when carbon compounds are produced in
photosynthesis
Light energy (sun) is transformed to chemical energy (carbon compounds) through the process of photosynthesis [1]
Oxygen, organic compunds [1]
Co2 and water [1]
This energy transformation supplies most of the chemical energy needed for life processes in ecosystems
Process of photosynthesis, written and formula
what is photolysis? [1]
1.3.2—Conversion of carbon dioxide to glucose in photosynthesis using hydrogen obtained by splitting water
C1.3.3—Oxygen as a by-product of photosynthesis in plants, algae and cyanobacteria
2H2O → 4H+ + 4e- + O [1]
Carbon dioxide + Water —> Glucose+ Oxygen [1]
Carbon dioxide is converted into glucose [1]
Oxygen is a byproduct of photosynthesis [1]
Hydrogen is used in the next few stages and will help produce glucose [1]
Photolysis involves the splitting of H2O [1] to produce hydrogen and oxygen [1]
Students should be able to write a simple word equation for photosynthesis, with glucose as the product
Students should know the simple word equation for photosynthesis. They should know that the oxygen produced by
photosynthesis comes from the splitting of water.
What are Cyanobacteria? [1]
what is chlorophyll ? [4]
are bacteria (a prokaryotes) that possess chlorophyll in their cytoplasm [1]
a photosynthetic pigment that absorbs light energy [1]
Pigments “capture” light energy which “excites” electrons within a pigment molecule [1]
The excited electrons will “drive” photosynthesis [1]
Transformation of light energy to chemical energy [1]
what is chloropast [2]
Chloroplasts contain photosynthetic pigments [1] called chlorophyll a and chlorophyll b [1]
What is a Action spectrum
C1.3.6—Similarities and differences of absorption and action spectra
plots photosynthesis activity against light wavelength [1]
(Activity can be measured through oxygen production, carbon dioxide absorption, biomass gained [1])
Shows which wavelengths of light is most efficient for photosynthesis [1]
Peaks in the action spectrum should occur at similar wavelengths to absorption spectrum (why)
Application of skills: Students should be able to determine rates of photosynthesis from data for oxygen production and
carbon dioxide consumption for varying wavelengths. They should also be able to plot this data to make an action
spectrum.
What is an absorption spectrum? [1]
An absorption spectrum displays the amount of light absorbed by a photosynthetic pigment for varying wavelengths of light [1]
Limiting factors of photosynthesis [8]
Temperature [1]
increase = More frequent and collisions between enzymes and substrate [1]
past an optimal point, enzyme will denature [1] expain more
Light intensity [1]
increase = photosynthetic pigments will absorb more light = photosynthetic rate to increase [2]
eventually pigments will be saturated with light and photosynthetic rate will plateau [1]
CO2 concentration [1]
increase = photosynthetic rate will increase bc more molecules to convert to organic molecules [2]
At a certain point, all enzymes will be fully saturated and the rate will plateau [1]
![<p><strong><u>Temperature [1]</u></strong></p><ul><li><p><strong>increase = More frequent and collisions between enzymes and substrate</strong> [1]</p></li><li><p>past an optimal point, enzyme will denature [1] expain more</p></li></ul><p><strong><u>Light intensity [1]</u></strong></p><ul><li><p><strong>increase = photosynthetic pigments will absorb more light = photosynthetic rate to increase</strong> [2]</p></li><li><p>eventually pigments will be <strong>saturated </strong>with light and <strong>photosynthetic rate will plateau [1]</strong></p></li></ul><p><strong><u>CO2 concentration [1]</u></strong></p><ul><li><p>increase = <strong>photosynthetic rate will increase </strong>bc more <strong>molecules to convert to organic molecules [2]</strong></p></li><li><p><strong>At a certain point, all enzymes will be fully saturated and the rate will plateau [1]</strong></p><p></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b5c1e18c-01ff-4818-988d-7e89dbb6a862.png)
What are specialised cells? [1]
specialized cells have a specific structures and functions (specific purpose) [1]
e.g. neuron, muscle fiber, etc.
