4-Parathyroid Hormone and Mineral Metabolism
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
94 Terms
Bone Tissue Composition
30% Organic
Cells 2%
Osteoblasts (form bone)
Osteocytes (encased within bone, signaling)
Osteoclasts (macrophages, bone reabsorption)
Matrix 98%
Collagen Type I
Proteins
70% Minerals
Ca2+
Phosphate
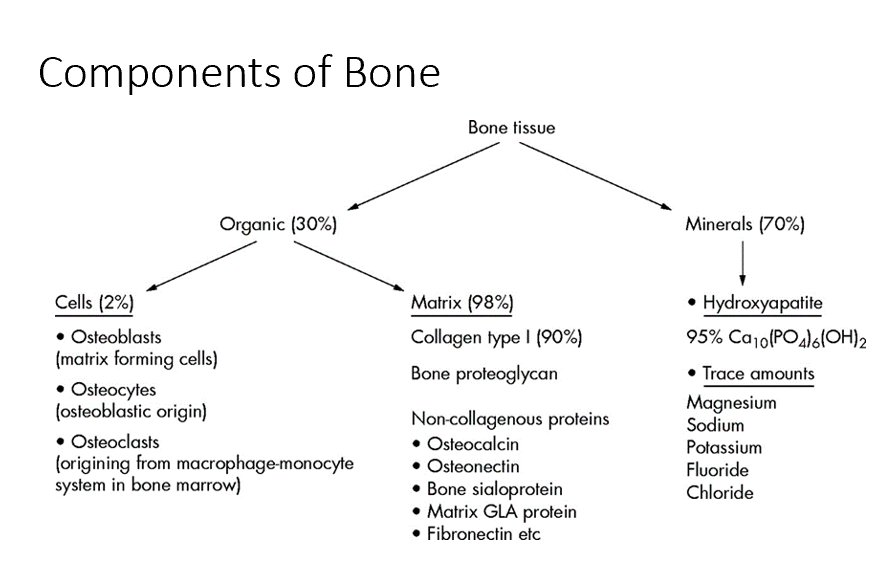
Type I Collagen
Crosslinks with itself to create a regularly reoccurring pattern that we see in bone, giving bone its strength.
Disrupting this pattern weakens bone.
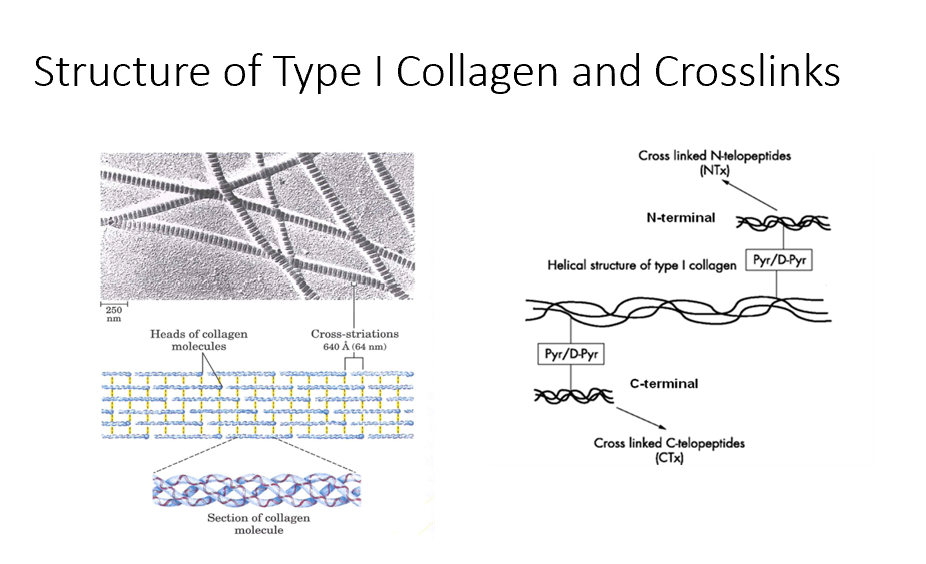
Bone Degradation
This is when the type I collagen crosslinks can be released.
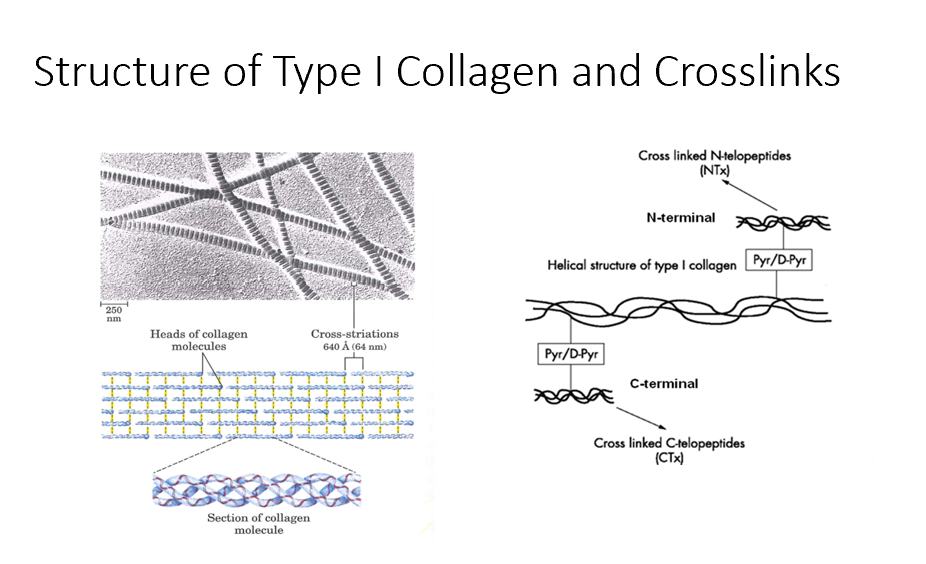
Bone Turnover Rate
Take an assay of N-telopeptides and C-telopeptides to get an idea of THIS.
Osteoblasts
Bone-forming cells that contain receptors for parathyroid hormone (PTH) and initiate the bone remodeling process.
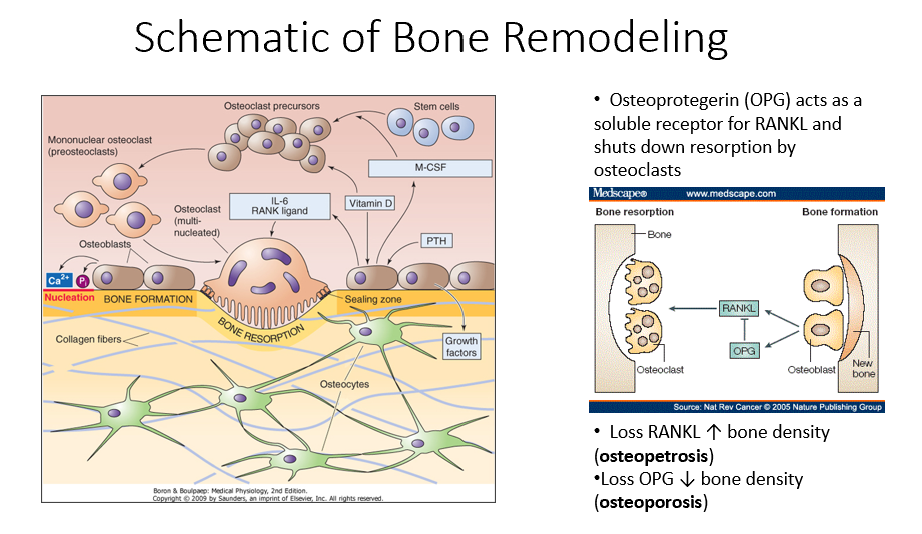
PTH
A hormone that signals osteoblasts to start the bone remodeling process.
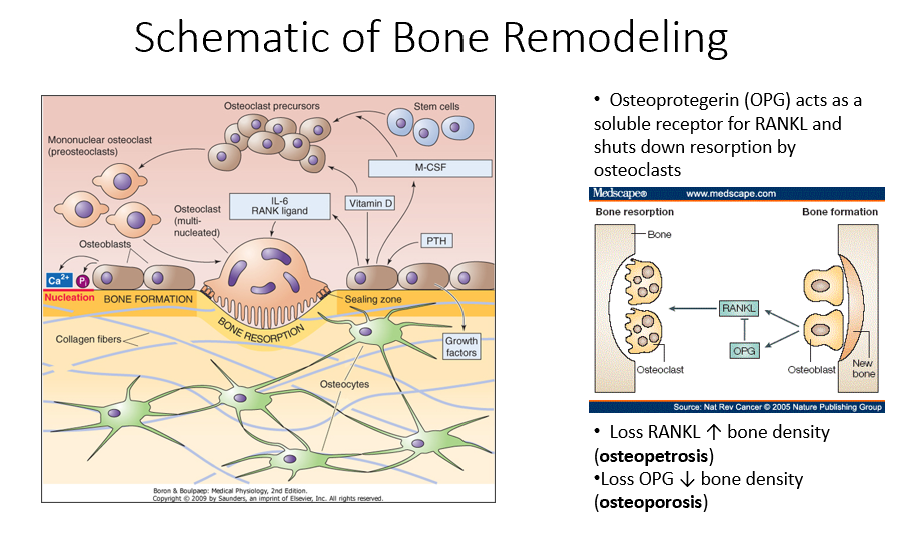
M-CSF (Monocyte Colony-Stimulating Factor)
A factor released by osteoblasts that helps recruit osteoclast precursors (STEM cells).
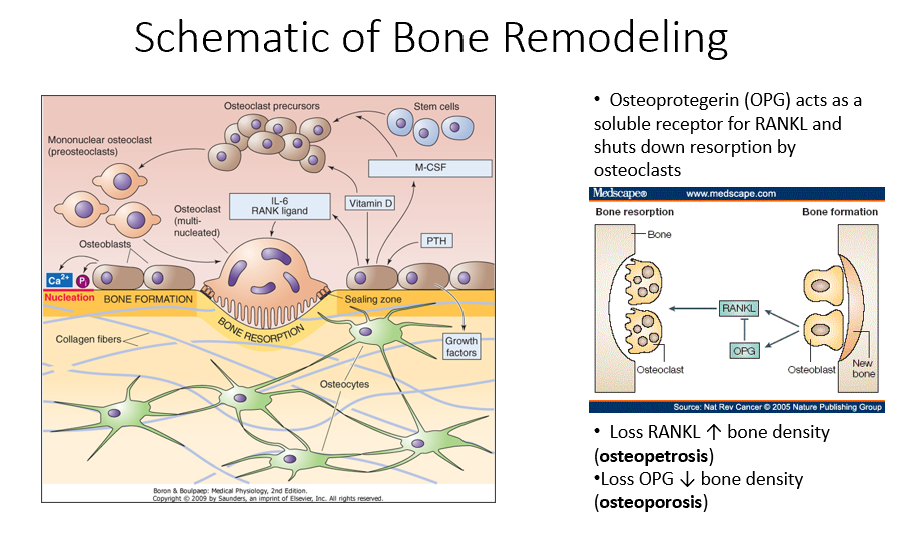
Vitamin D in Bone Remodeling
Works alongside M-CSF to promote osteoclast precursor differentiation.
