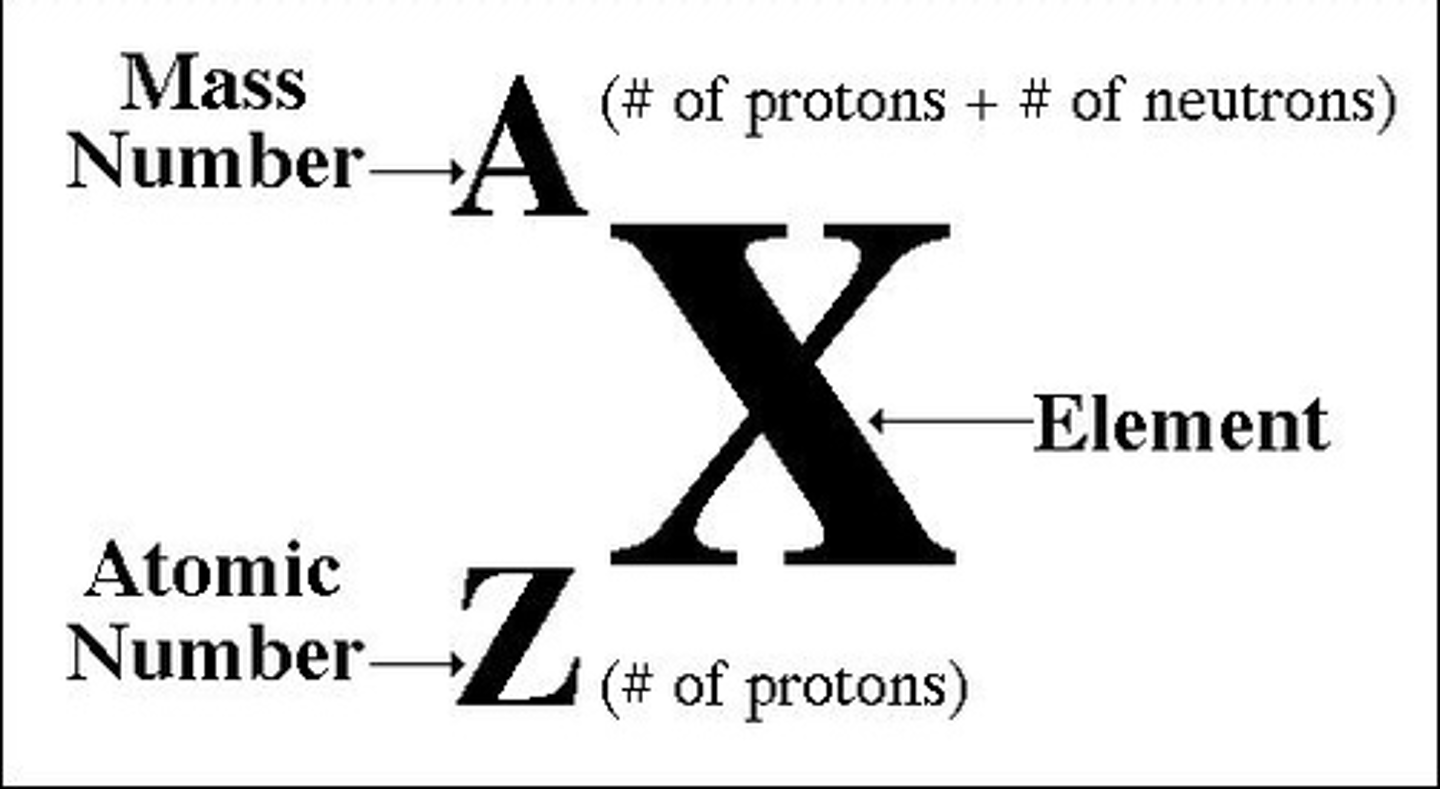nuclear chem
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
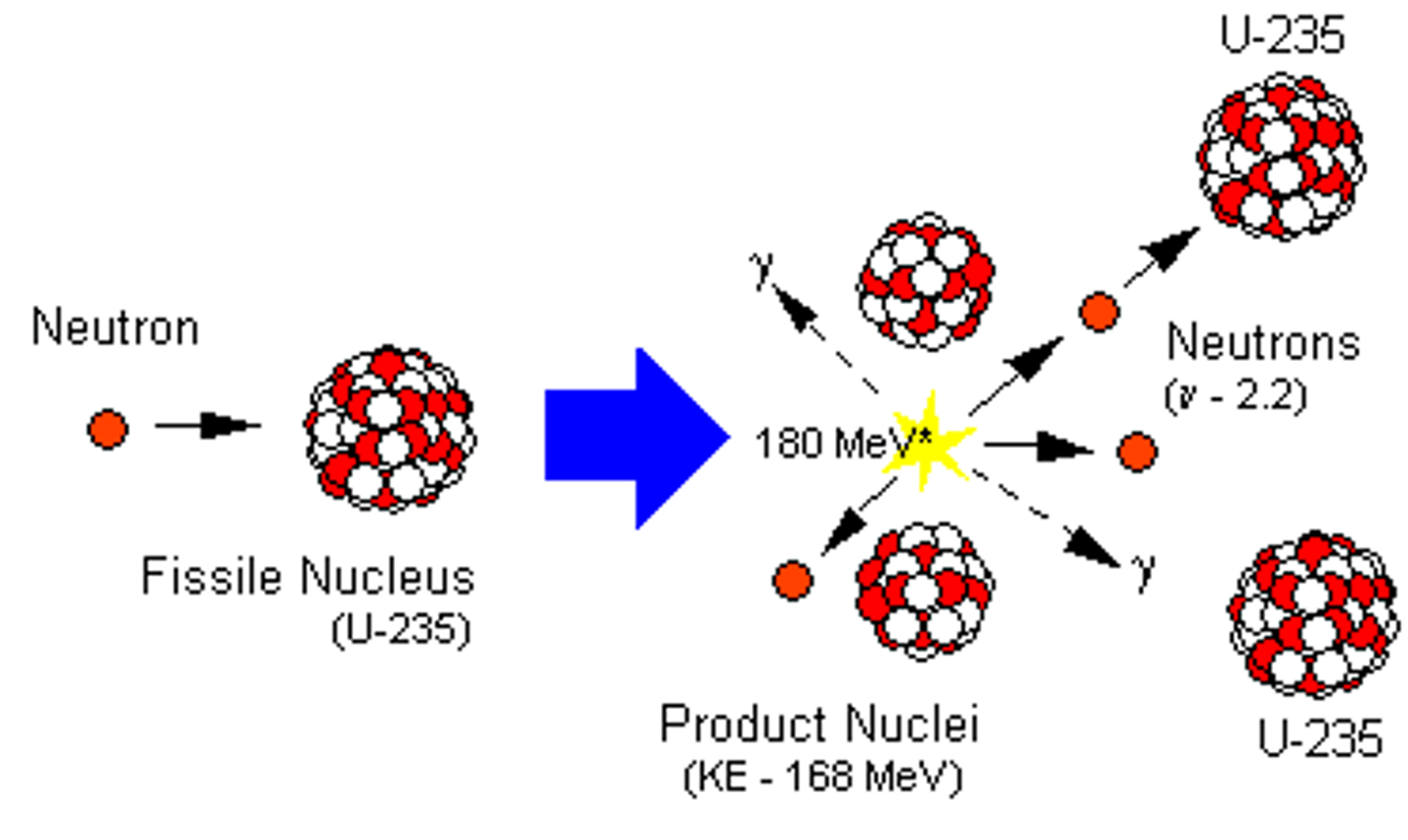
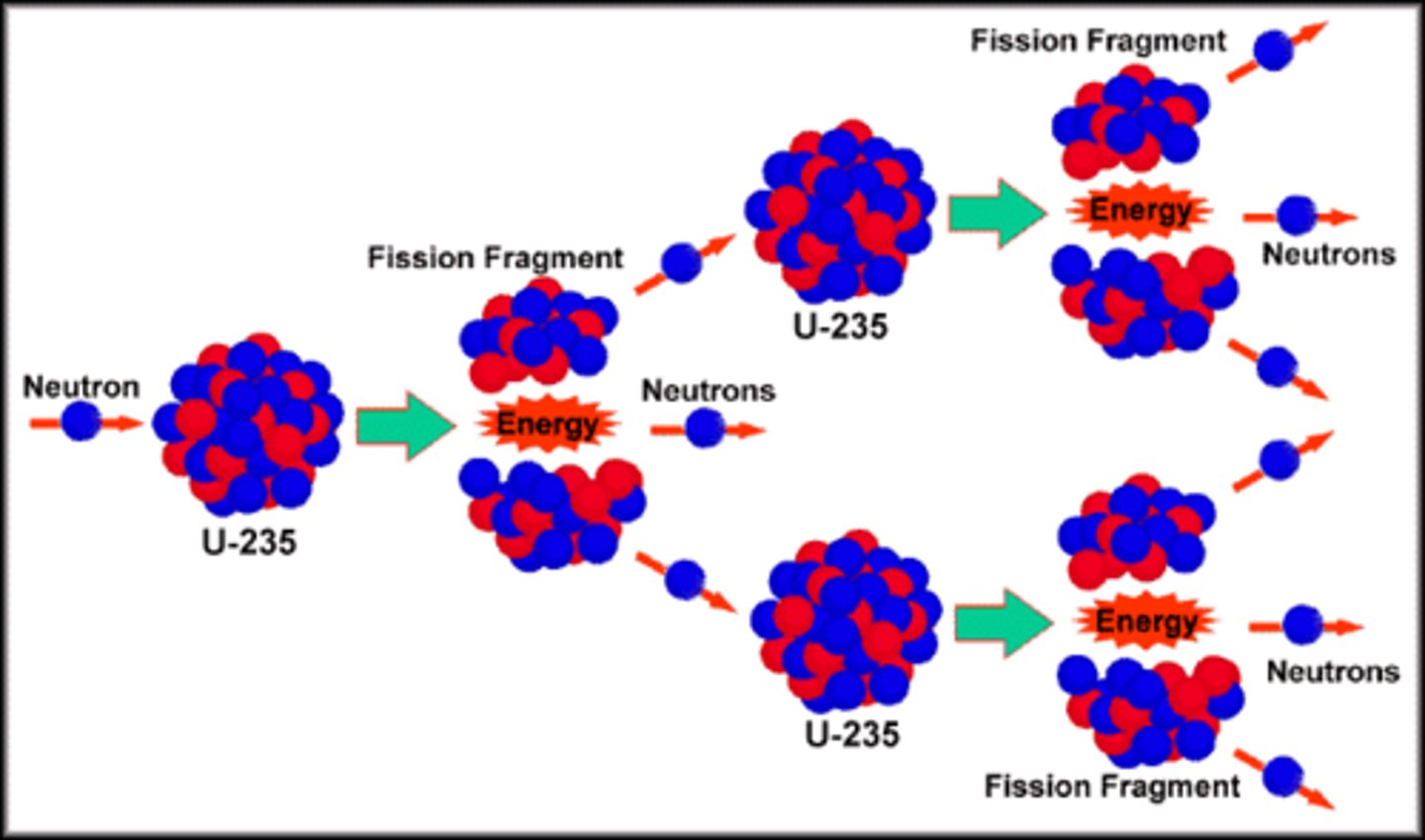
fission
