Aldehydes & Ketones
1/35
Earn XP
Description and Tags
Chapter 19 guide to aldehydes & ketones
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Aldehydes
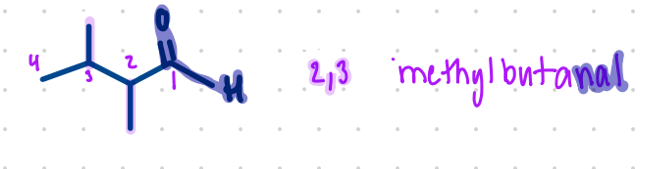
Nomenclature for what?
parent name is always the carbon carbonyl. It’ll be carbon one as well
the end of the name will end w/ -al
e.g.,
hexanal
2,3-dimethylbutanal (shown in picture)
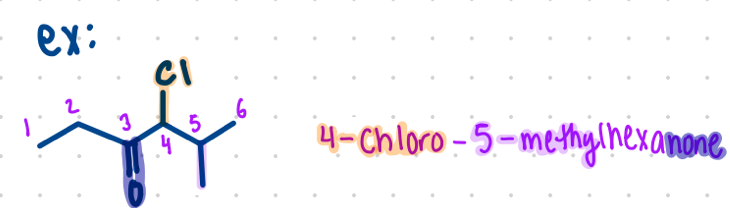
Ketones
Nomenclature for what?
carbon of this group should have lowest number possible
end of the name will end with -one
e.g.,
Butanone
4-chloro-5-methylhexanone (shown in picture)
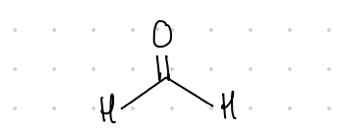
Formaldehyde
This aldehyde’s common name is what?
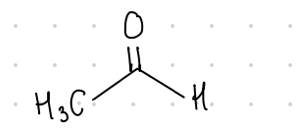
Acetaldehyde
This aldehyde’s common name is what?
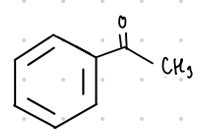
Acetophenone
This ketone’s common name is what?
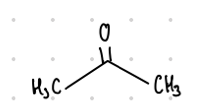
Acetone
This ketone’s common name is what?
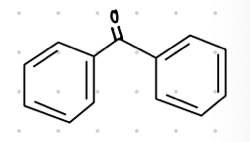
Benzophenone
This ketone’s common name is what?
Imine
Different C groups of what?
nitrogen double bonded

primary
An imine is formed by what kind of amines?
Enamine
Different C groups of what?
Nitrogen one away from double bond
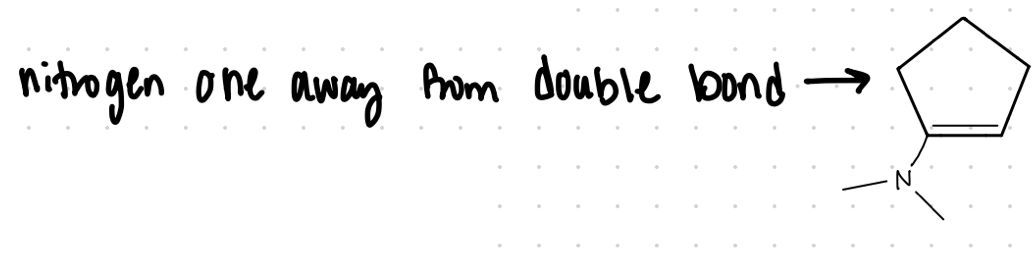
secondary
An enamine is formed by what kind of amines?
Acetal
Different C groups of what?
oxygen with 2 C groups coming off

Hemiacetal
Different C groups of what?
one alcohol and one oxygen w/ C group
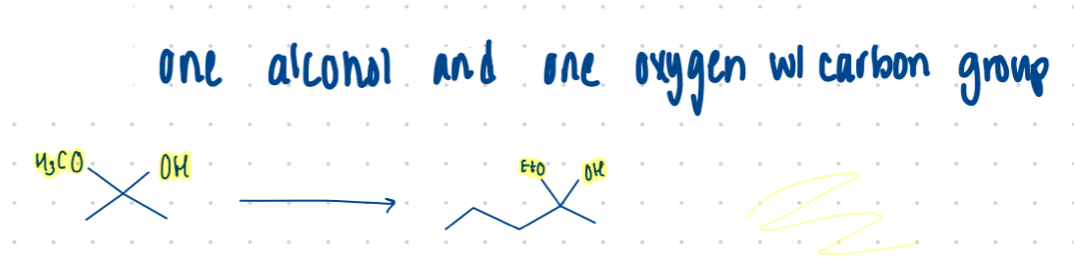
ketones
All secondary (2°) alcohols will oxidize into what?