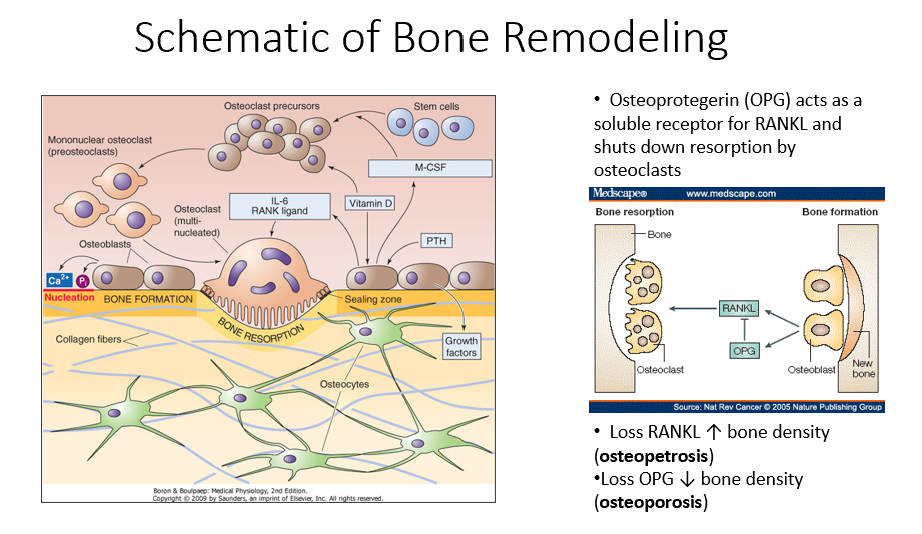
Osteoclast Precursors
Immature bone-resorbing cells that develop from hematopoietic stem cells.
Hematopoietic Stem Cells
The type of stem cells that differentiate into osteoclast precursors (not mesenchymal stem cells).
Multinucleated Osteoclasts
Fully matured osteoclasts with a ruffled border, responsible for bone resorption.
IL-6 (Interleukin-6)
A signaling molecule that helps activate multinucleated osteoclasts.
RANK Ligand
A key signaling factor that promotes osteoclast differentiation and activation.
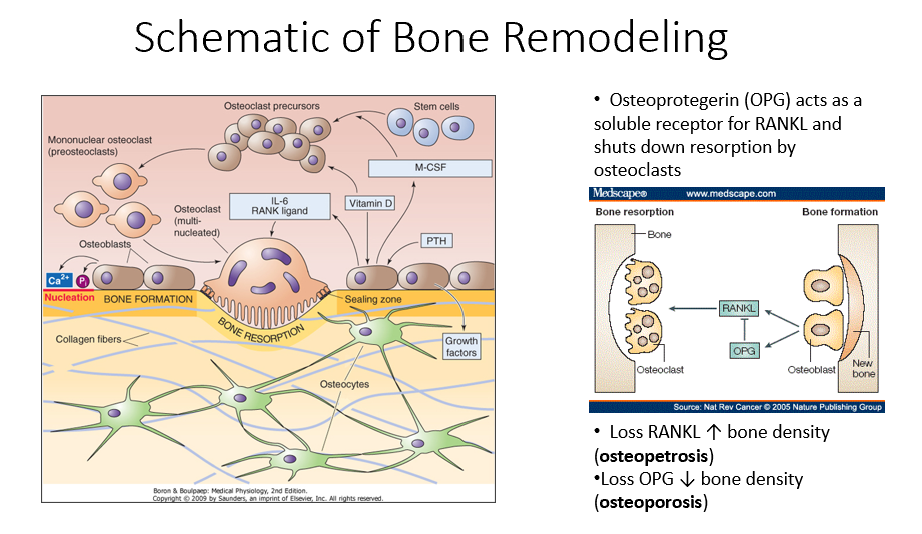
Bone Resorption
The process by which osteoclasts break down bone, releasing minerals and collagen fragments.
Enzymes Released by Osteoclasts
Chemical mediators that break down bone matrix and dissolve minerals.
Osteoblasts’ Role in Bone Remodeling
Follow osteoclasts and lay down new organic matrix, which later mineralizes into new bone.
Bone Matrix
The organic structure deposited by osteoblasts, which becomes mineralized to form new bone.
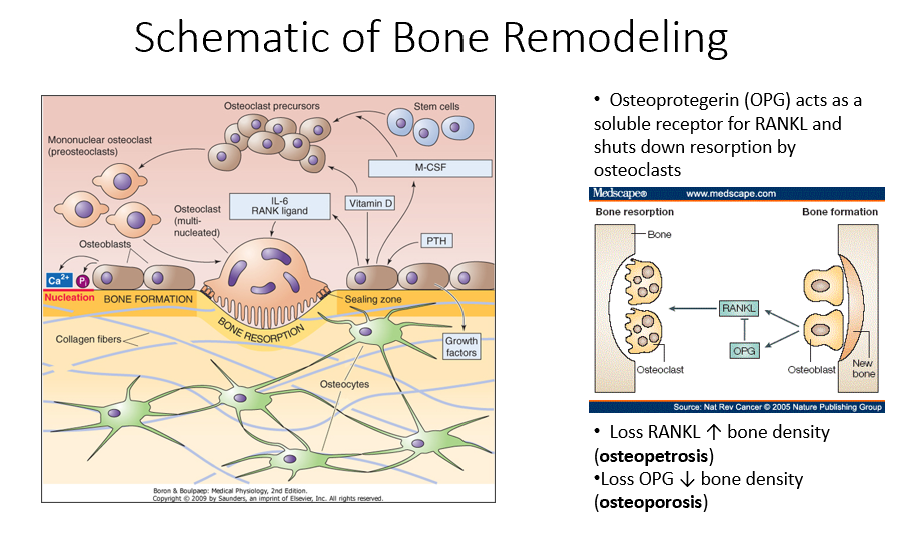
Osteocytes
Mature bone cells embedded in bone that monitor the environment and regulate bone formation and resorption.
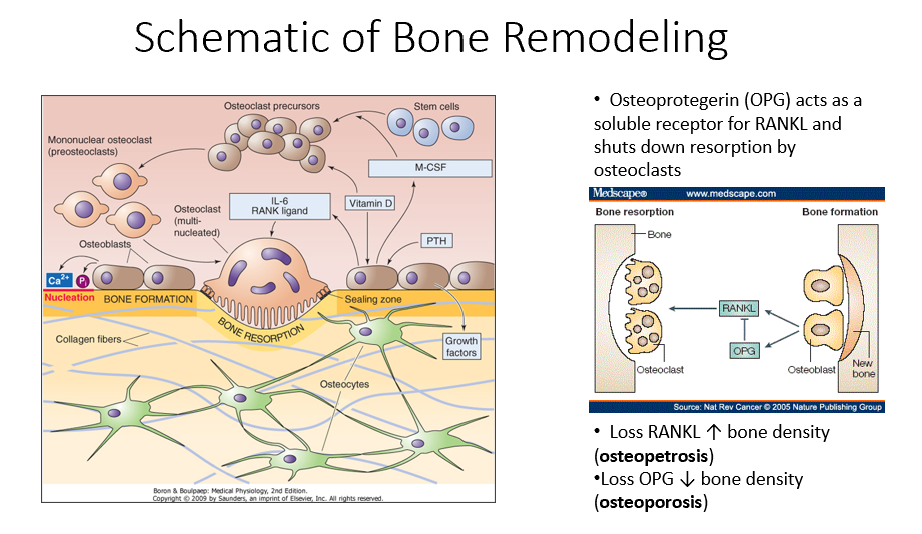
Bone Remodeling Process Steps
PTH signals osteoblasts to release M-CSF and vitamin D.
M-CSF and vitamin D recruit hematopoietic stem cells to become osteoclast precursors.
Osteoclast precursors mature into multinucleated osteoclasts with a ruffled border.