A nuclear reaction in which a massive nucleus splits into smaller nuclei with the simultaneous release of energy
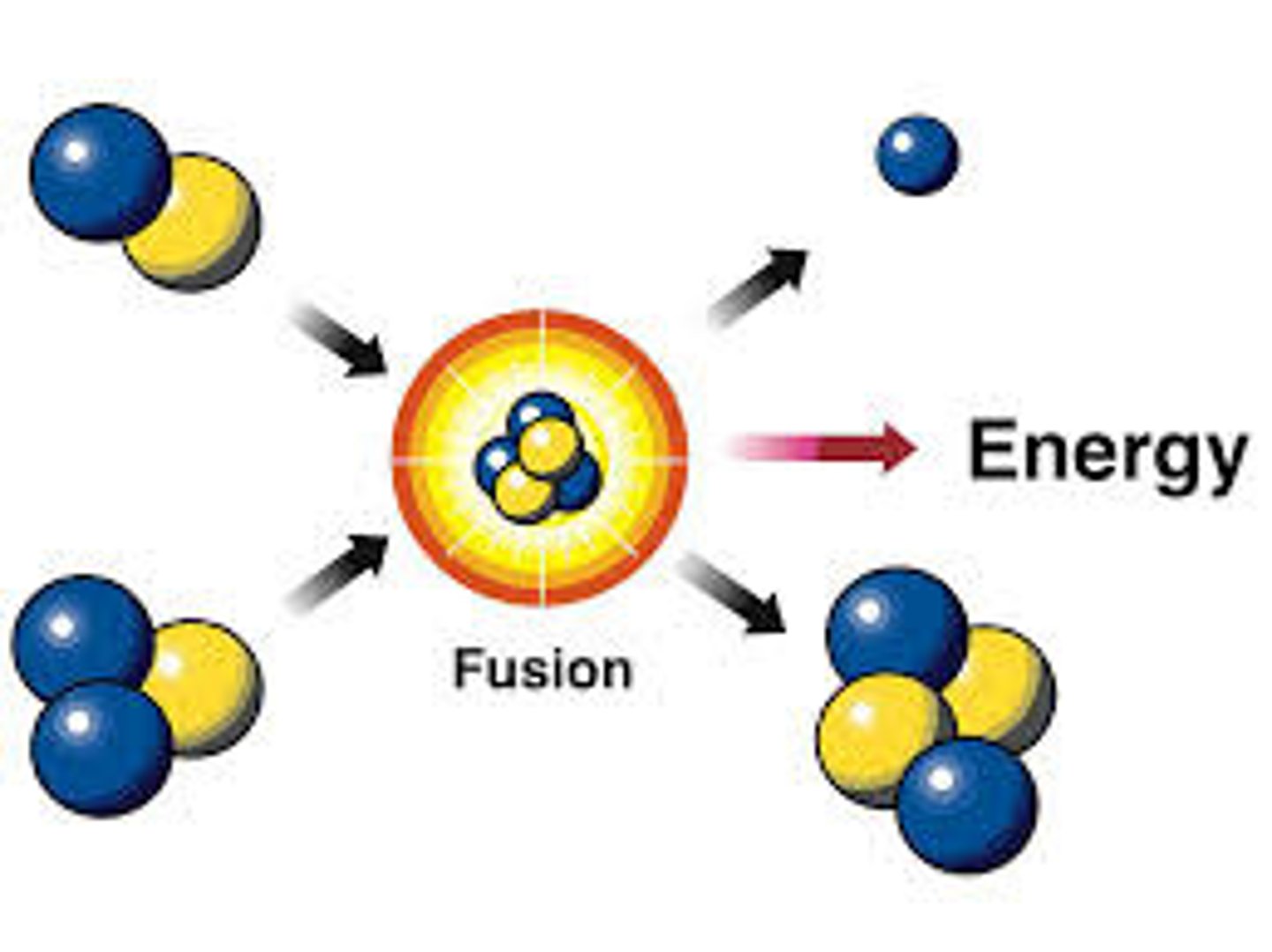
fusion
Creation of energy by joining the nuclei of two hydrogen atoms to form helium.
