(THERMO) Important Shit to Know in Chemical Engineering
1/44
Earn XP
Description and Tags
you should know this mofo
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
Zeroth Law
If 2 systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other
First Law
Conservation of energy! Energy cannot be created or destroyed.
When energy passes into/out of a system, the system’s internal energy changes in accordance to the law of conservation of energy.
Second Law
The sum of the entropies of interacting thermodynamic systems never decreases.
Third Law
A system’s entropy approaches a constant value as the temperature approaches absolute zero. With the exception of non-crystalline solids (glasses), the entropy of a system at absolute zero is typically close to zero.
Open vs. Closed System
Closed system: exchanges ONLY energy
Open system: freely exchanges energy AND matter with its surroundings
Isentropic
constant entropy
(Also, fun fact! A reversible adiabatic process is isentropic)
Adiabatic
no heat exchange with surroundings
Isobaric
constant pressure
Polytropic Processes: What is it? It Follows What Equation?
PV^n = C
where, n is the polytropic index & C is a constant
Polytropic processes are used to model real-world applications like engines and compressors where heat transfer occurs during compression or expansion.
Open System Frist Law Equation (Generalized)
change in energy = energy in - energy out + heat transfer - net/shift work
Ideal Gas Law: Main assumptions & Equation
Assumptions: gases have no volume or intermolecular forces
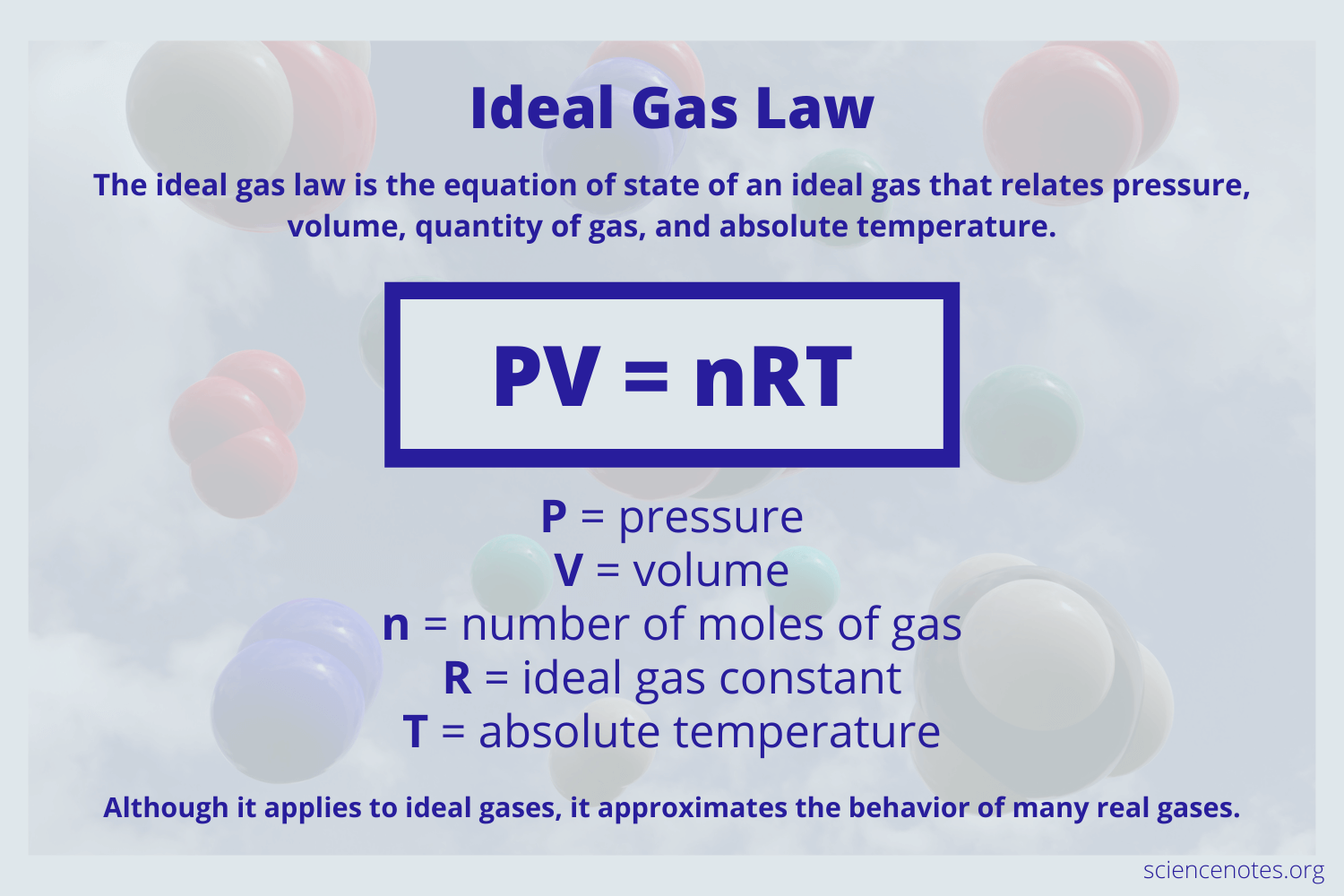
Generalized Compressibility Factor: Use & Equation
Use: modified version of ideal gas law used for REAL gases. This equation includes a correction factor, z. May be used for ALL phases.
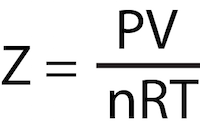
Virial EOS: Use & Equation
Use: bridge microscopic behavior of particles to macroscopic thermodynamic properties. Used to determine accurate description of REAL GASES by accounting for intermolecular interactions.
Values of B(T), C(T), etc. must be determined for EACH real gas at every temperature.
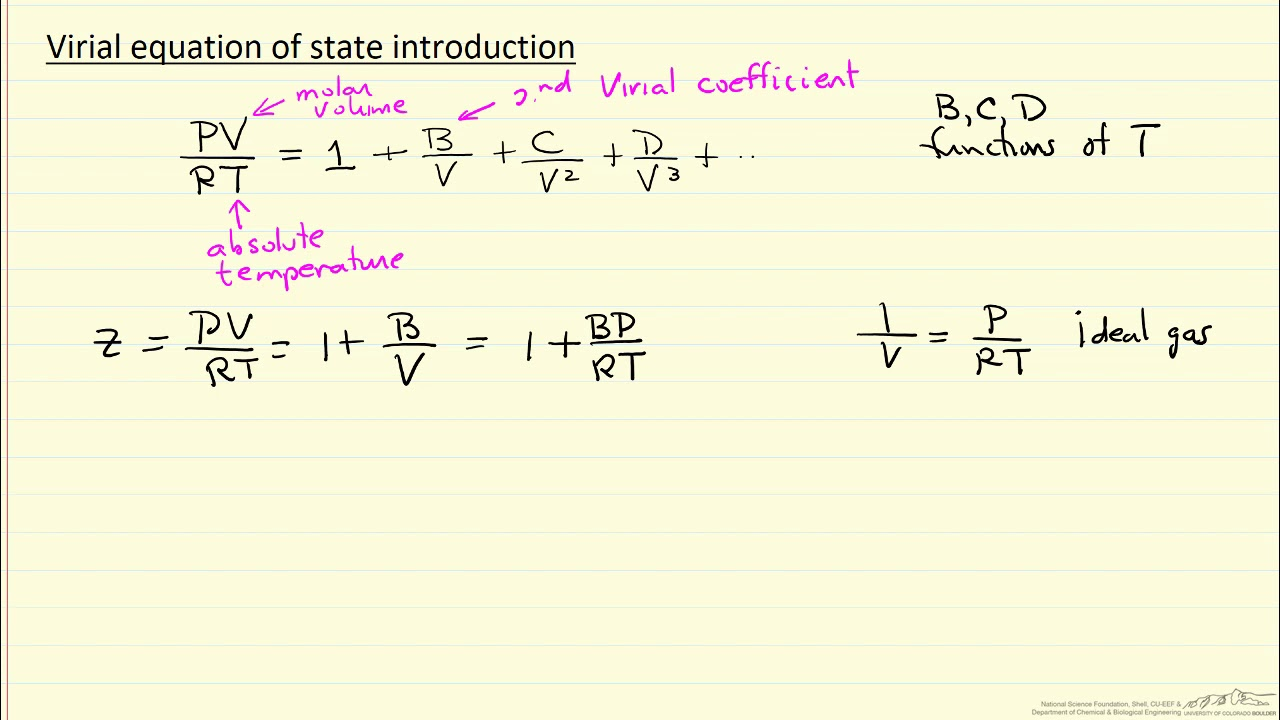
Cubic EOS (Redlich-Kwong): Use & Equation
Use: third-order polynomial that relates temperature, pressure, and volume of gases. More accurate than van der Waals and ideal gas EOS at temperature ABOVE critical temperature.