Activated osteoclasts break down bone, releasing minerals and collagen fragments.
Osteoblasts follow osteoclasts, laying down a new organic matrix that will mineralize into bone.
Osteocytes, embedded within the bone, monitor the environment and regulate both bone formation and resorption.
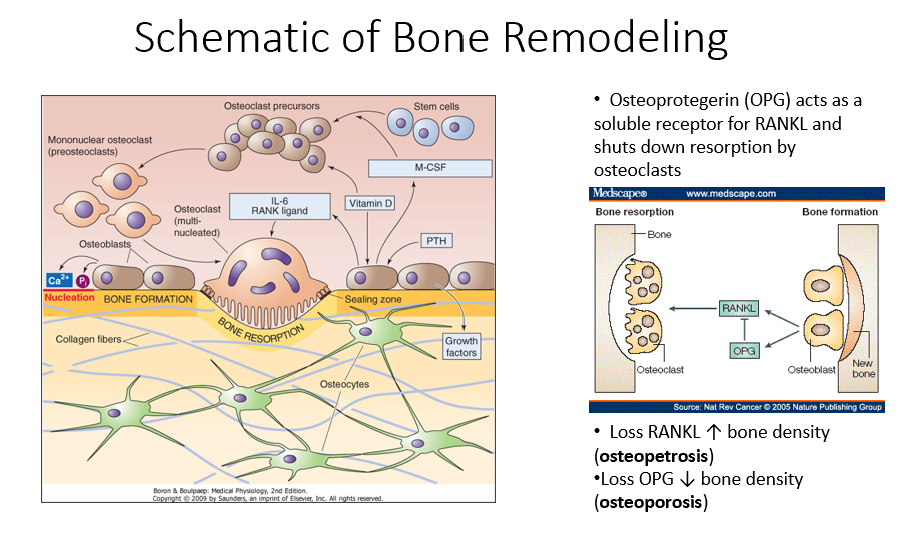
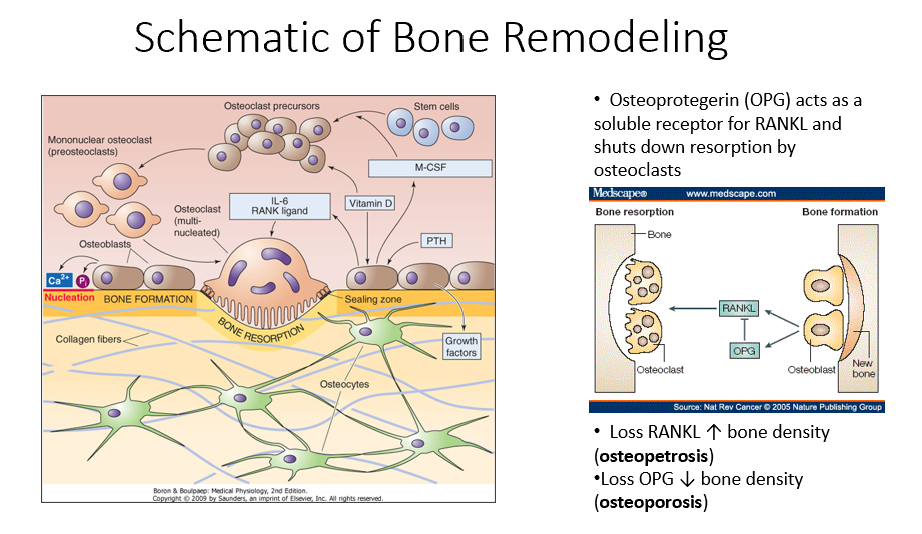
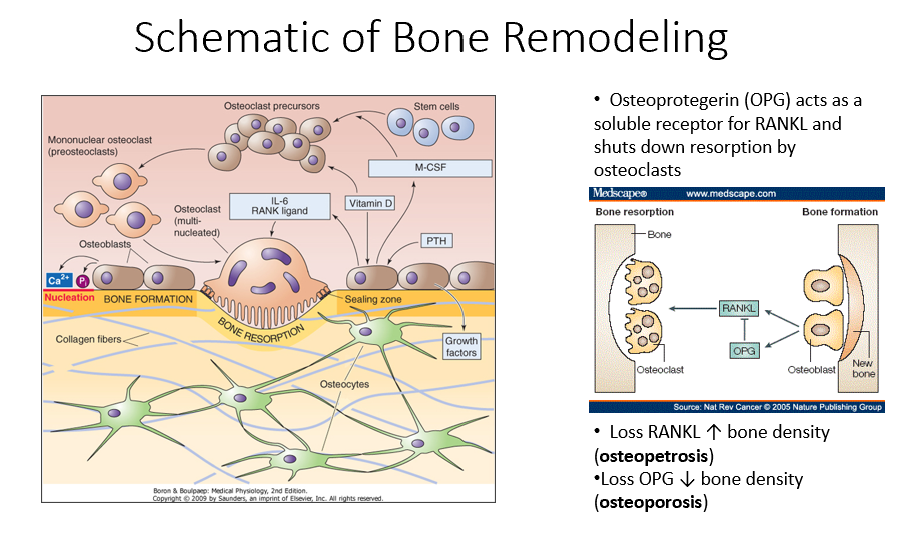
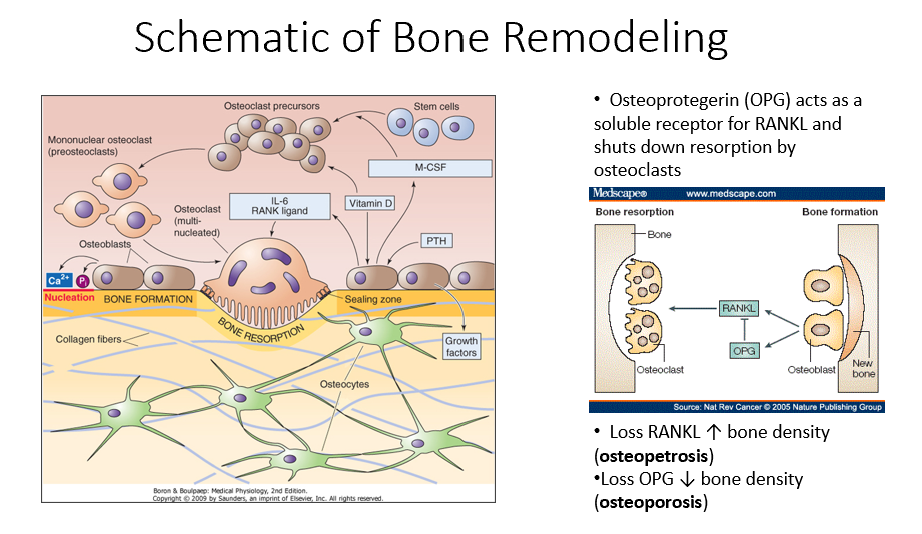
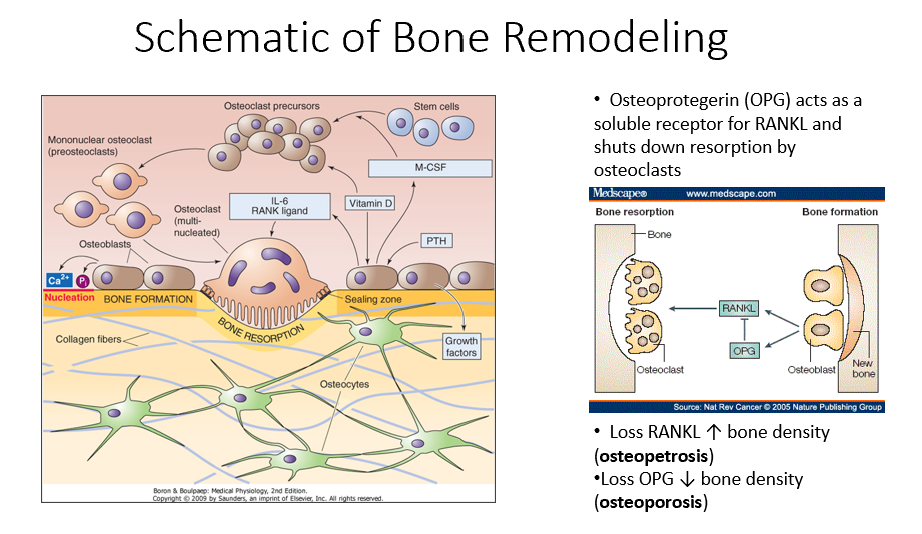
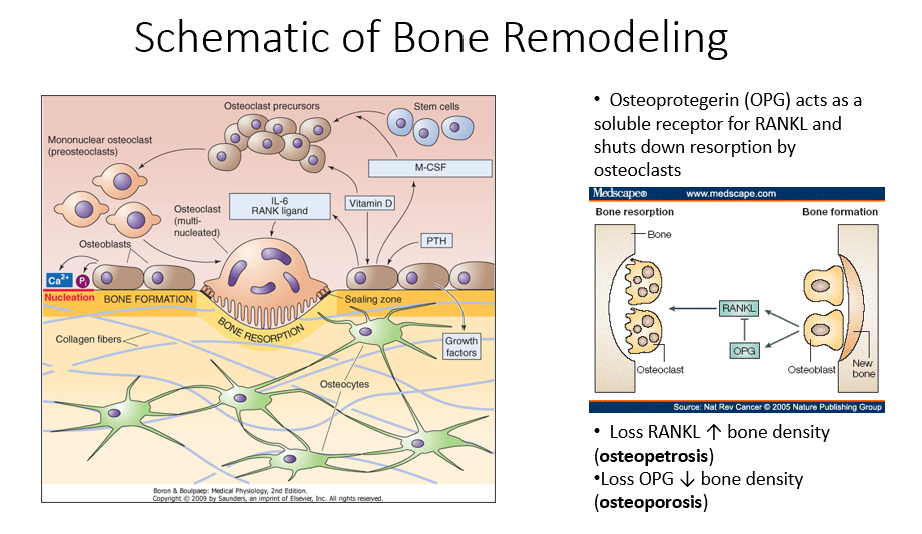
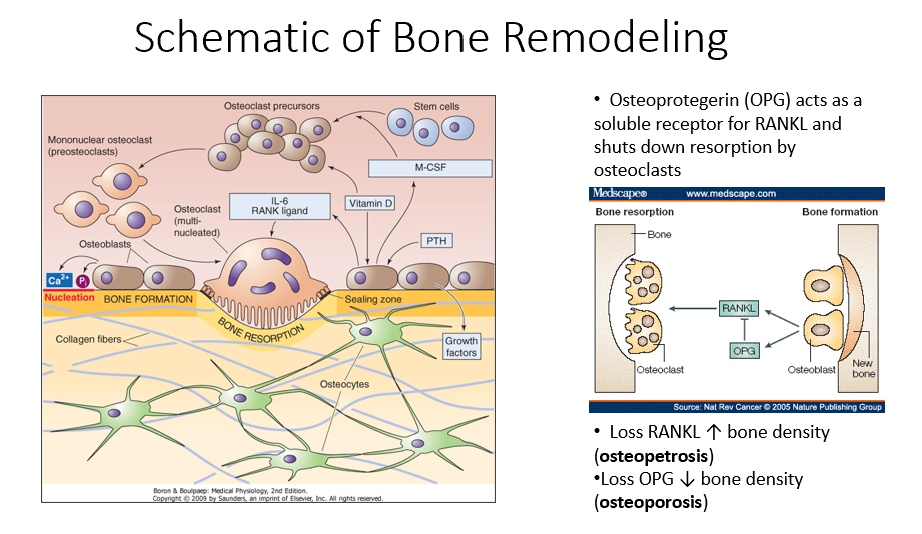
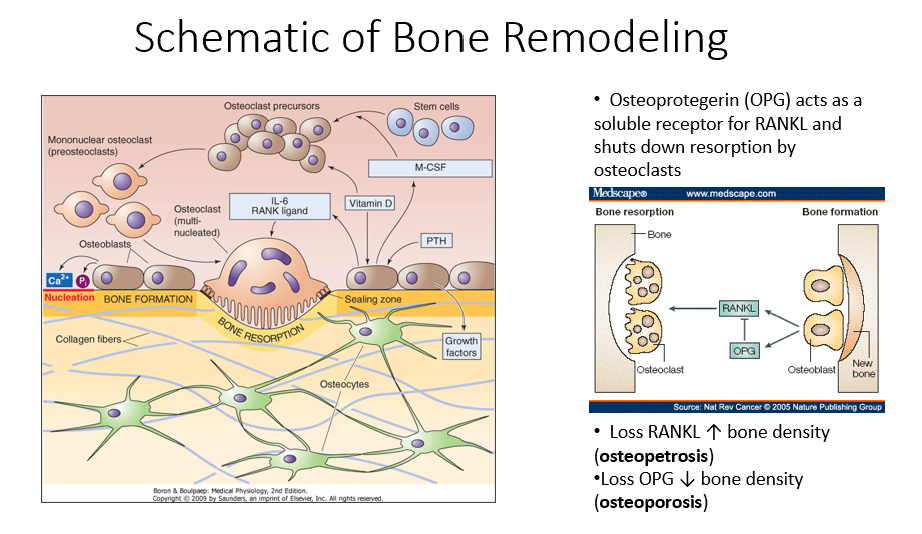
Osteoprotegerin (OPG)
Released by osteoblasts
Acts as a soluble receptor for RANKL
Shuts down resorption by osteoclasts in bone-forming process
No RANKL → ↑ bone density (osteopetrosis: no activity of osteoclasts)
No OPG → ↓ bone density (osteoporosis: RANKL is always around/on)
Hormones Regulating Mineral Metabolism (what are they? what do they act on?)
Parathyroid hormone (PTH), vitamin D, calcitonin, and fibroblast growth factor (FGF23)
Regulates levels of calcium and phosphate in the blood.
They act on cells in the intestine, kidney, bone, and parathyroid gland
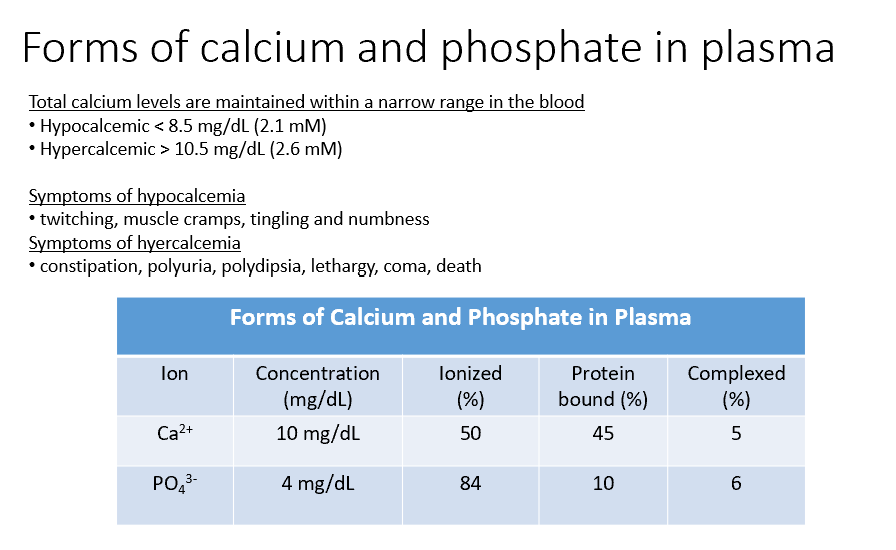
Ca and Phosphate
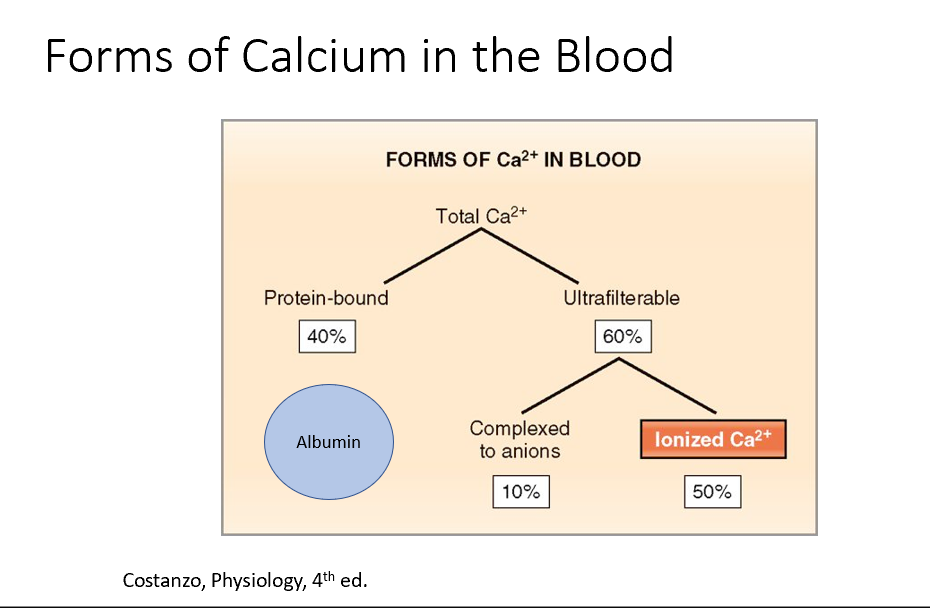
Both THESE have concentrations within the blood, but some of that is Ionized (50%, 84%), Protein Bound (45%, 10%), and Complexed (5%, 6%).