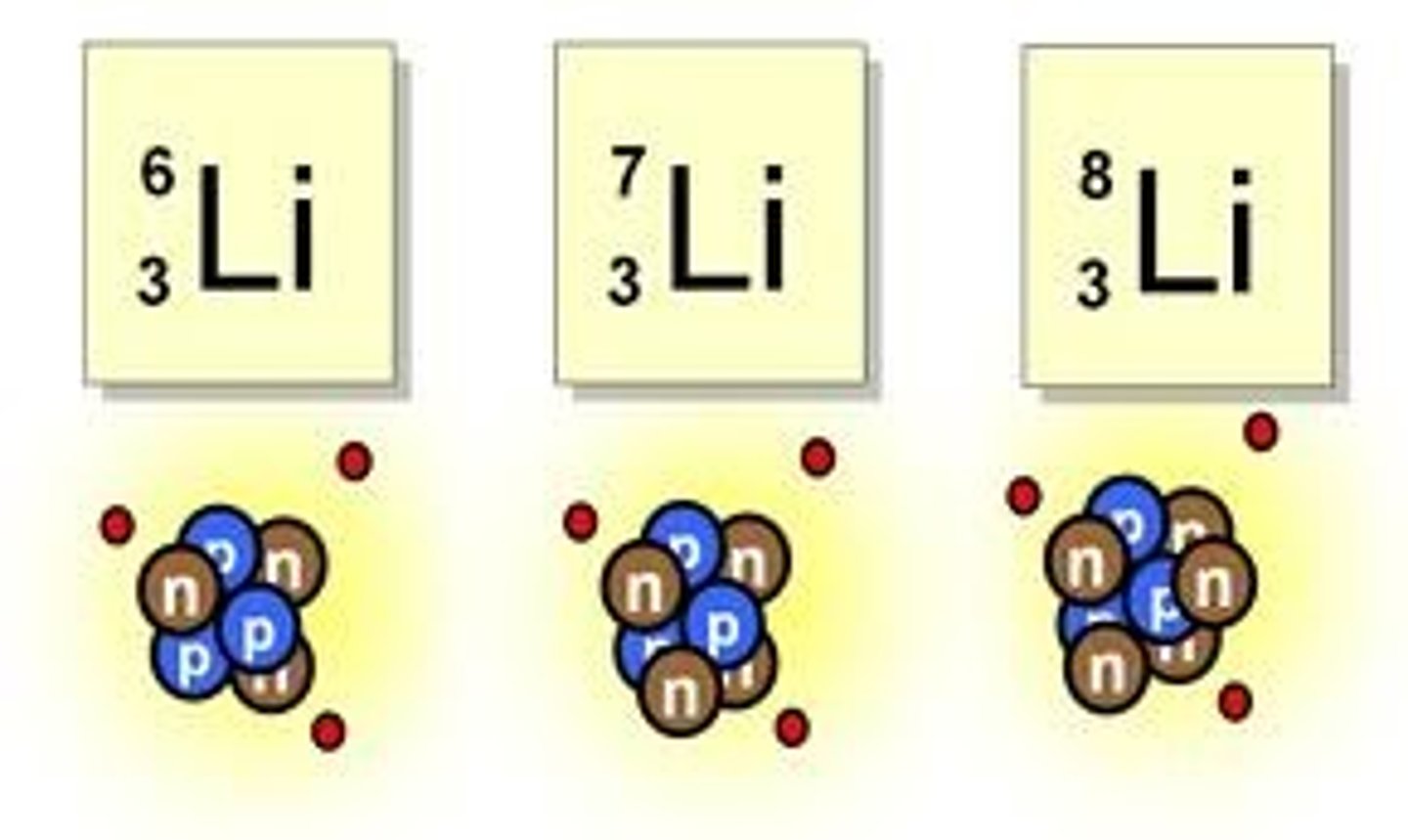
isotope
An atom with the same number of protons and a different number of neutrons from other atoms of the same element.
transmutation
the conversion of an atom of one element to an atom of another element
alpha decay
A nuclear reaction in which an atom emits an alpha particle consisting of two protons and two neutrons. This increases the atomic number by 2 and the mass number by 4.