Van der Waals EOS: Use & Equation
Use: relates the density of gas/liquids to the pressure, volume, and temperature conditions.
Adjustment to ideal gas law to take into account non-zero volume of gas molecules and inter-particle attraction using correction factors a & b.
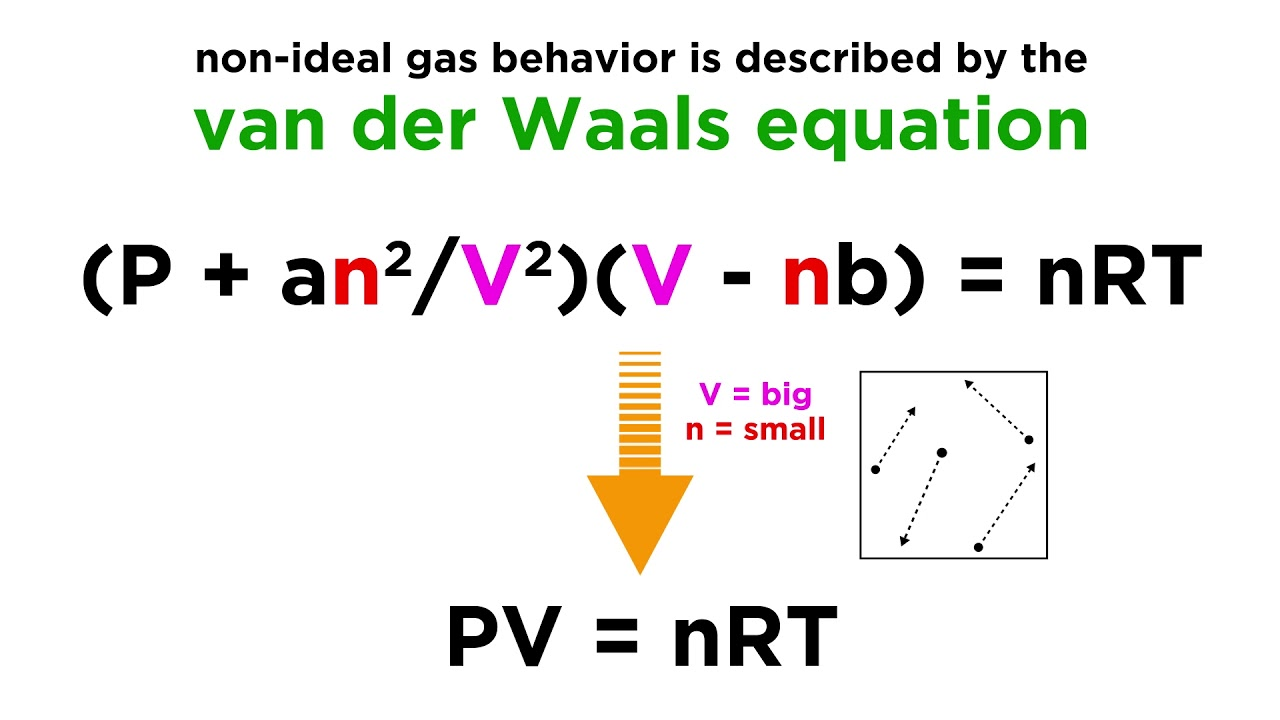
Theorem of Corresponding States
all fluids, when compared at the same reduced temperature and reduced pressure, have approximately the same compressibility factor and all deviate from ideal gas behavior to about the same degree
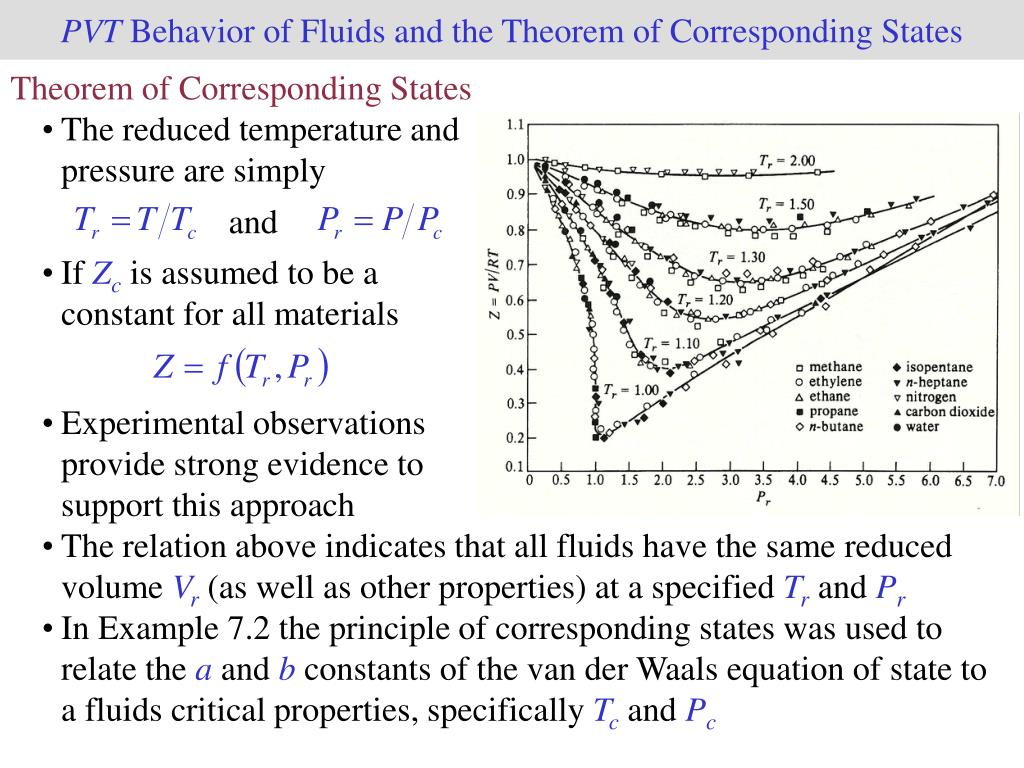
Charles’ Law
for a fixed mass of gas at constant pressure, the volume is directly proportional to its absolute temperature (V ∝ T)