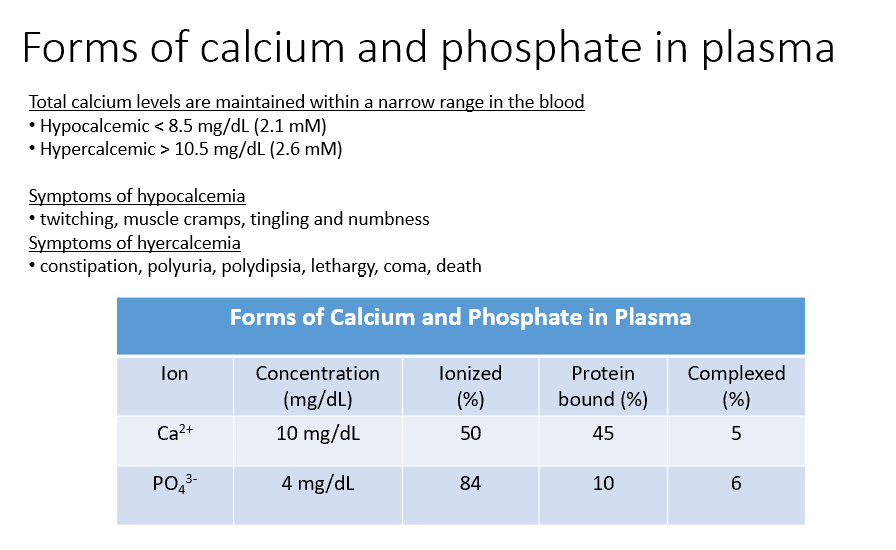
Hypocalcemic
< 8.5 mg/dL (2.1 mM)
Sx:
twitching
muscle cramps
tingling
numbness
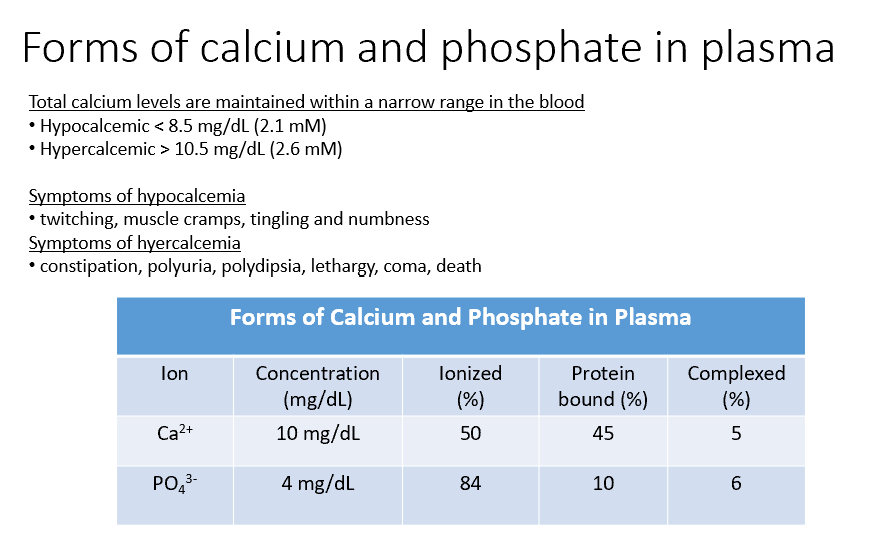
Hypercalcemic
> 10.5 mg/dL (2.6 mM)
Sx:
constipation
polyuria
polydipsia
lethargy
coma
death
Calcium and Parathyroid Hormone Connection
Calcium and parathyroid hormone (PTH)
tightly connected
regulate each other
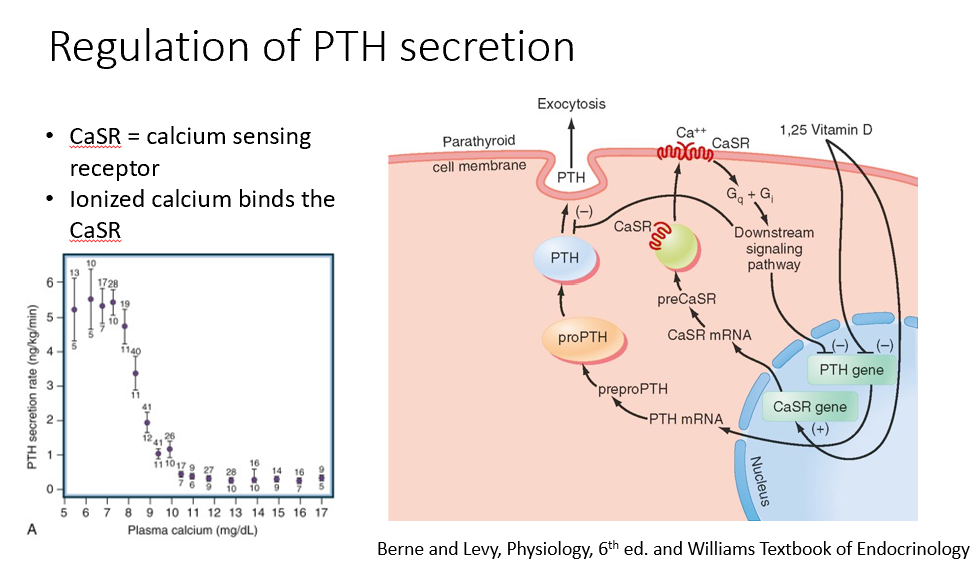
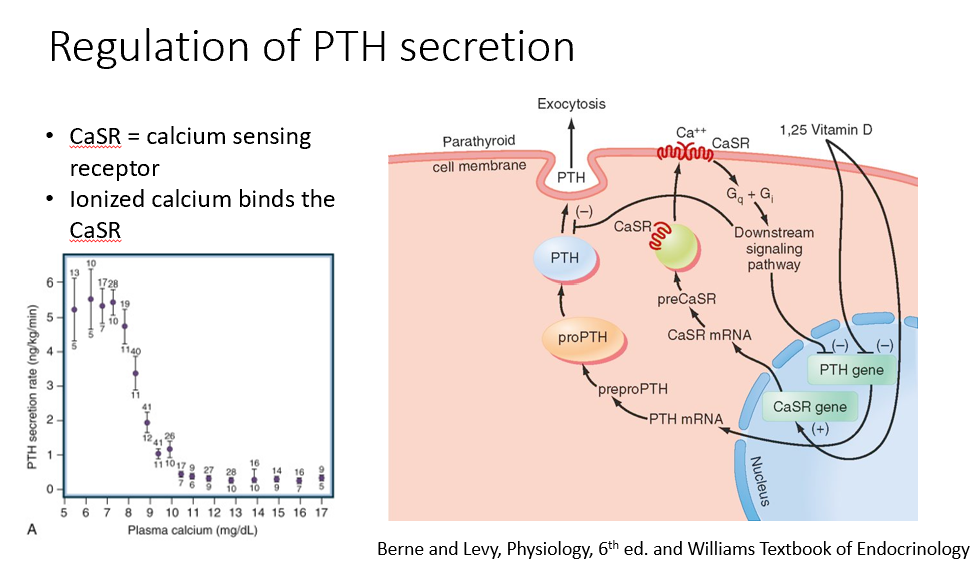
Steps in the Regulation of Parathyroid Hormone Expression
Calcium binds to the calcium-sensing receptor, suppressing PTH expression (inhibition).
Vitamin D (active form) also suppresses PTH expression (inhibition).
When calcium levels drop below 9, PTH expression increases.
Calcium dissociates from the receptor, removing the inhibition.
PTH mRNA is expressed, leading to the formation of the preprohormone→prohormone, which is cleaved into its active form and released.
Only ionized calcium binds to the calcium-sensing receptor, regulating PTH expression.
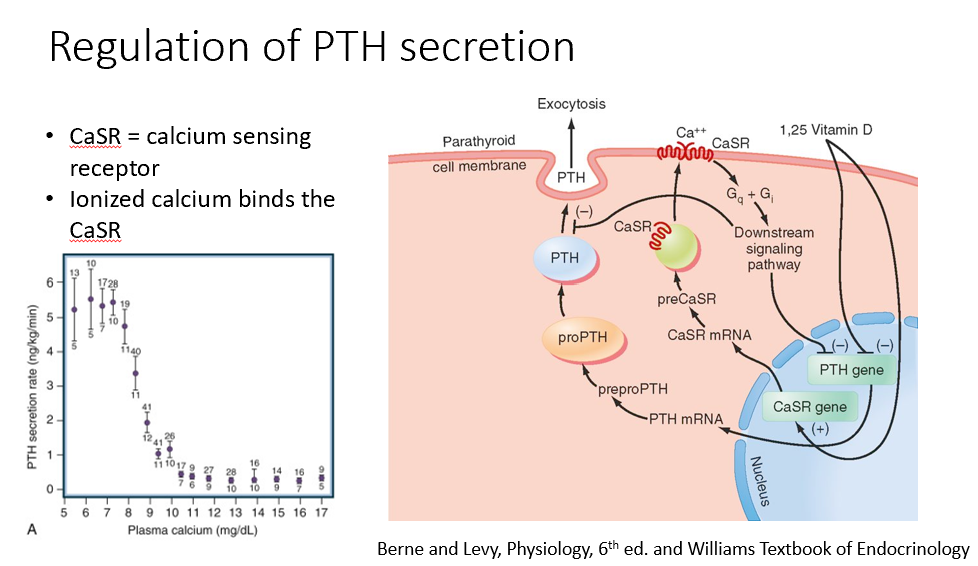
CaSR
Calcium-Sensing Receptor
Ionized Ca binds this!