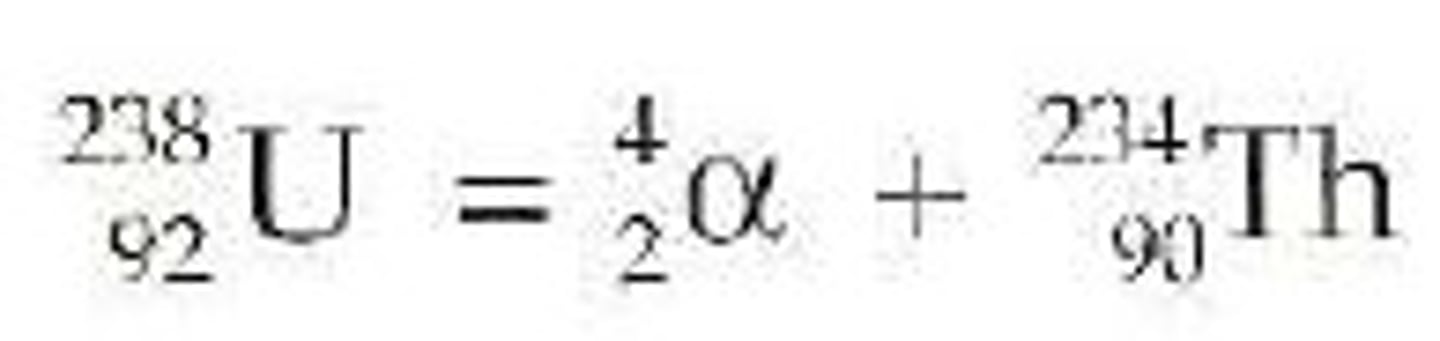
alpha particle
a type of nuclear radiation consisting of two protons and two neutrons (a helium nucleus)
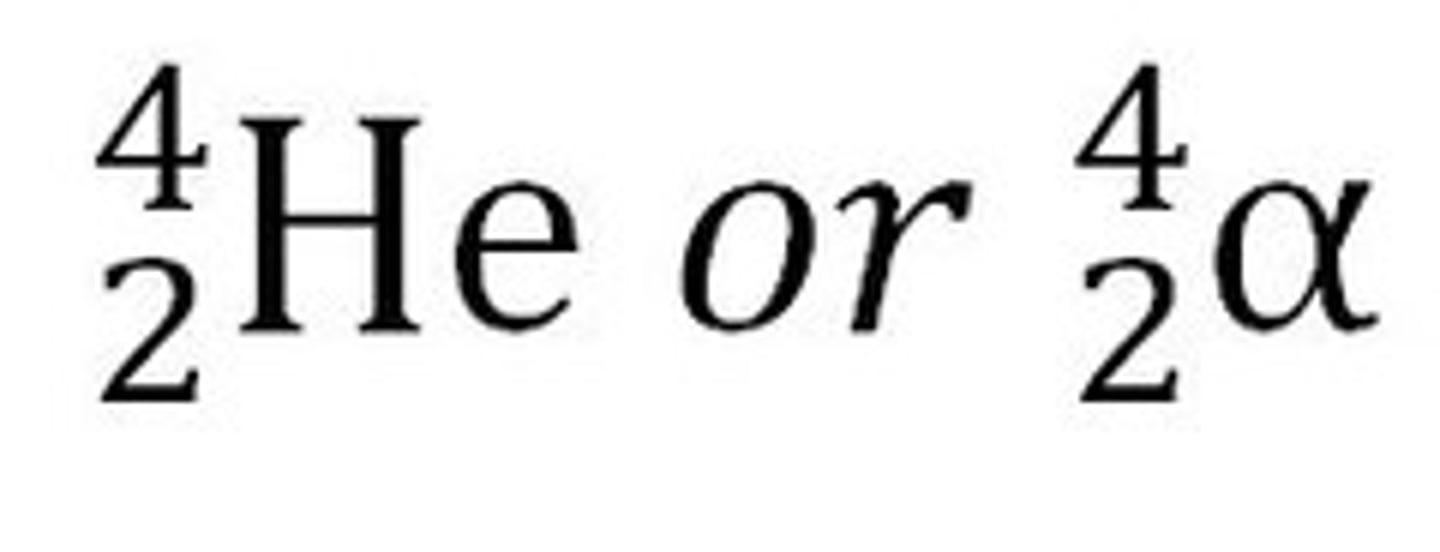
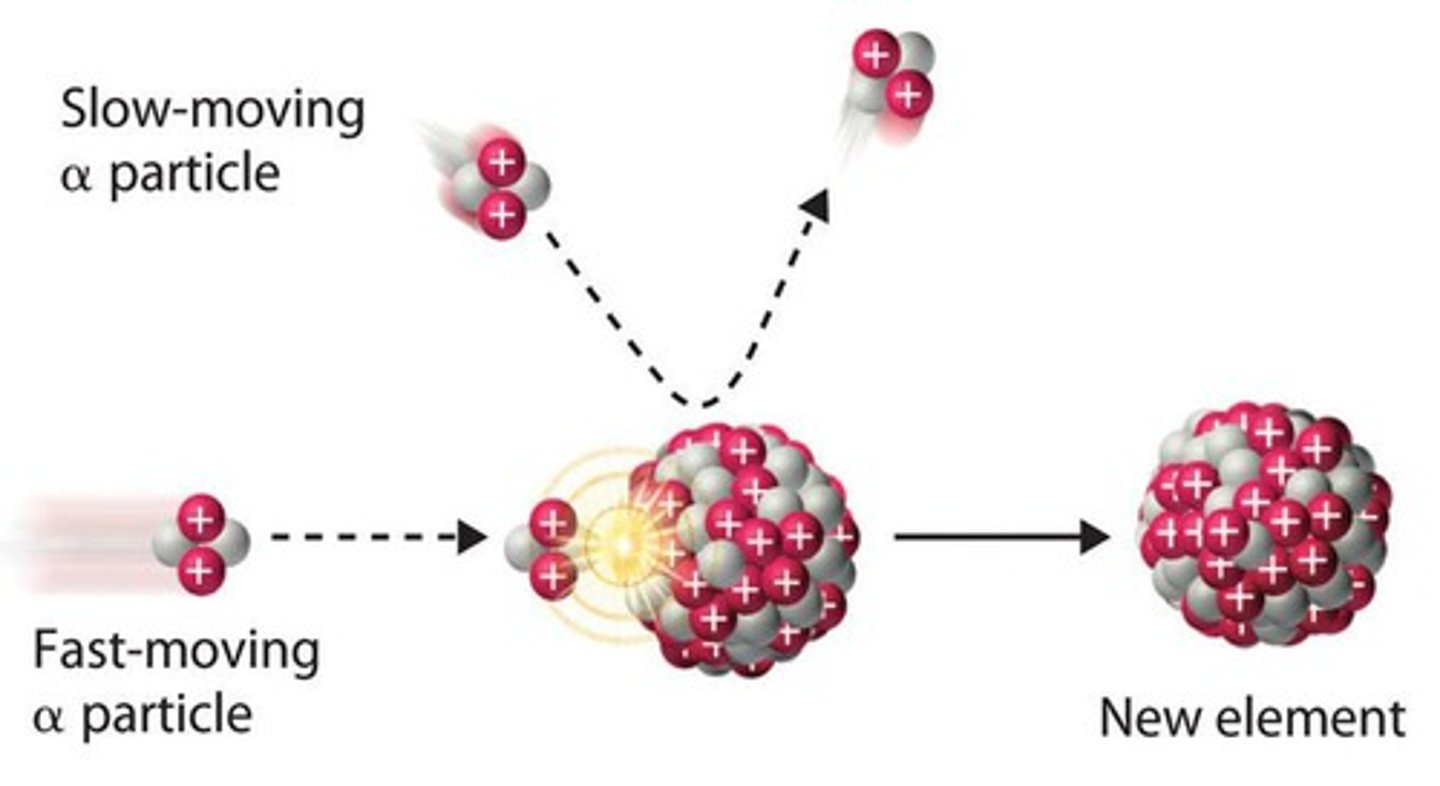
nuclear bombardment
Two nuclear particles collide to make one or more different nuclear particles
beta particle
