Chapter 6: Energy from Combustion (Quiz)
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Why is the mileage a vehicle gets less when burning E85 than 100 percent gasoline?
Ethanol has a lower heat of combustion
The general term for plant matter such as trees, grasses, agricultural crops, or other biological material is
biomass.
A calorie is defined as exactly 4.184 J. Therefore 1.000 Cal is exactly
4184 J
The conclusion that it is impossible to completely convert heat into work without making other changes in the universe is
the concept that increasing entropy characterizes all changes in the universe and the second law of thermodynamics.
Which is an advantage of using coal over petroleum as a source of energy in the United States?
Coal reserves in the United States are far greater than petroleum reserves
The Triple Bottom Line refers to benefits to:
Society, Economy, and the Environment
The following molecules contain only single bonds.
NH3(g) + 3F2(g) → NF3(g) + 3 HF(g)
The bond energies are: N-H 391 kJ/mol; F-F 158 kJ/mol; N-F 272 kJ/mol; H-F 566 kJ/mol. Which is the heat evolved or absorbed per mole of NH3 that reacts with F2?
-867 kJ/mol
Which of the following is not a step in fractional distillation of crude oil?
Fractions are mixed with natural gas to enrich the hydrocarbon content
The energy that flows from a warmer body to a colder body is called
heat
Calculate the amount of energy that is saved by replacing a 75-watt incandescent light bulb with an 18-watt fluorescent bulb during the 1500-hour life of the incandescent bulb. One watt is the equivalent of one joule of energy used per second.
308,000 kJ
Soft lignite (brown coal) is the lowest grade of coal. Since it has undergone the least change since burial, its chemical composition—and hence its heat of combustion—is most similar to that of
wood.
Which is not a known advantage of natural gas over other fossil fuels?
It is far more abundant than any other fossil fuel
If a snack cake contains 450 food Calories and Dr. Wattenburger is able to burn 250 Calories by running for one-half an hour, how long must she run to completely burn off the snack cake?
54 minutes
Compounds with the same chemical formulas but different molecular structures are called
isomers.
Combustion is a chemical process in which a fuel combines with __________ to release energy and form products.
oxygen
Which common process on earth is endothermic?
the production of oxygen by photosynthesis
Advertising claims sometimes state that adding something mechanical to a car's engine will allow it to recover 100 percent of the energy that comes from burning gasoline. You should be skeptical of such claims because they violate the
second law of thermodynamics.
Assume that an extremely inefficient electrical utility company delivers electrical energy to your home from a natural gas-burning power plant with an overall efficiency of only 31 percent and your furnace is 85 percent efficient in converting electrical energy into heat energy. What mass of natural gas the power plant must burn if heating your home requires 3.5 × 10^7 kJ? (The heat of combustion of natural gas is 50.1 kJ/g.)
2650 kg
Cracking is
the breaking of larger molecules into smaller ones.
The energy stored in the chemical bonds of fossil fuels is a form of __________ energy.
potential
Oxygenated gasolines are blends of petroleum-derived compounds with oxygen-containing compounds. Which of the following oxygenated compounds has been discontinued in some states because of potential health risks?
MTBE
Over the very long run, the energy source that has the greatest potential to meet humanity's needs is
solar (the sun).
The energy needed to initiate a chemical reaction is called the
activation energy.
Alternative energy sources are currently being researched in effort to replace our dependence on fossil fuels. Which is not a current research effort in this regard?
Reintroducing the use of tetraethyl lead to increase the octane rating of gasoline
What percentage of global energy consumption do renewable sources currently represent?
18%
Which is a fossil fuel?
Natural gas
A chemical reaction accompanied by a release of energy is called a/an __________ reaction.
exothermic
How is heat energy used to generate electricity in a modern power plant?
Heat boils water to make steam, which drives a turbine
The heat energy released or absorbed by a chemical reaction is generally determined by the difference between the energy that
must be put in to break the bonds in the reactants and the energy that is released upon making the bonds in the products.
Petroleum (crude oil) is a complex mixture of thousands of substances, the majority of which are
hydrocarbons.