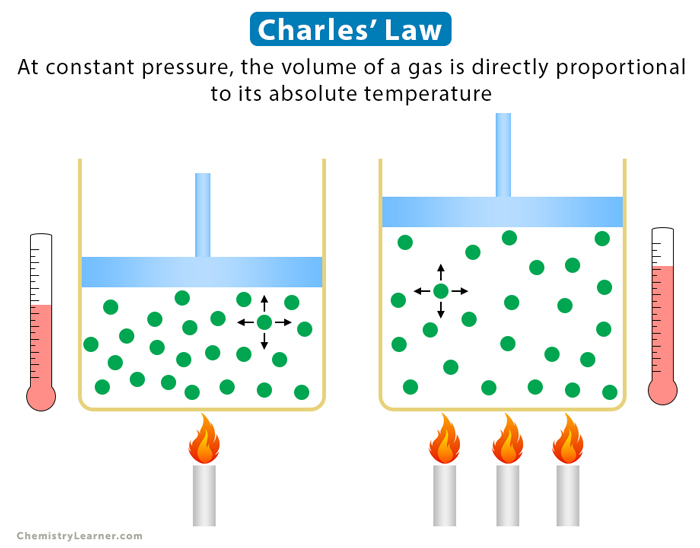
Boyle’s Law
The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain constant within a closed system
Isentropic Efficiency (η)
Real device performance vs. ideal, isentropic performance
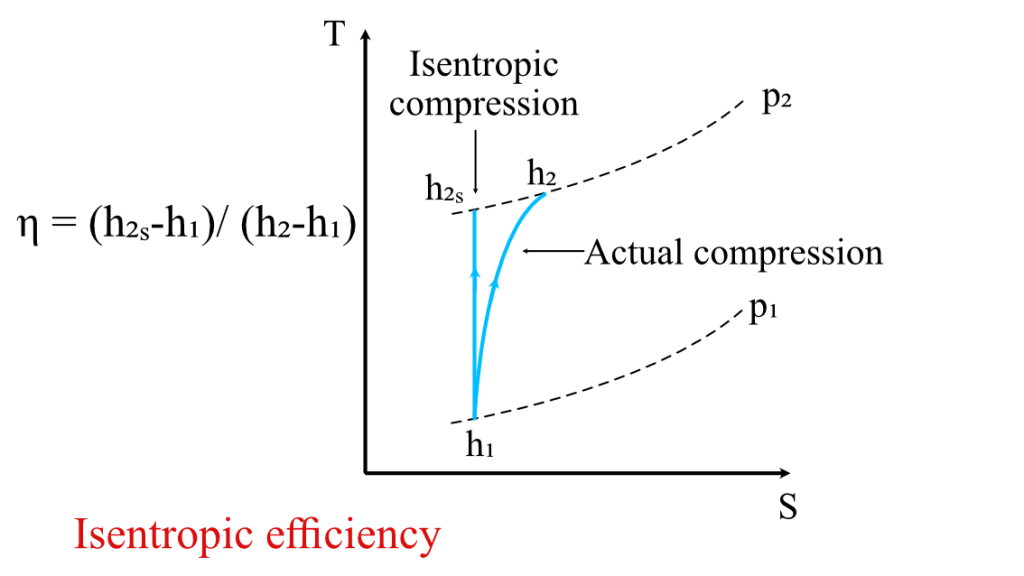
Coefficient of Performance (COP)
Useful heat/cooling output (heat moved) / required work input
Specific Humidity (ω)
(mass water vapor) / (mass dry air)
DENSITY of water in vapor
Relative Humidity (φ)
(Partial pressure water vaper) / (saturation pressure at temperature)
RATIO of water vapor in air vs. maximum amount air can hold at current temperature (percentage)… at 100% any cooling will cause water vapor to condense into a liquid
Thermal Energy Reservoirs
Body with large thermal capacity that can supply/absorb a finite amount of heat without changing its temperature (e.g. atmosphere, ocean, etc.)
Kelvin-Planck Statement
Heat engine operating on a cycle cannot convert all the absorbed heat → useful work… some heat MUST be rejected to a colder atmosphere
(Carnot Cycle has max efficiency)
Clausius’ Statement of Second Law
Heat CANNOT spontaneously flow from colder → hotter body without some external work being down ON system
(Carnot cycle has largest COP)
Gibbs
Energy released or absorbed in a reaction occurring reversibly at constant pressure and temperature

Helmholtz
Energy released or absorbed in a reaction occurring reversibly at constant volume and temperature.