a high-speed electron with a 1- charge that is emitted during radioactive decay
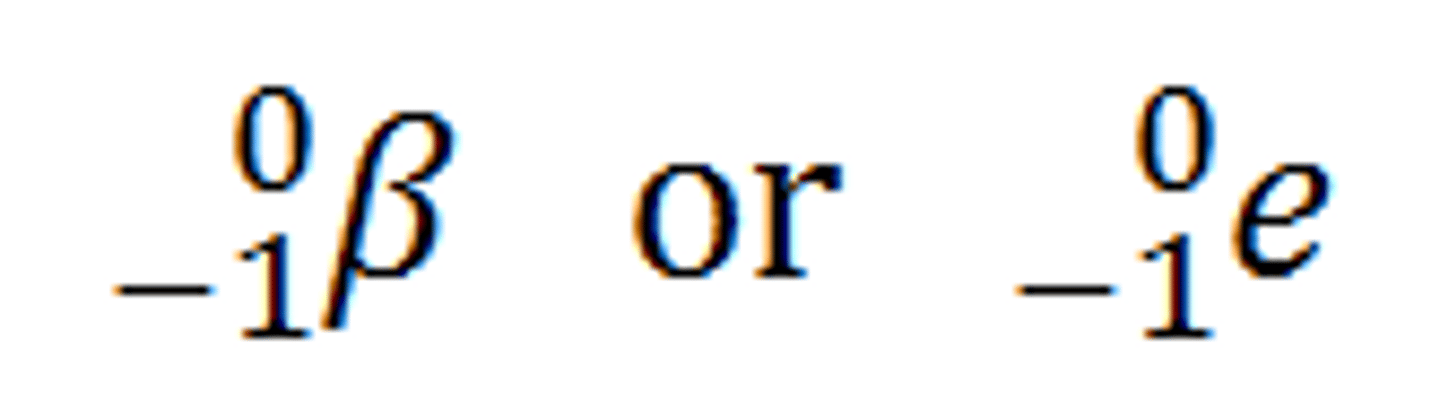
beta decay
Radioactive decay in which an electron is emitted. (A neutron changes into a proton in the nucleus and releases an electron)
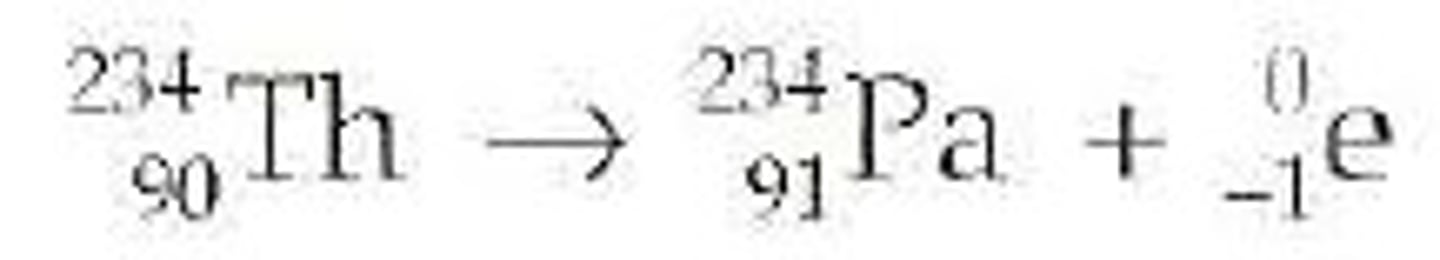
gamma ray
a high-energy photon emitted by a radioisotope
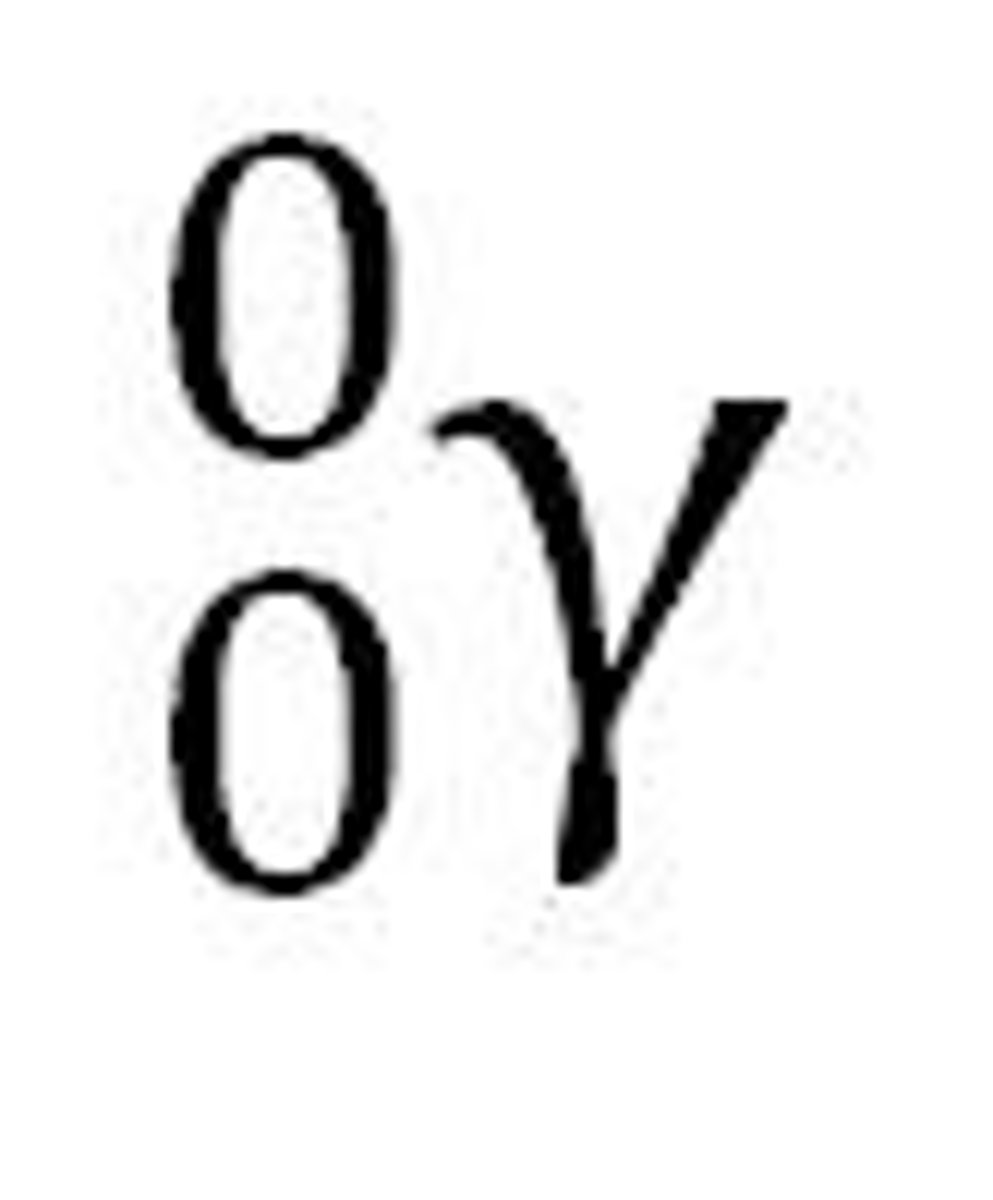