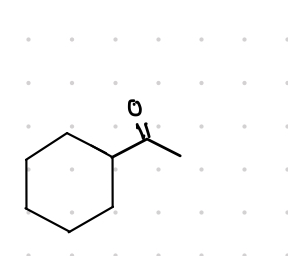
ring w/ ketone
What is the resulting product from these?
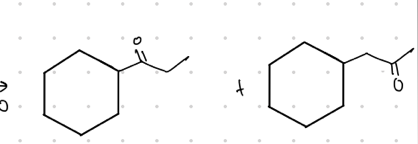
mixture of methyl ketone
What is the result product from these?
internal bond to _________.
Reagent:
HgSO4
—-—>
H2SO4, H20
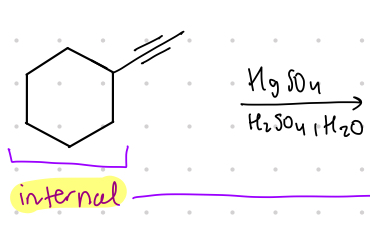
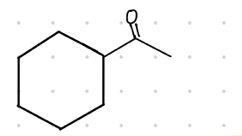
methyl ketone
What is the result product from this?
terminal bond to ____________.
Reagent:
HgSO4
—-—>
H2SO4, H2O
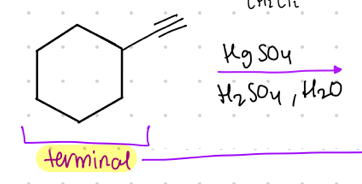
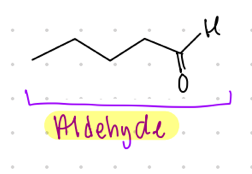
aldehyde
What is the result product from this?
terminal end to _____.
Reagent:
1.) 9-BBN
—-—>
2.) H2O2, NaOH
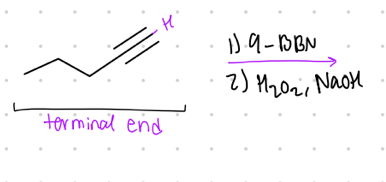
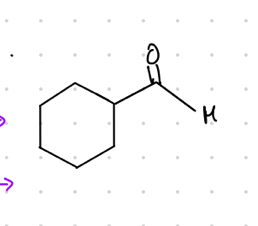
aldehyde substituent
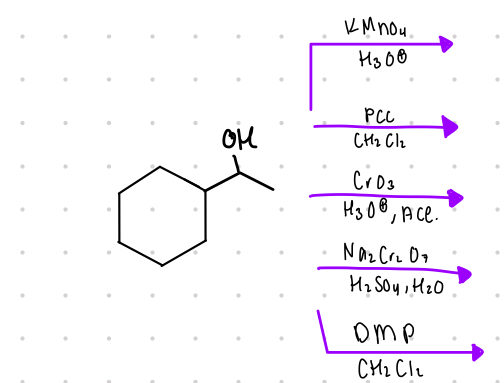
What is the result product from this?
from primary alcohols
Reagents:
DMP
—-—>
CH2Cl2
PCC
—-—>
CH2Cl2
CH2Cl2
—-—>
DMSO, Et3N
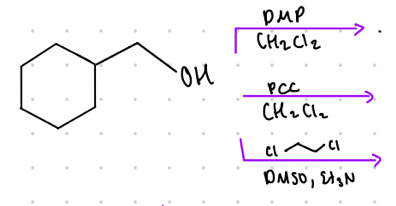
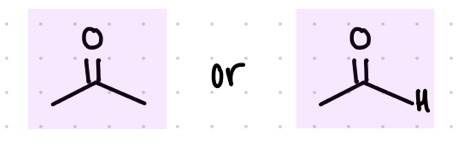
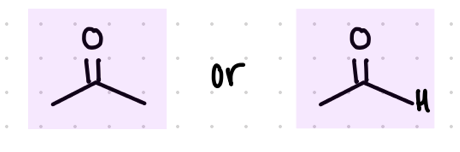
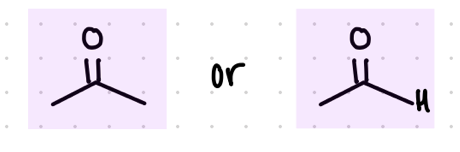
Aldehydes
What is more reactive?
aldehydes or ketones
aliphatic
What is more reactive?
