ch 20/21/22 reagents
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
H2, Pb/C
Removes any sigma bonds unless poisoned
triple → double → single
NaCCH3
Adds to C=O
X-CN
CN adds to C=O
CN replaces Br/halide
Chromic acid (H2CrO4)
primary alcohol → CBA
secondary alcohol → ketone
aldehyde → CBA
Better, but dangerous
PCC
primary alcohol → aldehyde
secondary alcohol → ketone
KMnO4
primary alcohol → CBA
secondary alcohol → ketone
alkenes → adds alcohol to both sides of bond (no more alkene)
CuI
Makes lithium dialkylcuprate (CuLi-X2) from an alkyllithium reagent (GILMAN REAGENT FORMATION)
ADDS TWO Li MOLECULES TOGETHER
mCPBA
double bond → epoxide (stereospecific)
X-MgBr
Adds -X to least substituted carbon
Grubbs Catalyst
double bonds become one double bond
Ring closing
H2
ketone → alcohol (adds two H)
ZnHg, HCl (H2O)
Removes carbonyl to just 2H
HSCH2CH2SH, H+ (Raney Ni H2)
Removes carbonyl to just 2H
Mg(s), ether
Makes grignard reagent (MgX)
Li(s), ether
Makes alkyllithium reagent (x→Li)
BH3, THF
Adds -OH anti-markovnikov (less substituded C)
H2, Raney Ni
aldehyde → primary alcohol
ketone → secondary alcohol
chloride → primary alcohol
nitrile (CtripleN) → primary amine (-NH2)
PBr3
alcohol → bromide (replaces -OH with -Br)
CO2, H3O+
carboxylation (creates CBA)
LAH
CBA → primary alcohol
acid chloride (C=O C-Cl) → primary alcohol
ester → primary alcohol
amide → amine with no C=O
Protonates cyanide to NH2 (CtripleN → C-NH2)
LDA
Moves carbonyl = to nearby carbon
NaOH
Adds to carbonyl C, kicking off LG if present
Can be saponification
n-BuBr
Adds n-Bu to other side of =
R-B(OR)2
R adds to halogen (R-X → R-R’)
R must be vinylic or aromatic
Uses Pd as a catalyst
SUSKI COUPLING - retains configuration
Pd(OAc)2, PPh3, NEt3
Heck coupling
PPh3X
X adds to carbonyl C
Umpoulung
NaH, CH3I
Williamson ether synthesis
-OH to -O-Ch3 or other
HOCH2CH3OH
Good protecting group for aldehydes
Prevents grignard reagents (X-MgBr) from reacting with itself if an aldehyde is present in the molecule
Intermediate is big pentane group with 2O
NaH, ClCH3OCH3 (MOM-Cl)
Good protecting group for alcohols
Intermediate is alcohol → ether
Hydropyran, H2SO4
Good protecting group for alcohols
Si-C(CH3)3 (TBS-Cl)
Good protecting group for alcohol
Intermediate is a cyclic silyl ether (TBAF)
Acetone, H2SO4
Good protecting group for diols (specifically two 1,3 alcohols)
KO-X
X adds to carbonyl C and kicks off ether
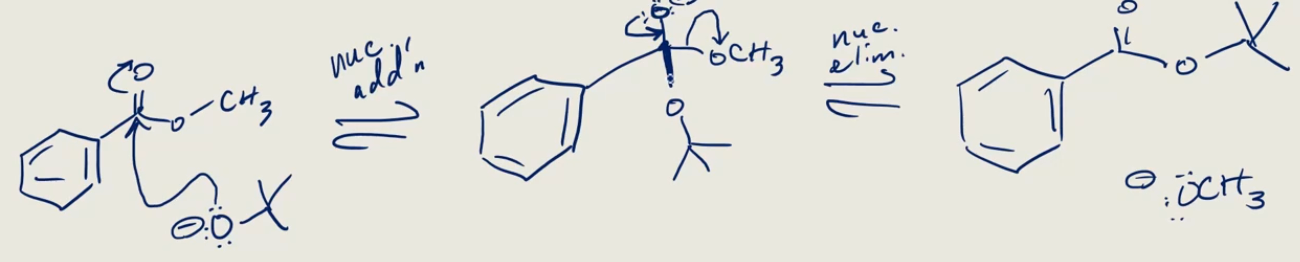
NaOCH3
-OCH3 replaces a halogen (kicks off good leaving group)
Stability Ladder (Best - worst leaving groups)
NH2 (amine) > OH (alcohol) / OR (ether) > CBA > Cl (halogen)
NH2NH2, KOH, heat
Wolff-Kischner reduction
Removes carbonyl entirely
phthalimide + 1) KOH/EtOH 2) R-X 3)KOH, H2O
Gabriel Synthesis - uses amine part
Creates a primary amine
1) Br2, NaOH, H2O 2) HCl, NaHSO3
ketone → CBA
breaks C-CBr3 bond, unusual
Haloform - requires 3 H to replace with 3 Br to make a good LG
NaBH4, EtOH
ketone & halogen → alcohol (kicks off halogen)
NO RXN WITH CBA
LiAlH(OtBu)3
acid chloride → aldehyde
DIBAl-H, HCl, H2O
ester → aldehyde