gamma decay
the release of energy as a gamma ray from a nucleus (no gain or loss of particles)
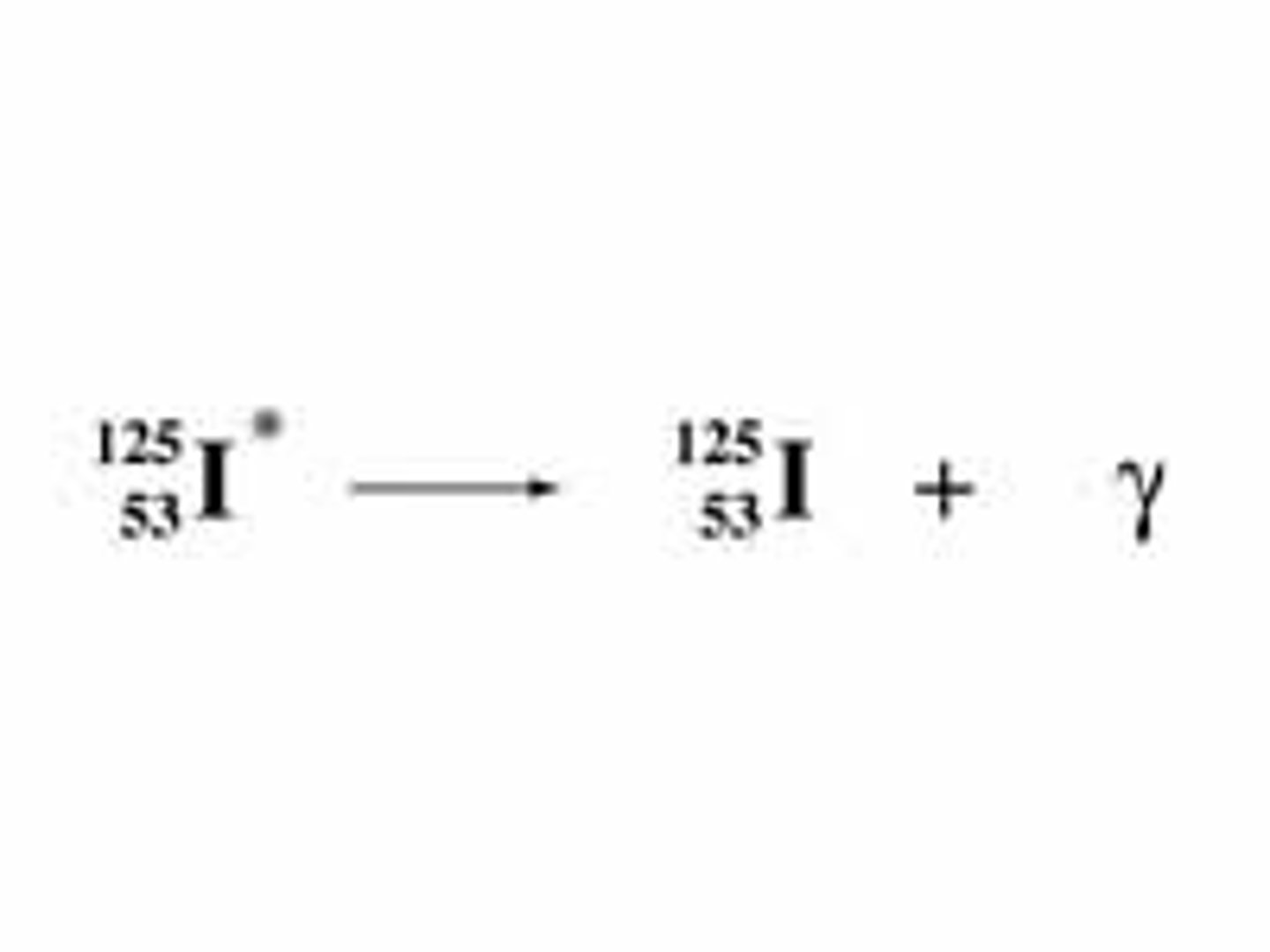
Radioisotopes
radioactive form of an element that gives off energy in the form of radiation; radioisotope
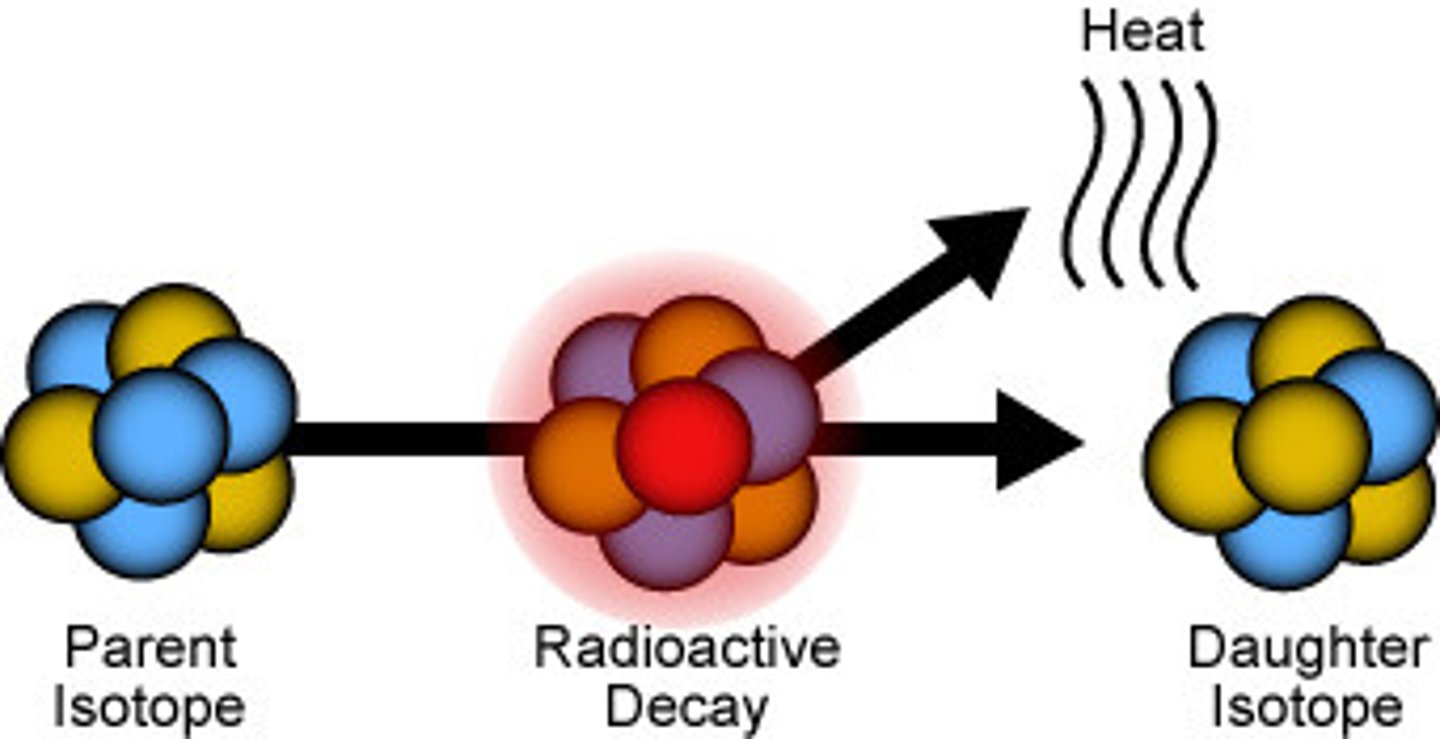
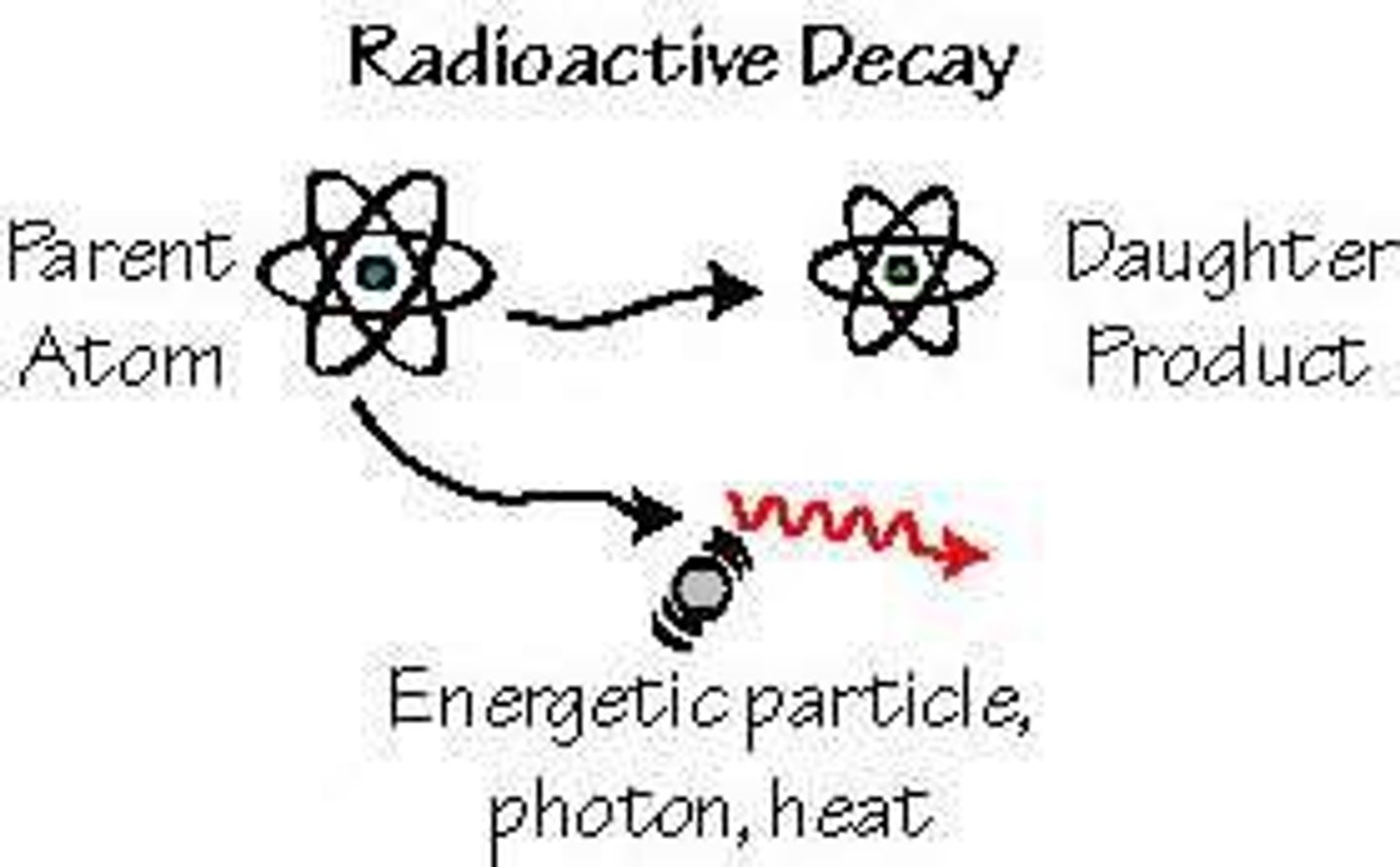
radioactive