Exergy
The maximum possible work that can be obtained from a cycle of a heat engine. The maximum possible work is obtained in a reversible process
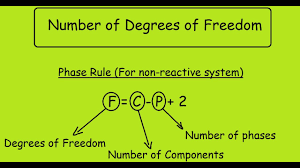
Degree of Freedom (DOF) Equation
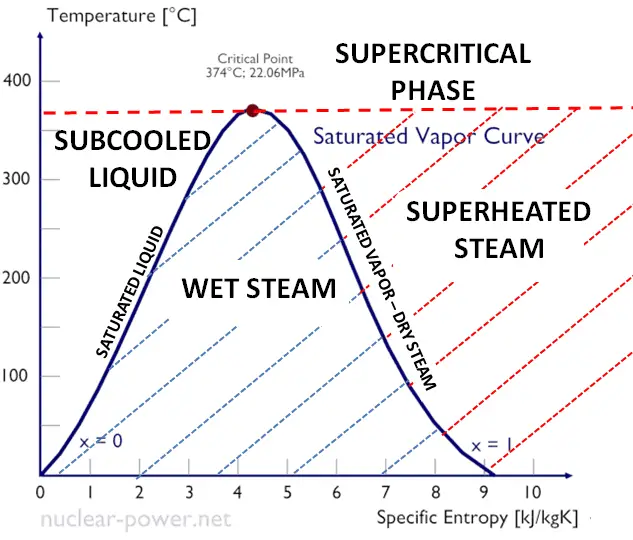
Saturated Vapor Curve (Sketch it!)
CLOSED System Energy Equation
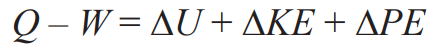
OPEN System Energy Equation
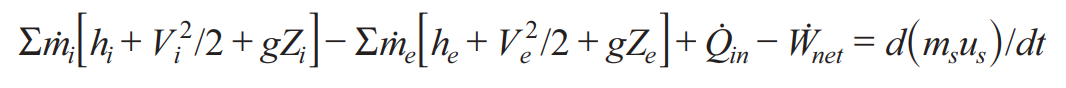
Heat
Energy transferred due to temperature difference and is considered positive if it is inward or added to the system
Work
Energy transferred when a force acts over a distance, moving energy across a system's boundary without a transfer of mass
Quality
The mass fraction of vapor in a liquid-vapor mixture
Compression Ratio
Measurement of how much a volume is compressed, typically comparing the largest volume to the smallest volume

Reversible Process
Every intermediate state between extremes is an equilibrium state, regardless the direction of change (e.g. melting ice, reversible gas expansion/compression, phase changes)
Irreversible Process
The intermediate states between extremes are not equilibrium states (e.g. mechanical work into frictional heat)
Label the following psychrometric terms: P, T, Twb, Pa, Pv, ω, φ
P: mixture of air-water mixture (normally 1atm)
T: dry-bulb temperature
Twb: wet-bulb temperature
Pa: partial pressure of dry air
Pv: partial pressure of water vapor
ω: specific humidity / humidity ratio / absolute humidity
φ: relative humidity (rh)
Label the following vapor-liquid equilibrium (VLE): h, Pi, xi, yi, P, Pi*
h: Henry’s Law constant
Pi: partial pressure of a gas in contact w/ liquid
xi: mol fraction of the gas in the LIQUID
yi: mol fraction of the gas in the VAPOR
P: total pressure
Pi*: VAPOR pressure of PURE component at the temperature of the mixture
What process? dQ = Tds
REVERSIBLE process
What process? dQ < Tds
IRREVERSIBLE process
Entropy, Enthalpy, & Internal Equations (cold air standard, constant heat capacities)

Enthalpy
thermodynamic quantity equivalent to the total heat content of a system

Entropy
thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system