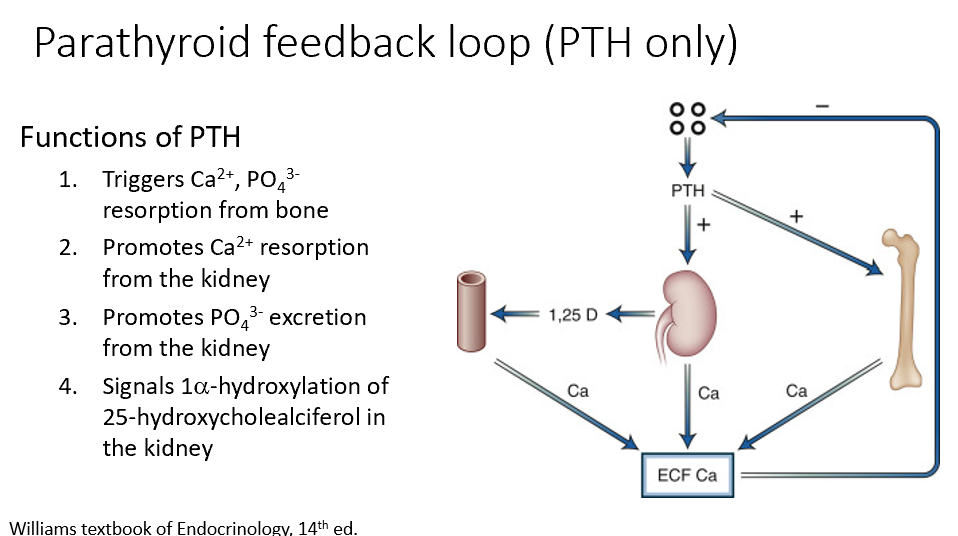
Key Functions of PTH
Triggers Ca2+, PO43- resorption from bone (increases Ca levels)
Promotes Ca2+ resorption from the kidney (increases Ca levels)
Promotes PO43- excretion from the kidney (minimizing calcification risk)
Signals 1alpha-hydroxylation of 25-hydroxycholealciferol in the kidney (increases Ca levels)
PTHrP (parathyroid hormone-related protein)
Can be made by many tissues
Has PTH-like actions
Associated with humoral hypercalcemia of malignancy
Locally expressed by tumors/lesions in the bone
Acts as calciotropic hormone during fetal period/lactation
Regulates cell proliferations/differentiation as an adult
PTH and Vitamin D
THESE are the 2 hormones associated with calcium regulation
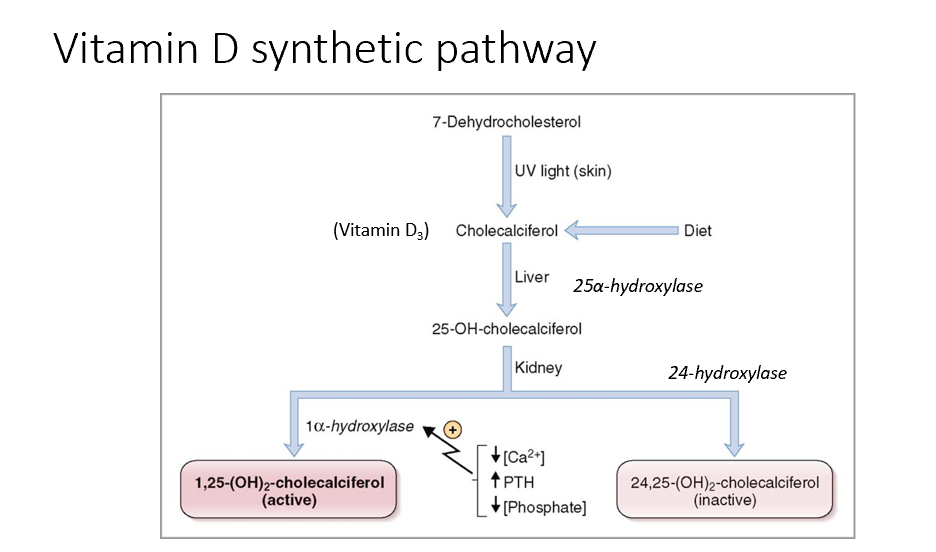
Steps in Vitamin D Synthesis and Regulation
7-dehydrocholesterol (cholesterol derivative) is exposed to UV light in the skin and converted to cholecalciferol (vitamin D3), can also come from the diet.
In the liver, cholecalciferol → 25-hydroxycholecalciferol (calcidiol).
In the kidney, 25-hydroxycholecalciferol is converted to:
Active form of Vit D (calcitriol) via 1-alpha hydroxylase
1,25 (OH)2- cholecalciferol
Upregulated by low calcium & high PTH & low phosphate
Inactive form of Vit D via 24-hydroxylase.
24, 25-hydroxycholecalciferol (calcidiol)
1-alpha hydroxylase and 24-hydroxylase are used in blood tests to assess Vit D levels!
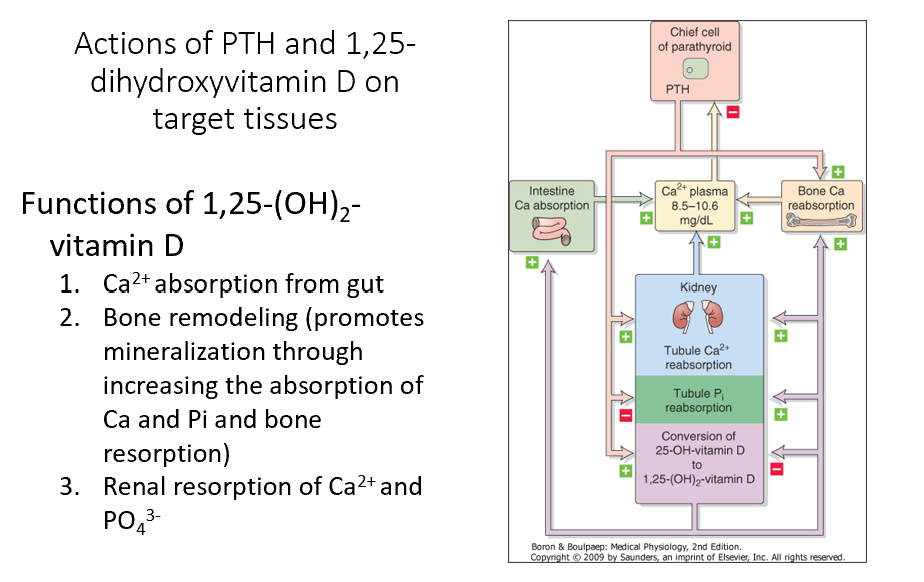
1,25-(OH)2-Vitamin D
Promotes Ca2+ absorption from gut
Shuts off its own synthesis (neg feedback)
Promotes bone remodeling (promotes mineralization through increasing the absorption of Ca and Pi and bone resorption)
Promotes renal resorption of Ca2+ and PO43- (bone remodeling)
Note: PTH promotes conversion of 25-OH-Vit D to 1,25(OH)2-Vit D
Absolutely NEED to have for bone formation!
Calcitonin
Regulates calcium homeostasis (uncertain in humans tho)
Made by non-follicular C cells in medullary thyroid cancer
Cancer marker!
Lowers blood Ca levels in experiments
Used to treat osteoporosis (salmon-derived) in the past (limited bone resorption)
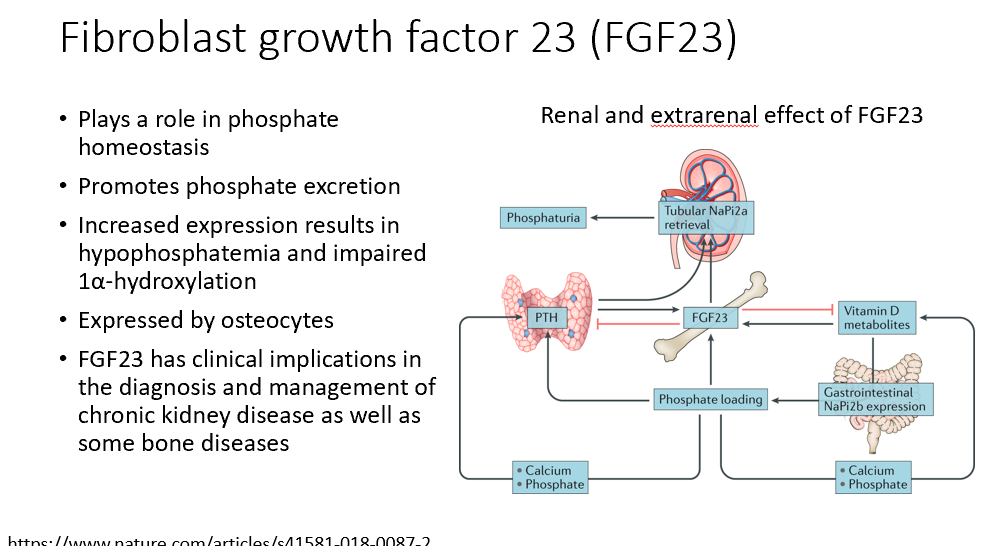
FGF23 (fibroblast growth factor 23)
Involved in phosphate homeostasis
Promotes phosphate excretion
Increased expression results in hypophosphatemia and impaired 1α-hydroxylation
Expressed by osteocytes
Clinical implications in the diagnosis and management of chronic kidney disease as well as some bone diseases
Inhibits Vit D and PTH, promotes loss of phosphate (causes phosphaturia when over-expressed)!
Additional hormone/growth factor regulators of bone homeostasis
Systemic hormones (e.g. GH, T3 and T4, insulin) increase bone formation
Glucocorticoids suppress bone formation
Gonadal hormones (estrogen and androgens) increase bone formation
Local regulators (e.g. cytokines related to the TNF subfamily) suppress bone formation
Growth factors (e.g. IGF, PDGF, etc.) stimulate bone formation
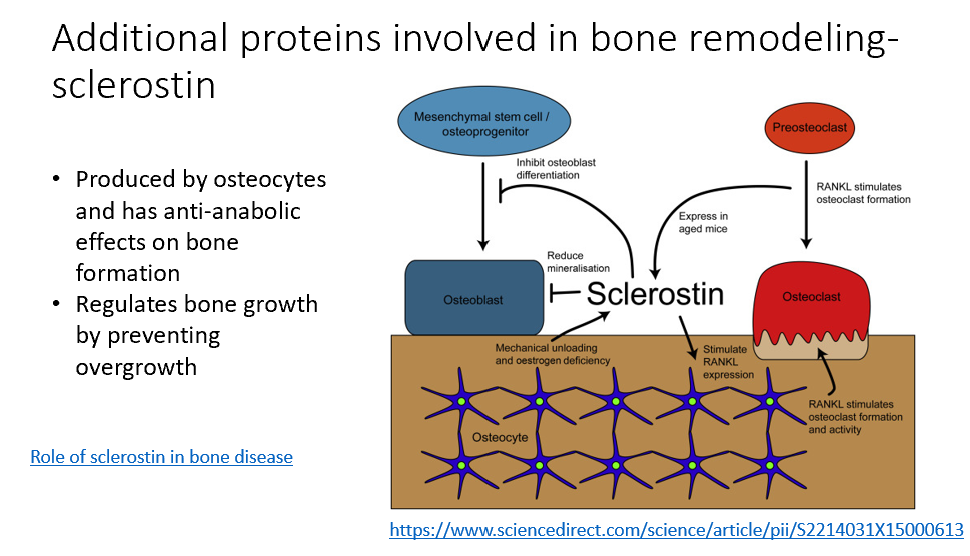
Sclerostin
Additional protein involved in bone remodeling
Made by osteocytes
Anti-anabolic effects on bone formation
Regulates bone growth, prevents overgrowth
Reduces bone mineralization (osteoblasts)
Target in treating osteoporosis!