A spontaneous process in which unstable nuclei lose energy by emitting radiation
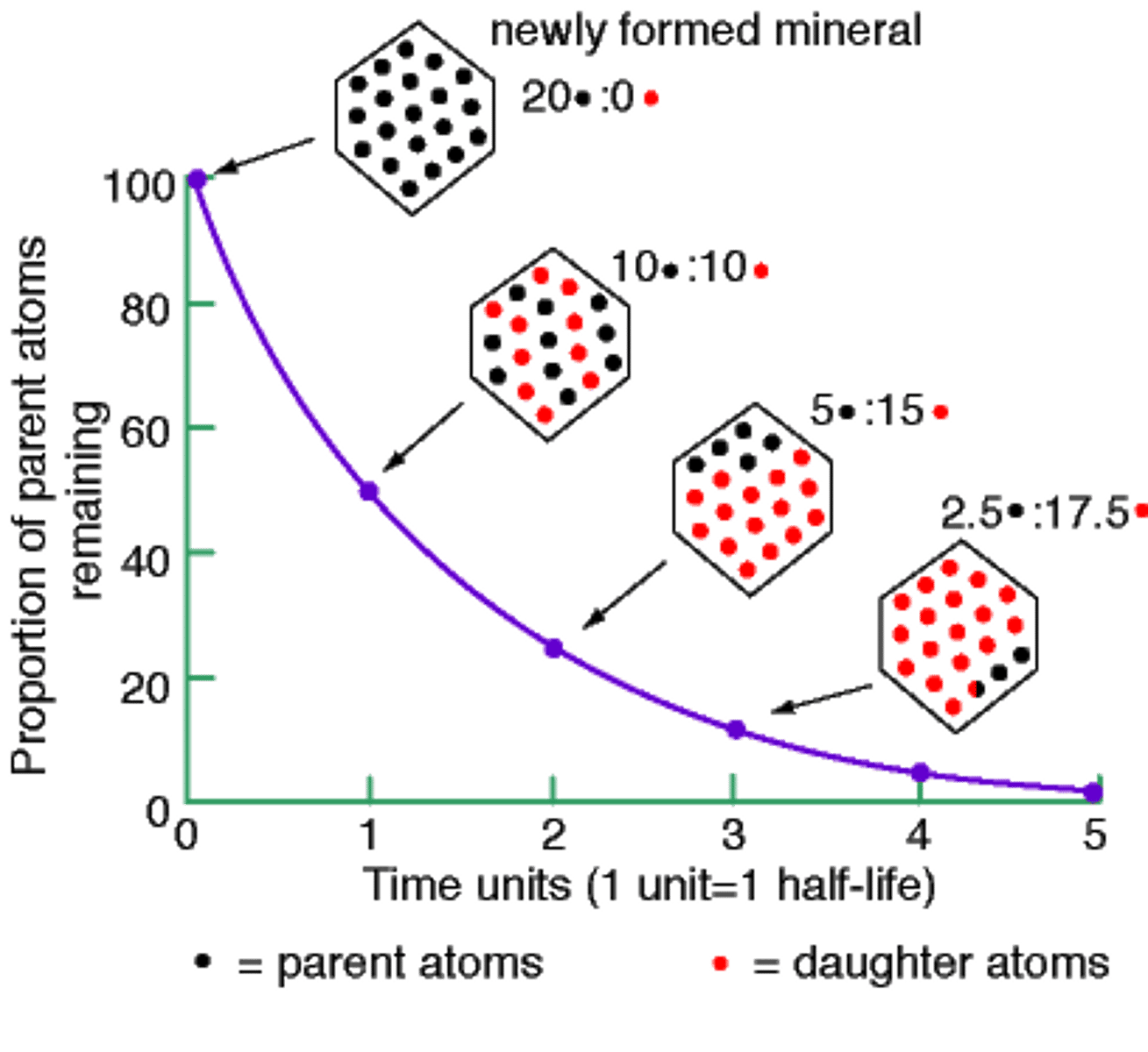
half-life
length of time required for half of the radioactive atoms in a sample to decay
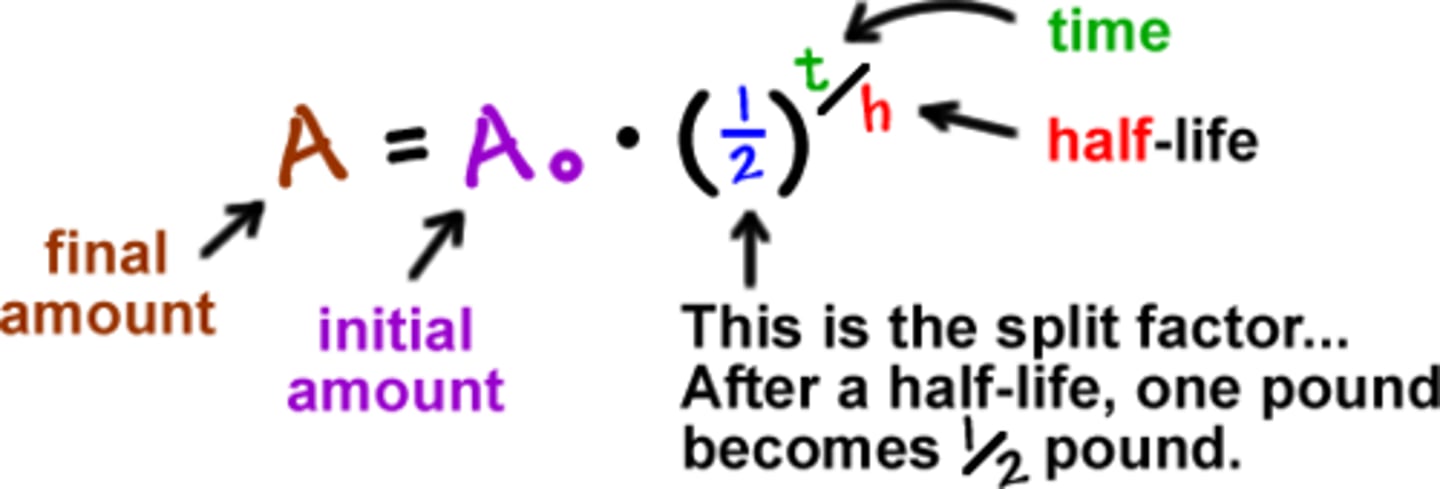
half life equation
proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
electron
A subatomic particle that has a negative charge and is found outside the nucleus of an atom
