Ionic bonding & ionic compounds
1/7
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
Explain what ionic bonding is.
A metal and a non-metal react, A metal loses electrons to form a positive ion and the non-metal gains that electron to form a negatively charged ion. The oppositely charged ions are strongly attracted by electrostatic forces.
How are ionic bonded elements shown?
Dot and cross diagram
What are 3 problems with the dot and cross diagram?
Don’t show the structure of the compound, size of the ions or how they are arranged
Draw a dot and cross diagram for Magnesium chloride ( MgCl2) with only the outer shells
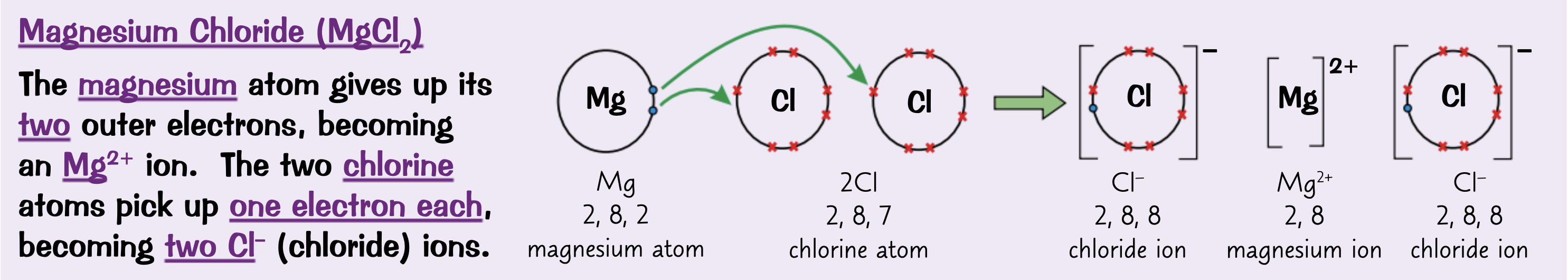
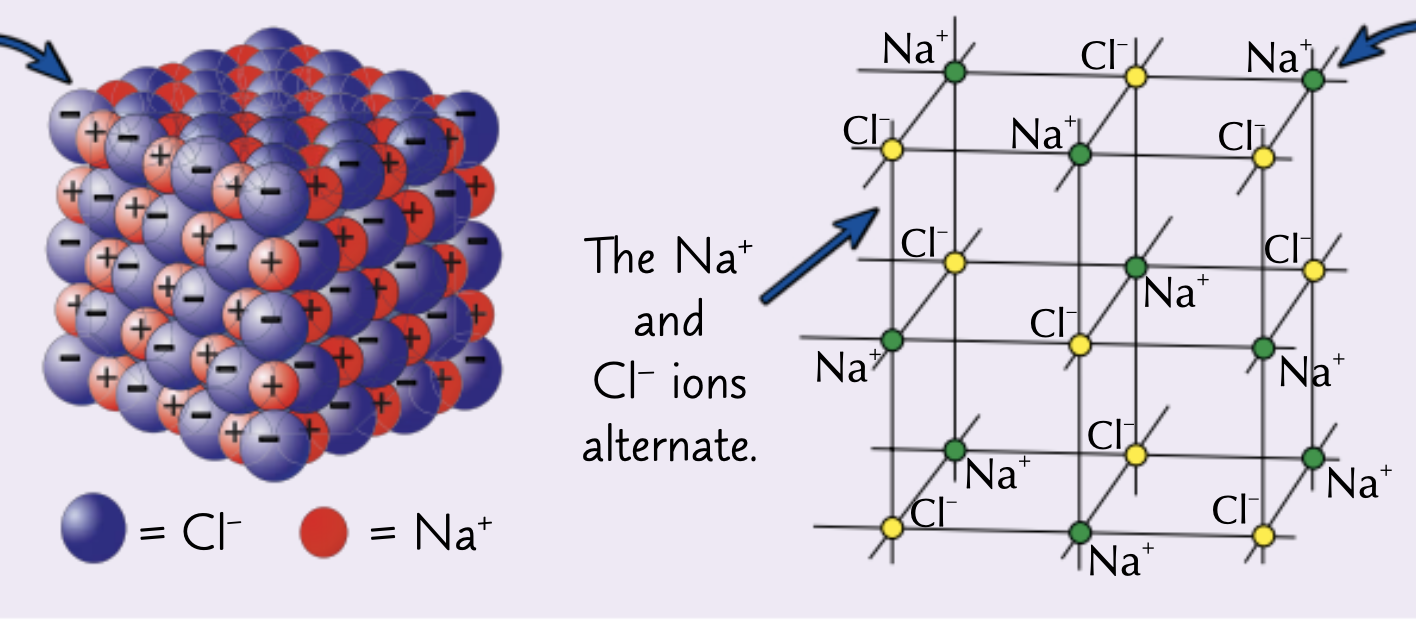
What structure do ionic compounds form?
Giant ionic lattice
Explain the properties and what an giant ionic lattice is.
The ions form a closely packed regular lattice arrangement with very strong electrostatic forces of attraction between the oppositely charged ions in all directions of the lattice
What are the properties of ionic compounds?
High mot and bot’s due to strong bonds between ions that require a lot of energy to break
Cannot conduct electricity as solids as ions are held in place
Can conduct electricity as a liquid when ions are free to move
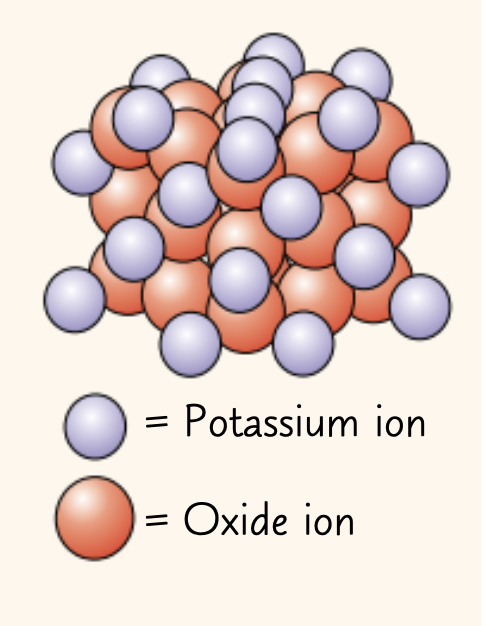
Find the empirical formula of the ionic compound in the image.