Metabolic Bone Disease Types
Osteoporosis
Osteomalacia & Rickets
Paget’s Disease
Osteogenesis Imperfecta
Osteoporosis
Characterized by increased bone fragility and risk of fracture
Risk factors fall into nonmodifiable and modifiable categories
Osteomalacia and Rickets
Disorders in mineralization of bone and most often associated with vitamin D deficiency
Paget’s disease
Characterized by excessive osteoblast activity and hyperactive bone remodeling
Osteogenesis Imperfecta
Condition characterized by weaking of bone due to genetic mutations in collagen
Markers for Bone Disease
Bone Densitometry
Biochemical measurements (formation/reabsorption)
Bone bx and imaging
Bone densitometry
T and Z scores can be obtained
T scores can be used to diagnose osteoporosis (<2.5 standard deviations is the criteria for diagnosis of osteoporosis)
Bone Formation Biochemical Measurements
Alkaline phosphatase, osteocalcin, N-terminal and C-terminal peptides of procollagen (PINP or PICP)
Bone Reabsorption Biochemical Measurements
Urinary hydroxyproline and collagen cross-links (NTX or N-telopeptides and CTX or C-telopeptides)
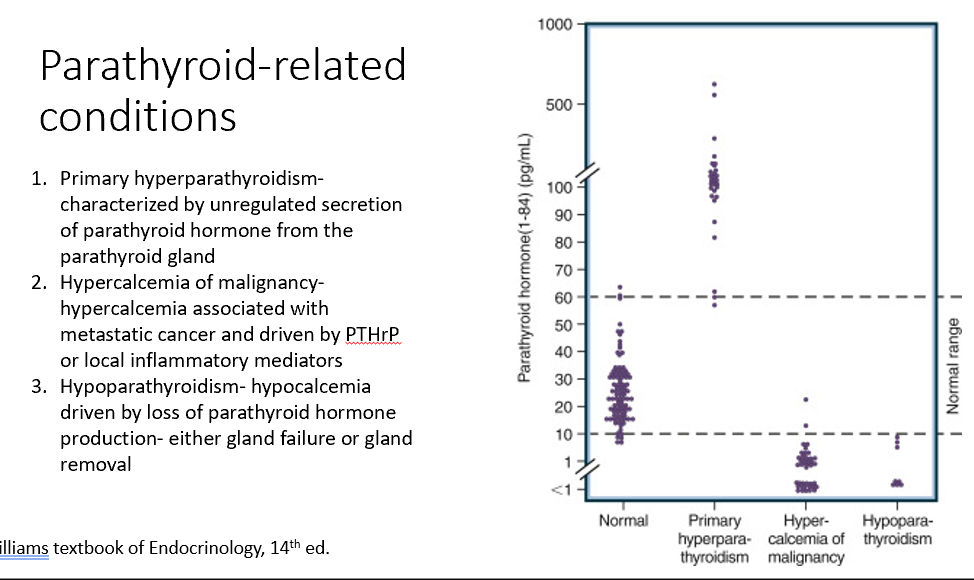
Parathyroid-Related Conditions
Primary hyperparathyroidism
Hypercalcemia of malignancy
Hypoparathyroidism
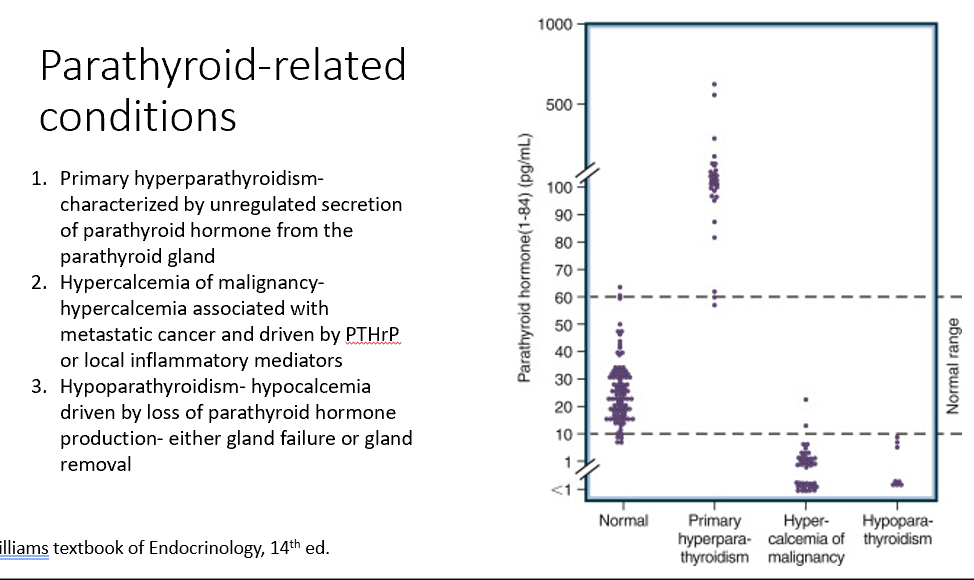
Primary hyperparathyroidism
Characterized by unregulated secretion of parathyroid hormone from the parathyroid gland (too much PTH)
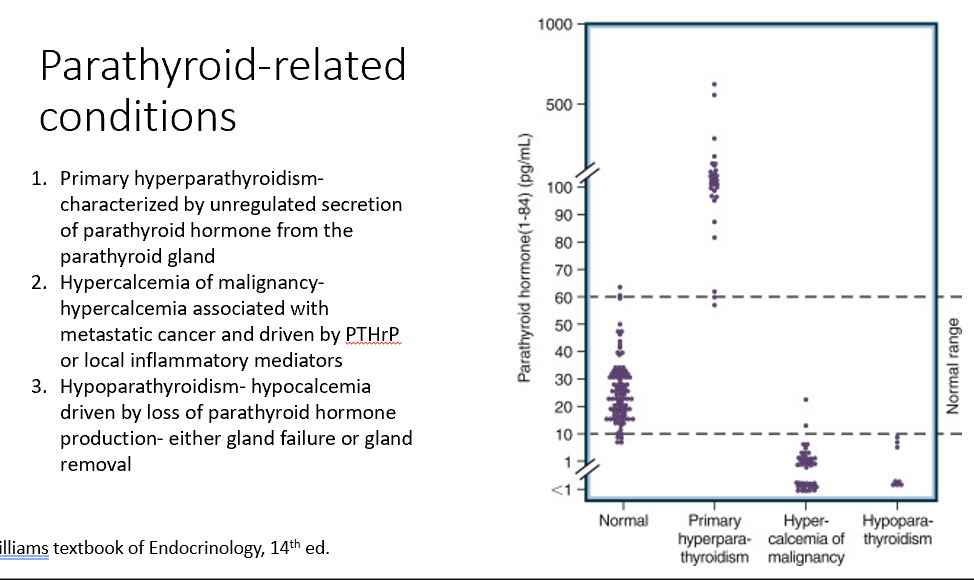
Hypercalcemia of malignancy
Associated with metastatic cancer and driven by PTHrP or local inflammatory mediators (too little PTH due to excess Ca)
Increases bone resoprtion (Ca increased), decreases PTH
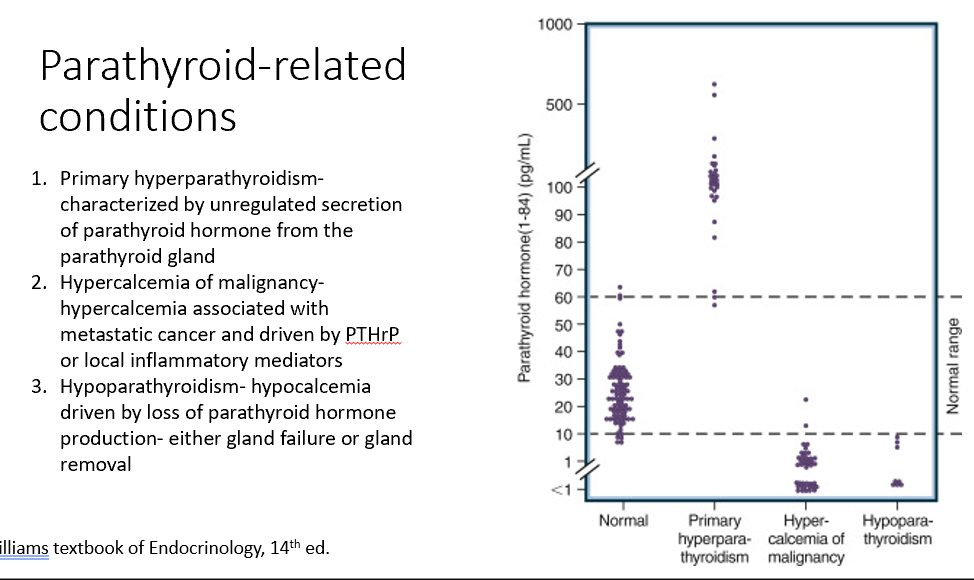
Hypoparathyroidism
Hypocalcemia driven by loss of parathyroid hormone production- either gland failure or gland removal (loss of PTH)
Bone Changes
Specifically seen in hyperparathyroidism!
PTH is secreted unregulated, too much PTH, driving bone resorption!
Decreased bone density!

Kidney Failure
Low blood Ca
High PTH
Issues with resorption, filtration, and excretion. Mainly no activation of Vit D! Causes low Ca, in response PTH rises!
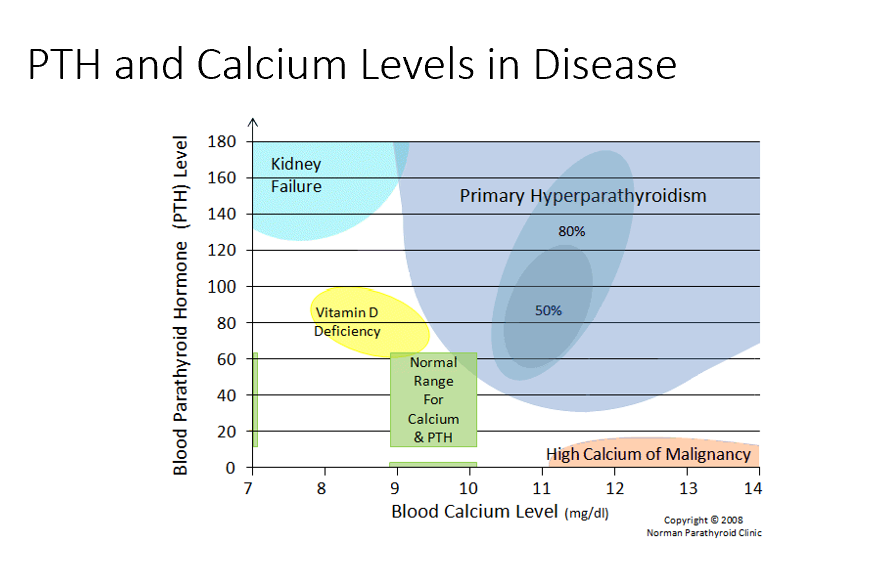
Vitamin D Deficiency
Low blood Ca
Semi-High PTH
Need vit d for Ca! Not enough vit d means not enough Ca resorption!