Formation of stem cells [4]
At the start of life
fusion (fertilization) of gametes (sex cells) into one cell called a zygote. [1] (sperm and egg)
The zygote undergoes cell division (mitosis) repeatedly to produce a solid ball of cells called a morula. [1]
All of these cells are unspecialized they do not have a defined function yet [1]
These are embryonic stem cells. [1]
Differentiation of Stem Cells [3]
Cell division (mitosis) continues and after 5-6 days the morula differentiates into a hollow ball of cells called the blastocyst [1]
the inner cell mass within the blastocyst and the trophoblast have begun differentiation [1]
specific layers have received signals about which organs, tissues, and cell types they will become [1]
![<ol><li><p>Cell division (mitosis) continues and <strong>after 5-6 days the morula differentiates into a hollow ball of cells called the blastocyst [1]</strong></p></li></ol><ol start="2"><li><p>the <strong>inner cell mass</strong> within the<strong> blastocyst</strong> and the <strong>trophoblast</strong> have begun differentiation [1]</p></li><li><p>specific layers have <strong>received signals about which organs, tissues, and cell types they will become [1]</strong></p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c7052e62-a809-4851-af8c-2c1b537c29c5.png)
What are morphogens
What are morphogen gradients [2]
B2.3.1—Production of unspecialized cells following fertilization and their development into specialized cells by differentiation
Morphogens are signal molecules that control
differentiation by changing gene expression. [1]
they are released from source cells creating a concentration gradient [1]
different regions of the early embryo have different concentrations of morphogens [1]
concentration of molecules that affect gene expression and thus the differentiation pathways. [1]
different concentrations affect gene expression differently causing differentiation along specific pathway [1]
Students should understand the impact of gradients on gene expression within an early-stage embryo
What are Stem cells and it’s proporties [3]
B2.3.2—Properties of stem cells
Unspecialized cells
Stem cell properties:
they have the capacity to divide endlessly [1]
they can differentiate along different pathways. [1]
Limit to the capacity of cells to divide endlessly and differentiate along different pathway
What are the four types of stem cells in decrecing order of potency and what differentiated cells do thay produce? [12]
B2.3.4—Differences between totipotent, pluripotent and multipotent stem cells
Note that cells in early-stage animal embryos are totipotent but soon become pluripotent,
whereas stem cells in adult tissue such as bone marrow are multipotent. [1]
Students should appreciate that cells in early-stage animal embryos are totipotent but soon become pluripotent, whereas stem cells in adult
tissue such as bone marrow are multipotent
![<p>Note that cells in early-stage animal embryos are <strong>totipotent</strong> but soon become <strong>pluripotent,</strong></p><p>whereas stem cells in adult tissue such as bone marrow are multipotent. [1]</p><div data-type="horizontalRule"><hr></div><p>Students should appreciate that cells in early-stage animal embryos are totipotent but soon become pluripotent, whereas stem cells in adult </p><p>tissue such as bone marrow are multipotent</p>](https://knowt-user-attachments.s3.amazonaws.com/368a6ec7-3423-42b9-8751-98c1d82f3861.png)
What is a Stem Cell Niche [5]
B2.3.3—Location and function of stem cell niches in adult humans
Refers to the microenvironment where adult stem cells are found. [1]
Interactions between adult stem cells and their niche regulate stem cell differentiation [1]
Maintain stem cells (self renewal)
promote proliferation and differentiation into specialized cells [3]
Limit to two example locations and the understanding that the stem cell niche can maintain the cells or promote their proliferation and differentiation. Bone marrow and hair follicles are suitable examples
What are three key factors that determine interactions in the stem cell niche? [3]
Cell to cell interactions
Interaction with the extracellular matrix
Signal molecules affecting gene expression
[3]
Bone marrow as a Stem cell niche example [5]
bone marrow (multipotent) [1]
blood stem cells create more stem cells or a variety of blood cells [2]