atomic number
the number of protons in an atom
atomic mass
Number of protons and neutrons
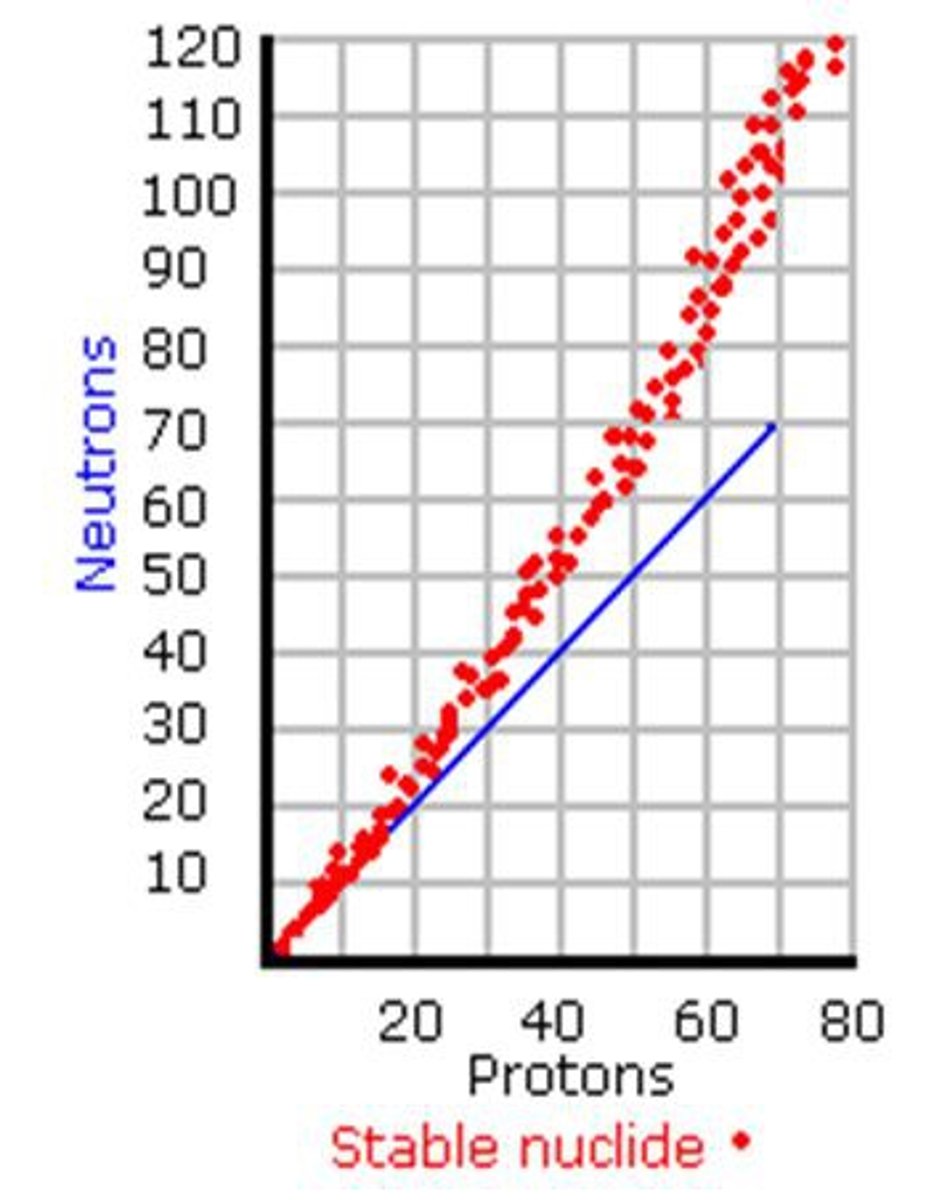
band of stability
the location of stable nuclei on a neutron-vs.-proton plot
isotope notation
The representation of a specific isotope. The atomic number is listed below the symbol, the mass number above.