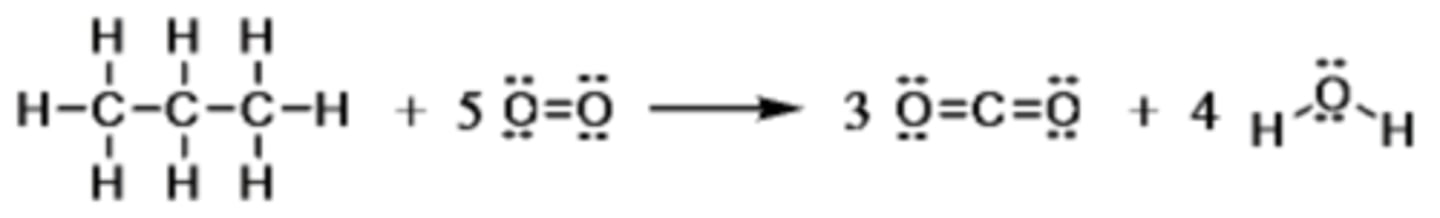
Consider the following equation that describes the complete combustion of propane, C3H8.
The bond energies are: C-H 416 kJ/mol; C-C 356 kJ/mol; O=O 498 kJ/mol; C=O 803 kJ/mol; H-O 467 kJ/mol. Which is the total amount of energy required to break all of the bonds in propane?
4040 kJ/mol
Which of the following is a proper use for a calorimeter?
Measuring heat of combustion
The process by which a solution is heated to its boiling point and the vapors are condensed and collected is known as
distillation.
The heat of combustion of ethane, C2H6, is 1560 kJ/mol. What is the heat of combustion of ethane, in kJ per gram?
51.9 kJ/g
Which of the following has the highest boiling point?
Octane
Which of the following are forms of kinetic energy?
Electrical energy
Consider the following equation that describes the complete combustion of propane, C3H8.
The bond energies are: C-H 416 kJ/mol; C-C 356 kJ/mol; O=O 498 kJ/mol; C=O 803 kJ/mol; H-O 467 kJ/mol. Which is the amount of energy gained on making all the bonds in carbon dioxide and water according to the equation?
8554 kJ/mol
The heat of combustion of methane, CH4, is 50.1 kJ/g. How much heat would be generated if 1.00 mol of methane undergoes complete combustion?
804 kJ
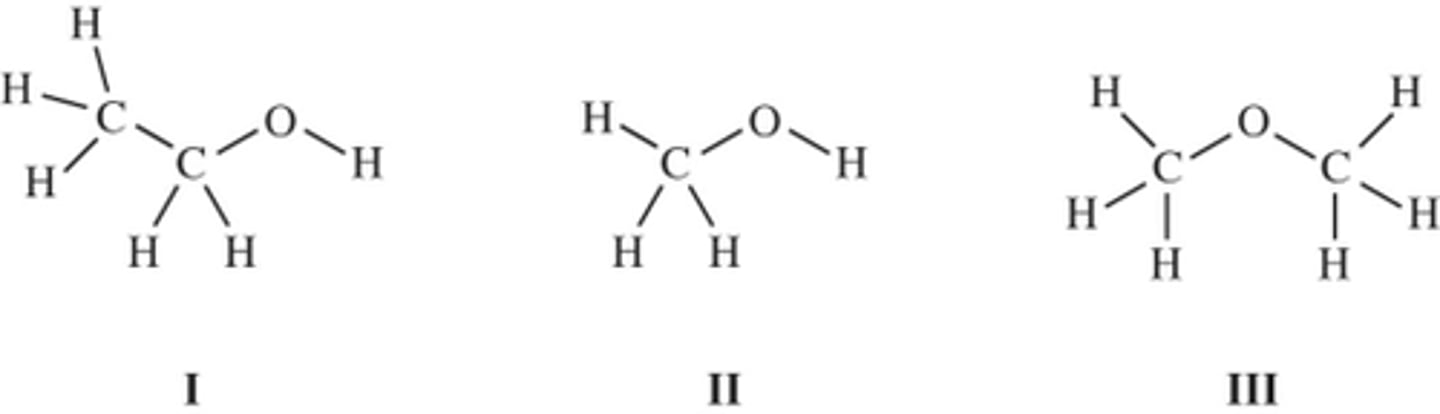
Consider these three compounds.
I and III only
During petroleum refining, catalysts play an extremely important role during the
cracking and reforming processes.