e.g. erythrocytes (RBCs), platelets, lymphocytes, leukocytes (differentiation pathways) [2] two examples
![<p><strong>bone marrow (multipotent) [1]</strong></p><ul><li><p>blood stem cells <strong>create more stem cells </strong>or a <strong>variety of blood cells [2]</strong></p></li></ul><p>e.g. erythrocytes (RBCs), platelets, lymphocytes, leukocytes (differentiation pathways) [2] two examples</p>](https://knowt-user-attachments.s3.amazonaws.com/c8364bb6-bb49-4b15-8b08-498d19fda5df.png)
Hair follicles as a Stem cell niche example [3]
Hair follicles have cycles of degeneration, growth and rest [0.5]
Stem cell niche found at the “bulge” [1]
Multipotent cells involved in hair follicle regeneration [0.5]
Also involved in production of oil producing (sebaceous) glands and epidermal skin cells [1]
![<p>Hair follicles have cycles of <strong>degeneration, growth and rest [0.5]</strong></p><ul><li><p><strong>Stem cell niche</strong> found at the <strong>“bulge</strong>” [1]</p></li></ul><ul><li><p><strong>Multipotent cells </strong>involved in hair follicle regeneration [0.5]</p></li></ul><ul><li><p>Also involved in <strong>production of oil producing (sebaceous) glands and epidermal skin cells [1]</strong></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/25bbd0b5-6c4a-4bf6-a955-ba5e1aedf7bb.png)
Draw Neuron Structure [10]
[10]
![<p>[10]</p>](https://knowt-user-attachments.s3.amazonaws.com/1a003f7b-ba7e-458d-be52-fe23d7166376.png)
Neuron Function [6]
2.2.1—Neurons as cells within the nervous system that carry electrical impulses
A neuron can be stimulated by other neurons or receptor cells [1]
transmits an electrical impulse (signal) along dendrites and the axon [2]
releases a chemical signal to signal other neurons or effector cells (eg muscles) [1]
the chemicals released are called neurotransmitters [1]
the neurotransmitters are released into the synapse [1]
unidirectional: only transmits dendrite → axon → synapse [1]
Students should understand that cytoplasm and a nucleus form the cell body of a neuron, with elongated nerve fibres of varying
length projecting from it. An axon is a long single fibre. Dendrites are multiple shorter fibres. Electrical impulses are conducted
along these fibres.
![<p>A neuron can be stimulated by other <strong>neurons or receptor cells [1]</strong></p><ul><li><p>transmits an <strong>electrical impulse (signal) </strong>along <strong>dendrites and the axon [2]</strong></p></li><li><p>releases a <strong>chemical signal to signal other neurons or effector cells (eg muscles) [1]</strong></p><ul><li><p>the chemicals released are called <strong>neurotransmitters [1]</strong></p></li><li><p>the <strong>neurotransmitters</strong> are <strong>released</strong> into the <strong>synapse</strong> [1]</p></li></ul></li><li><p>unidirectional: only transmits dendrite → axon → synapse [1]</p></li></ul><div data-type="horizontalRule"><hr></div><p>Students should understand that cytoplasm and a nucleus form the cell body of a neuron, with elongated nerve fibres of varying </p><p>length projecting from it. An axon is a long single fibre. Dendrites are multiple shorter fibres. Electrical impulses are conducted </p><p>along these fibres.</p>](https://knowt-user-attachments.s3.amazonaws.com/556e03e5-44a0-46a7-b8b6-7064bcec730b.png)
Membrane Resting Potential? [1]
How is it achieved? [4]
C2.2.2—Generation of the resting potential by pumping to establish and maintain concentration gradients of sodium and potassium ions
electrical charge difference (-70mV) between the inside and outside of its cell membrane [1]when it is not transmitting an impulse. It is achieved by
pumping positively charged sodium ions (Na+) out of the cell and positively charged potassium ions (K+) into the cell [2]
3:2 ratio of Na+:K+ by sodium-potassium ion pump [1]
(pumps use ATP)
This creates a difference in charge which is a form of electrical voltage. [0.5]
The outside is considered positively charged and the inside negatively charged [0.5]
Students should understand how energy from ATP drives the pumping of sodium and potassium ions in opposite directions across
the plasma membrane of neurons. They should understand the concept of a membrane polarization and a membrane potential and also reasons that the resting potential is negative.
