Lab 4: Solid-Liquid Extraction-Isolation of Trimyristin from Nutmeg
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is solid-liquid extraction?
Extraction of a compound form a solid using a liquid media
(coffee example applies here where you are extracting caffeine from solid coffee grounds with hot liquid=the media)
What does solid liquid extraction depend on?
A compound’s solubility in the particular solvent (aka there must be a difference in solubility between the desired compound the rest of the components)
What is liquid-liquid extraction?
Separation of compounds based on their relative solubilities in two different immiscible liquids (ex: aqueous solvent with an organic layer or two organic layers differing in density). This will be seen in a later lab (focus on solid-liquid extraction here)
What is a reflux?
A process of boiling reactants while also cooling and condensing the vapor, returning back to the flask to boil again. It is beneficial when the boiling point of the solvent is less than the heat needed for the reaction and you can heat mixtures for an extended period of time at high temperatures to extract compounds or carry out a chemical reaction (without boiling off all of your solvent, hence why it is helpful here)
When does reflux start?
When the solution is boiling (dont forget boiling chips)
Where does the water tubing go for a reflux?
At the bottom of the condenser-in
At the top of the condenser-out

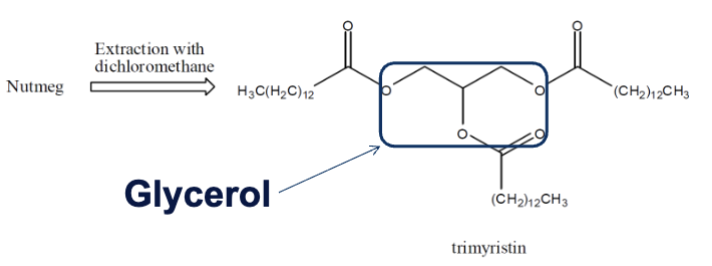
What are we trying to extract?
Trimyristin from nutmeg
Why are we using recrystallization again?
So we can isolate and obtain our trimyristin from our solvent post distillation
What is extraction?
A physical/chemical process of separating a single part from a complex mixture
ex: extracting caffeine from coffee beans or coffee powder
What is nutmeg and how much trimyristin does it contain?
A spice and contains 20-25% trimyristin by weight
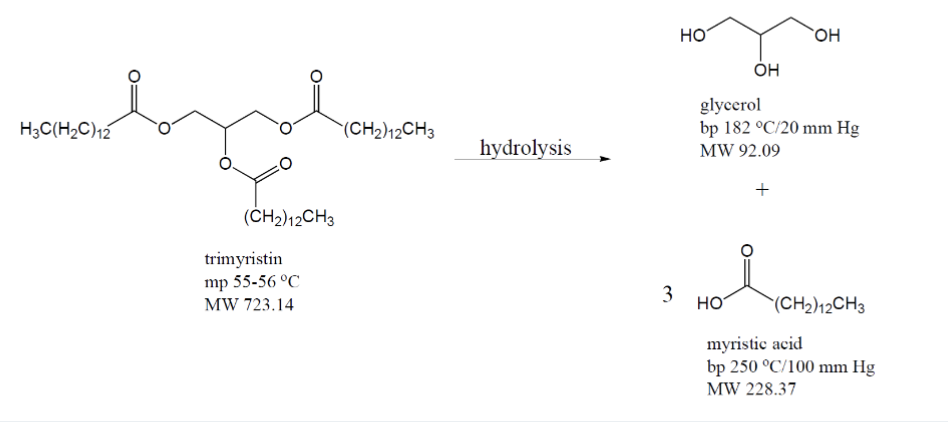
What is trimyristin?
A triglyceride of myristic acid (14-carbon fatty acid), and you can get this via extraction of nutmeg using organic solvent
True or false: trimyristin can be saponified?
True
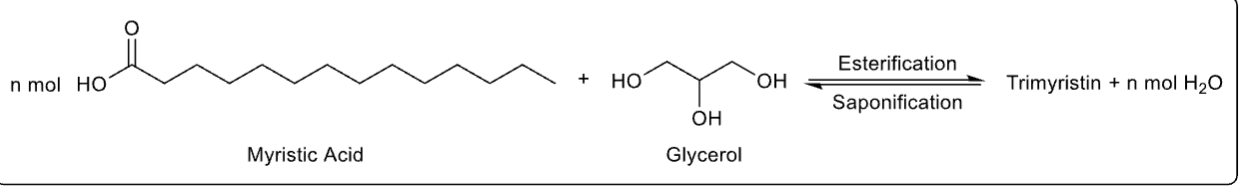
What is the chemical equation for extracting nutmeg?
see photo
Techniques used in this lab?
Extraction (solid/liquid), reflux, simple distillation, gravity + vacuum/suction filtration, and melting point determination
Components involved?
Acetone, methylene, and trimyristin
Why do we reflux?
this is where the extraction takes place
General procedure: solid/liquid extraction via reflux
get your nutmeg and put it in a round bottom flask (with boiling chips)
Add your solvent (diethyl ether?)
Reflux for 30 mins (timer begins when boiling starts)
Let apparatus cool
Hot filter the solution to a second round bottom flask (removes big nutmeg particles)
General procedure: simple distillation and filtration
Add solvent to round bottom flask
Distill (simple) the solvent
Product is in the distillaiton flask while your solvent was boiled off because they prevent the crystals from forming/they melt or denature the crystals (you will boil a lot off)
Let round bottom flask cook in the ice bath
Recrystallization occurs
Add acetone to wash the product/soluble impurities from crstyals
Suction filtration: get crystals (pale yellow powder)
wash again with acetone
Let run through vacuum filtration for 5-6 minutes
Math involved
Percent recovery
Why does the melting point differ from literature melting point?
Impurities—broaden and deepen melting point range (inhbit bonds for crystal lattice, so less energy is required to break IMFs)
What would happen if you did this process without using a reflux condenser?
During solid-liquid extraction, heat helps extract trimyristin from the solid nutmeg. The liquid does contain the desired product, so if you boil it during distillation without a refluc, we would lose our ethyl acetate (with the trimyristin)