aliphatics (non-aromatic) or aromatics
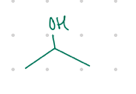
add OH
Starting product: aldehyde or ketone
Reagents:
LiAl4
—-—>
H3O+
(LiAl4 is a strong reducing agent)
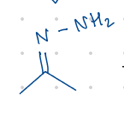
add N-NH2
Starting product: aldehyde or ketone
Reagent:
NH2NH2, [H+]
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>NH<sub>2</sub>NH<sub>2</sub>, [H+]</p>](https://knowt-user-attachments.s3.amazonaws.com/c7b567c4-33ac-41d5-ae3c-5248696c7350.png)
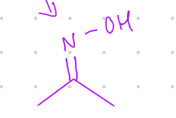
add N-OH
Starting product: aldehyde or ketone
Reagent:
NH2OH, [H+]
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>NH<sub>2</sub>OH, [H+]</p>](https://knowt-user-attachments.s3.amazonaws.com/4a94cf51-da39-4f0a-98c1-1d2c83376929.png)
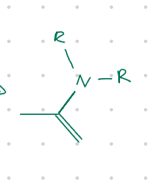
move double bond, add N with 2 R groups
Starting product: aldehyde or ketone
Reagent:
R2NH, [H+]
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>R<sub>2</sub>NH, [H+]</p>](https://knowt-user-attachments.s3.amazonaws.com/e55cdbd5-8ba5-48a2-baa9-19dfb07f8993.png)
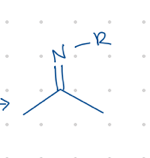
add N-R
Starting product: aldehyde or ketone
Reagent:
RNH2, [H+]
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>RNH<sub>2</sub>, [H+]</p>](https://knowt-user-attachments.s3.amazonaws.com/e76821dd-0471-4400-a601-22cb42dd22a7.png)
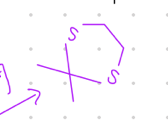
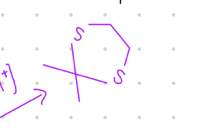
Connect 2 S groups by bridgehead
Starting product: aldehyde or ketone
Reagent:
HS/\/SH, [H+]
—-—>
-H2O
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>HS/\/SH, [H+]</p><p>—-—></p><p>-H2O</p>](https://knowt-user-attachments.s3.amazonaws.com/57b84dd4-7982-4380-8cd9-6c62de23321d.png)
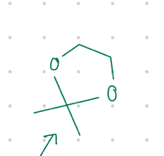
two O’s connected with a bridgehead
Starting product: aldehyde or ketone
Reagent:
HO/\/OH, [H+]
—-—>
-H2O
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>HO/\/OH, [H+]</p><p>—-—></p><p>-H2O</p>](https://knowt-user-attachments.s3.amazonaws.com/eba18ef8-002a-4d8a-be13-bd99337b0cfe.jpg)
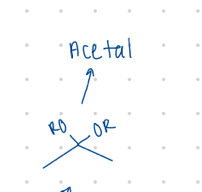
Acetal (OR and OR)
Starting product: aldehyde or ketone
Reagent:
[H+]
—-—>
ROH, -H2O
(R is any carbon/H group)
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>[H+]</p><p>—-—></p><p>ROH, -H2O</p><p>(R is any carbon/H group)</p>](https://knowt-user-attachments.s3.amazonaws.com/04fa21ce-528f-4985-9d2f-aead388c11da.png)
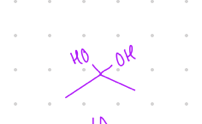
add 2 OH groups
Starting product: aldehyde or ketone
Reagent:
[H+], H2O
![<p>Starting product: aldehyde or ketone</p><p>Reagent:</p><p>[H+], H2O</p>](https://knowt-user-attachments.s3.amazonaws.com/5a6518e3-b223-48cc-bd90-52293da3920f.png)
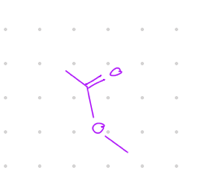
replace a group with O-
Starting product: aldehyde or ketone
Reagent:
RCO3H
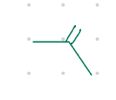
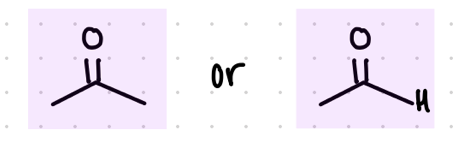
remove the O
Starting product: aldehyde or ketone
Reagent:
H2C=PPh3
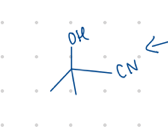
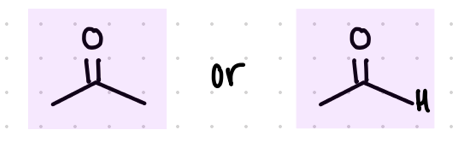
add an OH and a CN
Starting product: aldehyde or ketone
Reagent:
KCN, HCl
or
KCN, H3O+
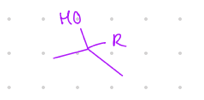
add an OH and an R group
Starting product: aldehyde or ketone
Reagent:
1.) RMgBr
—-—>
H3O+
(MgBr is a reducing agent)
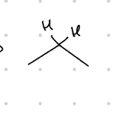
replace with 2 H’s
Starting product: =N-NH2
Reagent:
NaOH, H2O
—-—>
heat
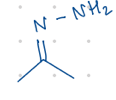

replace with 2 H’s
Starting product: 2 S groups connected by a bridgehead
Reagent:
Raney Nickel