![<p><strong>electrical charge difference (-70mV) between the inside and outside of its cell membrane </strong>[1]when it is not transmitting an impulse. It is achieved by</p><div data-type="horizontalRule"><hr></div><p>pumping positively<strong> charged sodium ions (Na+) out of the cell </strong>and <strong>positively charged potassium ions (K+) into the cell [2]</strong></p><p>3:2 ratio of Na+:K+ by <strong>sodium-potassium ion pump [1]</strong></p><p><strong>(pumps use ATP)</strong></p><p>This creates a difference in charge which is a form of electrical voltage. [0.5]</p><p>The outside is considered positively charged and the inside negatively charged [0.5]</p><div data-type="horizontalRule"><hr></div><p>Students should understand how energy from ATP drives the pumping of sodium and potassium ions in opposite directions across </p><p>the plasma membrane of neurons. They should understand the concept of a membrane polarization and a membrane potential and also reasons that the resting potential is negative.</p>](https://knowt-user-attachments.s3.amazonaws.com/0dbf026b-73aa-4a03-9db0-010d5212875e.png)
What is membrane polarization [1]
Polarization is the unequal distribution of charges: more positive ions outside & fewer positive ions inside (during resting potential) [1]
What is depolarization? [2]
What are Electrical Impulses aka Action Potentials? [1]
C2.2.2
C2.2.3—Nerve impulses as action potentials that are propagated along nerve fibres
Depolarization is caused by the movement of positively charged sodium ions when a signal is sent [1]
sodium channels open and allow sodium ions to diffuse into the neuron [1]
This makes the inside more positive than the outside (+30mV) → depolarization [1]
the propagation of the area of depolarization (moves along the nerve) [1]
They should understand the concept of a membrane polarization and a membrane potential and also reasons that the resting potential is negative.
Students should appreciate that a nerve impulse is electrical because it involves movement of positively charged ions
What is myelin and how does it affect the neuron? [4]
Myelin acts as an insulator and amplifies the effect of the ion concentration gradient + faster speed of transmission [1]
(prevent leakage and in the node of ranvier all the sodium ion pumps are concentrated there, as the sodium diffuses through and changes the charge it will activate the next channel to open. Leading to the action potential to “jump” across the nodes which is faster than without them) [3]
![<p>Myelin acts as an <strong>insulator</strong> and <strong>amplifies the effect of the ion concentration gradient</strong> + faster speed of transmission [1]</p><p>(<strong>prevent leakage</strong> and in the node of ranvier all the <strong>sodium ion pumps are concentrated there</strong>, as the sodium diffuses through and changes the charge <strong>it will activate the next channel to open</strong>. Leading to the <strong>action potential to “jump” across the nodes which is faster than without them</strong>) [3]</p>](https://knowt-user-attachments.s3.amazonaws.com/83c9660c-020f-47d9-bc1d-2bf3b88d3e3e.png)
What is a synapse and synaptic cleft? [2]
C2.2.5—Synapses as junctions between neurons and between neurons and effector cells
synapse is the area/junction between two neruons [1]
synaptic cleft:The gap between the postsynaptic and presynaptic neruon dendrite [1]
Limit to chemical synapses, not electrical, and these can simply be referred to as synapses. Students should understand that a signal can only pass in one direction across a typical synapse.
Draw the diagram of Synaptic Transmission [9]
C2.2.6—Release of neurotransmitters from a presynaptic membrane
[9]
Include uptake of calcium in response to depolarization of a presynaptic membrane and its action as a signalling chemical inside a neuron
![<p>[9]</p><div data-type="horizontalRule"><hr></div><p>Include uptake of calcium in response to depolarization of a presynaptic membrane and its action as a signalling chemical inside a neuron</p>](https://knowt-user-attachments.s3.amazonaws.com/fd244494-fee2-4325-949b-5a55ee59cc9e.png)
acetylcholine as an example of a neurotransmitters [6]
C2.2.7—Generation of an excitatory postsynaptic potential
Chemical signals/neurotransmitters released in neuromuscular junctions [1] (a specialized synapse where a motor neuron communicates with a muscle fiber) [1]
initiate muscle contraction [1]
skeletal muscle
cardiac muscle
smooth muscle (eg peristalsis) [3]
Include diffusion of neurotransmitters across the synaptic cleft and binding to transmembrane receptors. Use acetylcholine as an example. Students should appreciate that this neurotransmitter exists in many types of synapse including neuromuscular junctions.
What are effector cells and examples? [4]
cells in muscles, glands, and organs that respond to nerve stimulus. [1]
skeletal muscle cells contract when neurons signal them [1]
adrenal glands release epinephrine (adrenaline) when stimulated [1]
the heart beats faster when signalled by neurons from the brain [1]