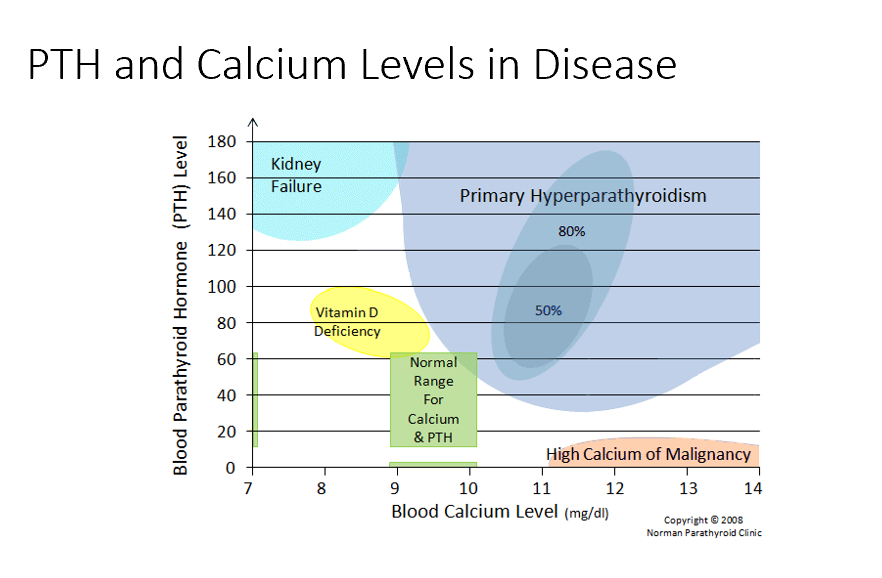
Normal Ranges
Ca 9-10 mg/dL
PTH 20-40
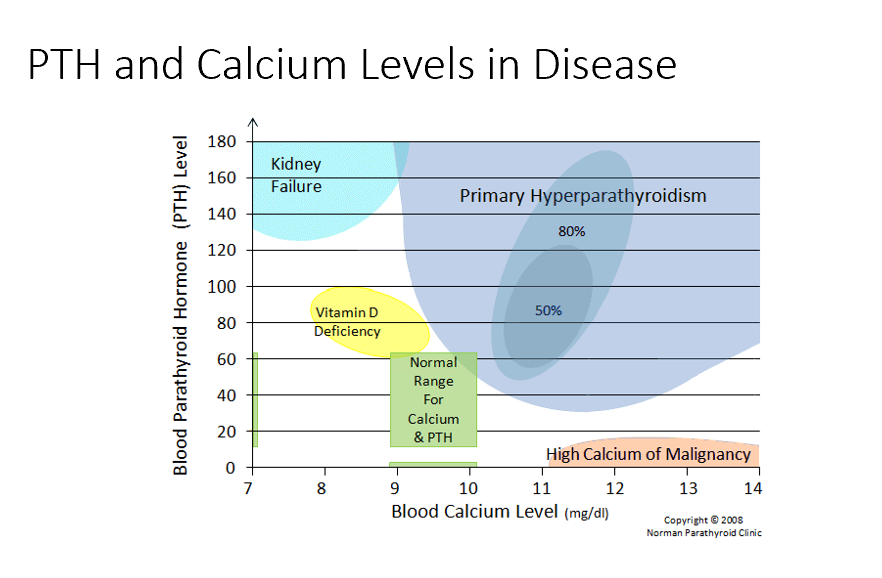
Primary Hyperparathyroidism
High blood Ca
High PTH
Too much PTH → too much bone resorption → too much blood Ca
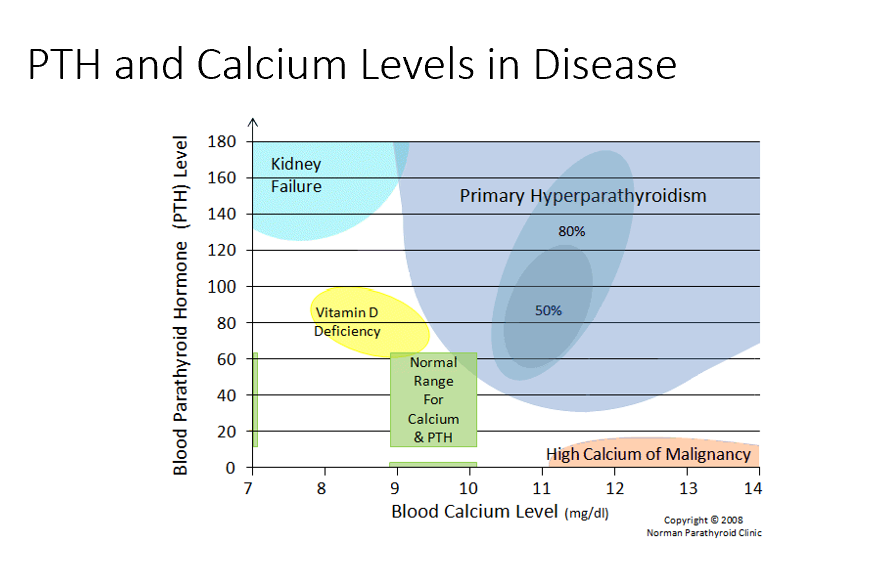
High Calcium of Malignancy
Very High Ca (caused by malignancy)
Low/No PTH
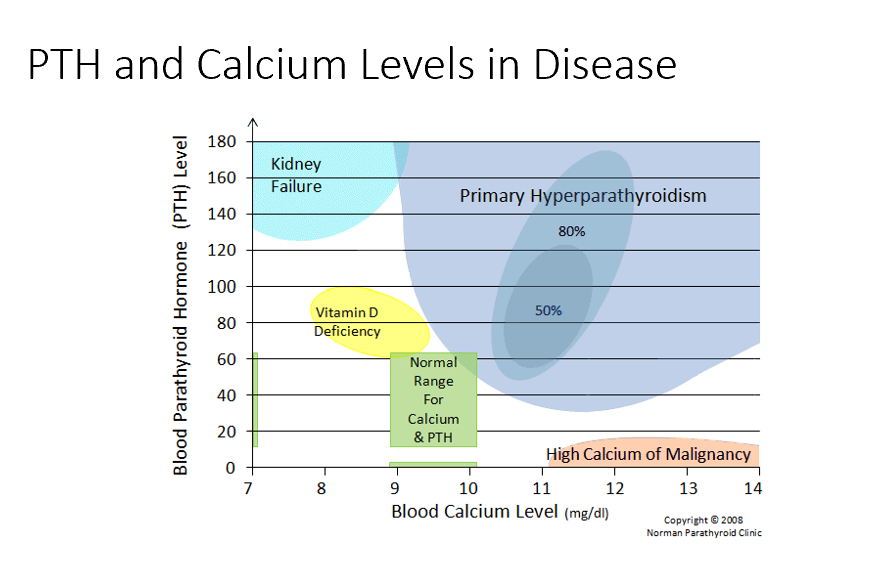
Hypercalcemic Disorders
PTH Dependent
PTH Independent
PTH Dependent Hypercalcemic Disorders
Primary hyperparathyroidism (increased PTH)
Familial hypocalciuric hypercalcemia
Heterozygous inactivating mutation in the CaSR (Ca sensory receptor)
Increased PTH and serum calcium
Low urine calcium
PTH Independent Hypercalcemic Disorders
Hypercalcemia of malignancy (not due to PTH)
Thiazide diuretics
Immobilization
Acute renal failure
Granulomatous disease
Serum PTH
THIS is key test in the differential diagnosis of hypercalcemia.
Causes of Hypocalcemia
Vitamin D Deficiency
Chronic Renal Failure
Liver Failure
Hypoparathyroidism
Pseudohypoparathyroidism
Genetic disorder causing resistance to PTH
C. Elevated PTH levels
A heterozygous inactivating mutation in the CaSR results in:
a. Hypercalciuria
b. Low levels of 1,25-dihydroxyvitamin D
c. Elevated PTH levels
d. Hypophosphatemia
C. Inhibiting secretion of SOST (sclerostin) by osteocytes
Intermittent treatment with PTH improves bone density through:
a. Stimulation of RANKL by osteoblasts
b. Induction of cell death by osteocytes
c. Inhibiting secretion of SOST (sclerostin) by osteocytes
d. Decreasing osteoprotegerin production by osteoblasts
B. Increase intestinal Ca2+ absorption
The primary action of 1,25-dihydroxyvitamin D to increase serum Ca2+ is to:
a. Stimulate osteoblast RANKL production
b. Increase intestinal Ca2+ absorption
c. Increase renal phosphate excretion
d. Stimulation PTH production
A. PTH 5 pg/mL, phosphate 6 mg/dL, alkaline phosphatase 600 U/L
A 73-year-old woman is admitted to the hospital following a bout of severe vomiting and generalized weakness. Initial laboratory values reveal elevated Ca2+levels. The referring physician tells you that she has breast cancer, and her bone scan indicates metastasis to bone. Which of the following laboratory values would be compatible with this clinical scenario?
a. PTH 5 pg/mL, phosphate 6 mg/dL, alkaline phosphatase 600 U/L
b. PTH 90 pg/mL, phosphate 6 mg/dL, alkaline phosphatase 30 U/L
c. PTH 5 pg/mL, phosphate 2 mg/dL, alkaline phosphatase 20 U/L
d. PTH 3 pg/mL, phosphate 2 mg/dL, alkaline phosphatase 100 U/L
Note:
PTH (nl, 10-65 pg/ml))
Ca (nl, 8.5-10.5 mg/dL)
Phosphate (nl, 3.0-4.5 mg/dL)
Alkaline phosphate (nl, 25-125 U/L)
25- OH Vitamin D and PTH
Measurement of THESE are the key tests in the differential diagnosis of hypocalcemia.
Low
High
_ urine Ca would indicate familial form of hypoparathyroidism
_ urine Ca would indicate primary form of hypoparathyroidism
B. ionized Ca
Carl has unregulated secretion of PTH due to an adenoma of the parathyroid gland. Under physiologic control, which form of calcium stimulates PTH secretion?
a. Total calcium
b. Ionized calcium
c. Protein-bound calcium
d. Anion-complexed calcium
Overall readings of Ca
What can you conclude about normal Ca levels if albumin is elevated?
Albumin binds Ca. If Albumin levels start to change, it can change _.
Forms of Ca in the blood:
40% is protein bound (to Alubumin!)
60% is ultrafilterable (50% of this is Ionized Ca, the other 10% is complexed to anions)
A. osteoblast cells
In patients with mild hypercalcemia, observation may be appropriate for treatment. Observation should include monitoring blood calcium and creatinine every 6 months and yearly bone densitometry. On which cell type do the PTH receptors reside?
a. Osteoblast cells
b. Osteoclast cells
c. Osteocyte cells
d. Chrondrocytes
B. net bone resorption
What is the outcome of increased PTH secretion on the bone homeostasis?
a. Net bone deposition
b. Net bone resorption
c. No net change
Increased osteoblast activity due to increased PTH (increased M-CSF) and increased RANKL (both of which increase bone resorption)
What causes increased alkaline phosphatase levels in hyperparathyroidism?
PTH signals to osteoblasts to release all STEM cell factors (M-CSF) to recruit osteoclasts.
PTH also stimulates RANKL production.
These both signal bone resorption! So, bone resorption is increased, but why is alkaline phosphatase?
Alkaline phosphatase is produced by osteoblasts and gives us an idea of what’s happening with bone formation.
So, bone resorption and bone formation are increased and linked together.
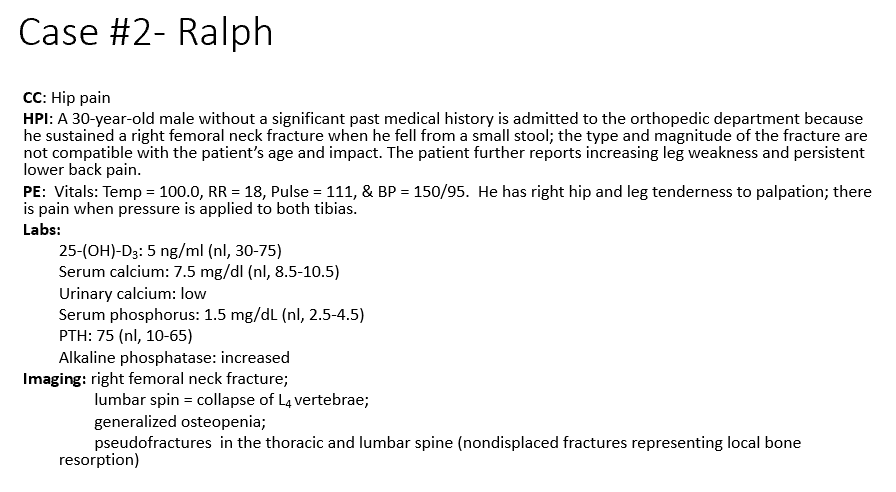
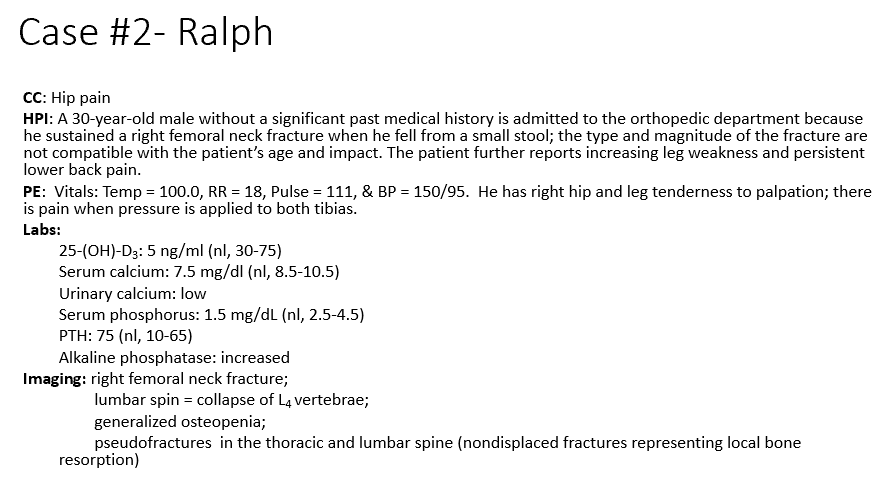
B. Vit D Deficiency
What is driving Ralph’s hypocalcemia?
a. Lack of calcium intake
b. Vitamin D deficiency
c. Phosphorus deficiency
d. Increased alkaline phosphatase levels
e. Elevated PTH

Phosphate (increases excretion)
What do the laboratory values tell us about processes occurring in the bone?
25-(OH)-D3 = 5 ng/ml (nl = 30-75)
Serum calcium = 7.5 mg/dl (nl = 8.5-10.5)
Serum phosphorus = 1.5 mg/dL (nl = 2.5-4.5)
Phosphorus decreased due to low Ca -> increases PTH to increase Ca retention-> increases PTH which decreases _

PTH
How will the loss of 25-(OH)-D3 impact bone homeostasis and change other laboratory values such as PTH?
Hypocalcemic, missing vit d: Net bone resorption which we can see through the increased THIS trying to maintain Ca levels but it isn’t enough without the vit d (leading to the osteopenia and the fractures)

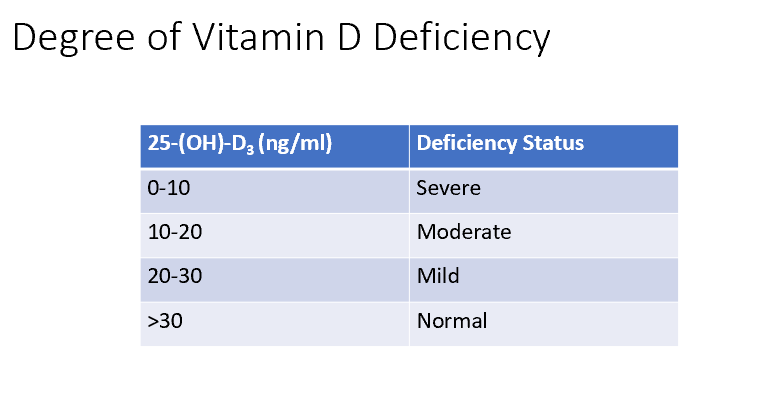
Classifications for Vit D Deficiencies
0-10 = Severe
10-12 = Moderate
20-30 = Mild
>30 = Normal
A. osteomalacia (lack of Ca and lack of Vitamin D = lack of bone mineralization)
Which of the following bone disorders does Ralph have?
a. Osteomalacia
b. Osteonecrosis
c. Osteoporosis
d. Osteopetrosis
e. Paget’s disease
Celiac Disease! Fat and fat-vitamins are not absorbed! Vit D deficiency!
Which GI conditions may result in similar findings to having decreased vitamin d?
Vitamin D is a fat-soluble vitamin. It’s absorbed the same way lipids are (emulsify, enzymes (lipase), micelles, diffusion, apolipoproteins added, chylomicrons, lacteals, circulation).
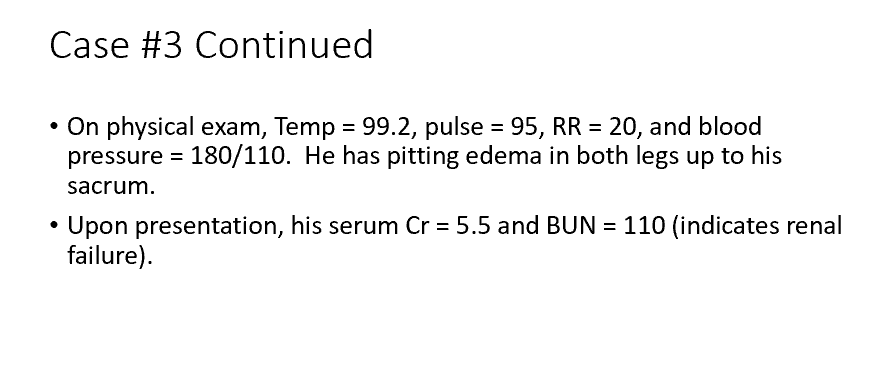
D. A decrease in 1α-hydroxylase activity (without this, no vit d in active form, so no Ca)
Calcium levels in this case of renal failure may be decreased due to which cause?
a. A decrease in serum PTH levels
b. An increase in urinary phosphate levels
c. Inability to produce 25-hydroxyvitamin D
d. A decrease in 1α-hydroxylase activity
B. Renal phosphate clearance decreases in renal failure (because of a decrease in urinary output, phosphate isn’t being cleared, no phosphate cleared means no Ca can be resorbed. Ca and phosphate complex and that leads to renal distruction!)
Bone loss associated with renal failure occurs in part because:
a. The failing kidney is no longer capable of PTH production
b. Renal phosphate clearance decreases in renal failure
c. The failing kidney increases activation of vitamin D
d. Renal calcium clearance increases in renal failure
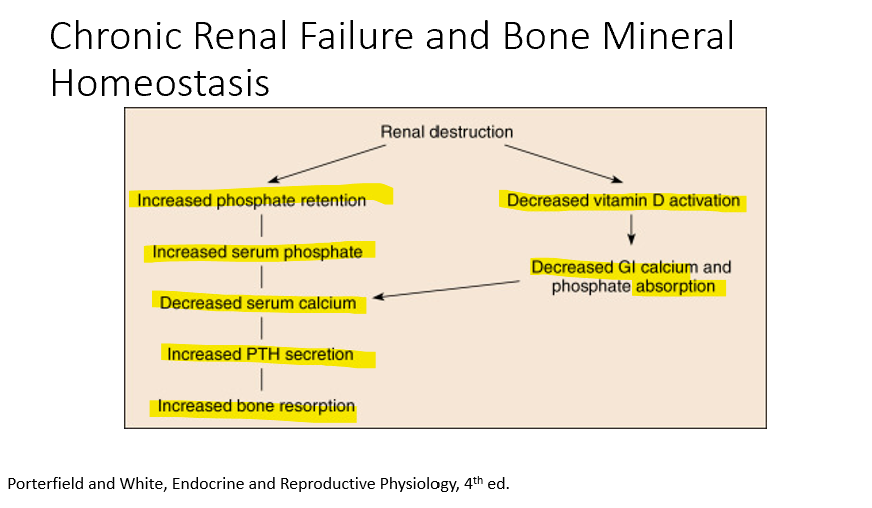
Chronic Renal Failure leads to:
Decreased vitamin d activation
decreased GI calcium absorption
Also
Increased phosphate retention
increased serum phosphate
decreased serum Ca
Increased PTH secretion
Increased bone resorption
B. Autoimmune destruction of her parathyroid glands. (no functioning glands means no PTH)
Someone with hypothyroidism has low serum calcium levels due to:
a. autoimmune destruction of her pancreas.
b. autoimmune destruction of her parathyroid glands.
c. inadequate calcium in her diet.
d. inappropriately low 25(OH) vitamin D level.
e. PTH resistance.
Bone resorption
Increased alkaline phosphatase & urine n-telopeptides means increased_

Osteoporosis
Promoted by:
age, loss of estrogen (post-menopausal), smoking, not enough Ca intake
Prevented by:
exercise, 25-hydroxy vit d is normal, weight gain (the weight gain and resistance is putting stress on bone, promoting more bone formation (hence the increased n-telopeptides))
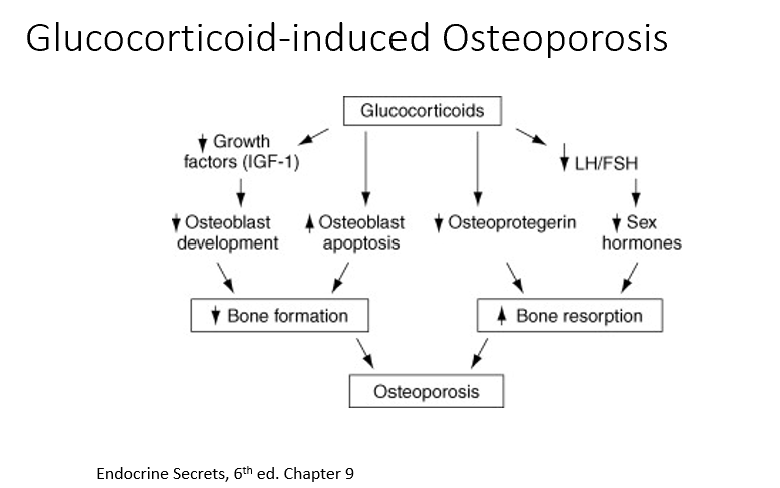
Glucocorticoids
Have detrimental effect on bone mass.
We need the perfect amount of cortisol.
THESE can:
Decrease growth factors
Decrease osteoblast development
Increase osteoblast death
Decreases osteoprotegrin (normally shuts down bone resorption)
Overall, they decrease bone formation, increase bone resorption, result = osteoporosis!
Pharmacologic Osteoporosis Treatments
Two categories:
Antiresorptive: inhibit bone resorption (i.e. bisphosphonates)
Anabolic: increase bone formation (i.e. subcutaneous PTH injections)
Don’t use these 2 together! Increased formation increases resoprtion and vice-vera (balancing act).
Bone Resorption
Why is it important to check vitamin D levels prior to starting an osteoporosis treatment?
With the treatments, we’re inhibiting _ (which limits serum Ca leves). Low Vit D would do the same thing. Not good.
Main targets for treating osteoporosis:
Maintain bone mass
Inhibit bone loss
RANKL
Sclerostin (antibodies against this inhibit bone resorption) (net formation effect)
Alkaline Phosphatase
Increased _ means unregulated osteoblast activity!
Osteoblasts
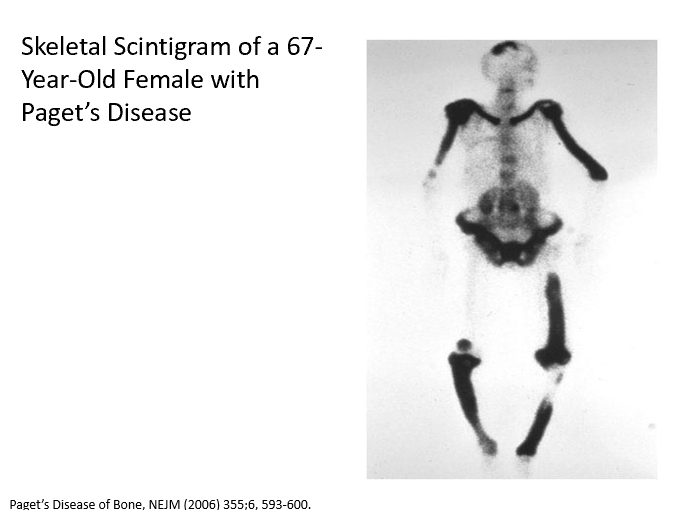
Origin of Paget’s?
THESE!
Releasing too much alkaline phosphatase

Bone formation
What causes progressive pain in the shins, knees, and left arm in Paget’s Disease?
Due to increased bone density! Osteoblasts aren’t regulated. Too much_
Bone weakness
Hypervasculature
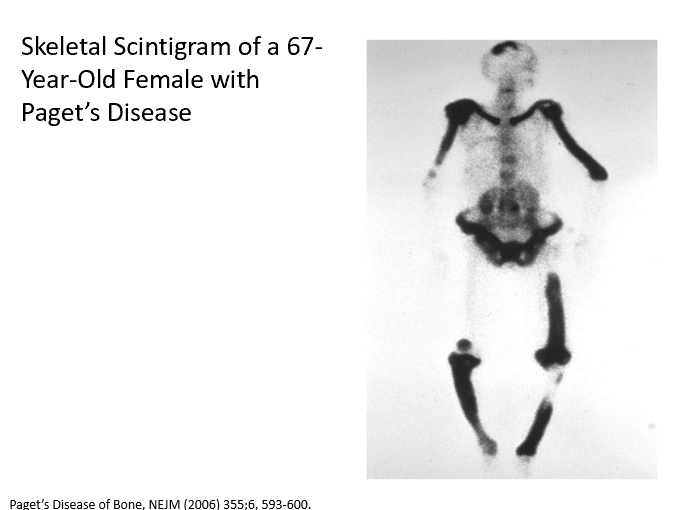
What causes tenderness above the left elbow, enlarged, bowed shins and warmth over both shins in Paget’s disease?
Increased _ in this case because of haphazard bone formation!
Hence bowing and pain!
Warmth due to _ (more blood)
D. Inhibition of bone resorption
Considering currently available therapeutics, which option should be utilized in the treatment plan for Paget’s?
a. Calcium supplementation
b. Increase in urine output
c. Inhibition of bone formation
d. Inhibition of bone resorption
e. Inhibition of PTH secretion
PTHrP
Ca
Why does this pt have hypercalcemia?
_ related to malignancy
Why are the PTH levels 2?
PTH is low because _ is high!
