unit 2 test review NOTES
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
nuclear notation
A = mass, biggest number on top
Z = atomic #, under mass
X = name
C = charge, like an exponent
how to find atomic #?
number of protons (+) or atomic name in periodic table
how to find mass?
protons + neutrons
how to find # of neutrons?
mass - atomic #
E ↑ f ↑ λ ↓
n = 1, 2, 3..
ℓ = 0 to (n-1)
mℓ = -ℓ to ℓ
ms = ± 1/2
how to find avg mass?
add percents * mass number and divide by 100
turn percents > decimal then multiply by mass numbers
how to find percent composition of each isotope?
x + y = 1
1: replace w/ the avg mass
y = 1 - x
new: x + (1-x) = 1
isolate x
y = 1 - x
then turn percentage
subtract from 100 to get x’s percentage
paramagnetism
unpaired electrons
attracted by magnetic field
diamagnetism
paired e-
not affected by magnetic field
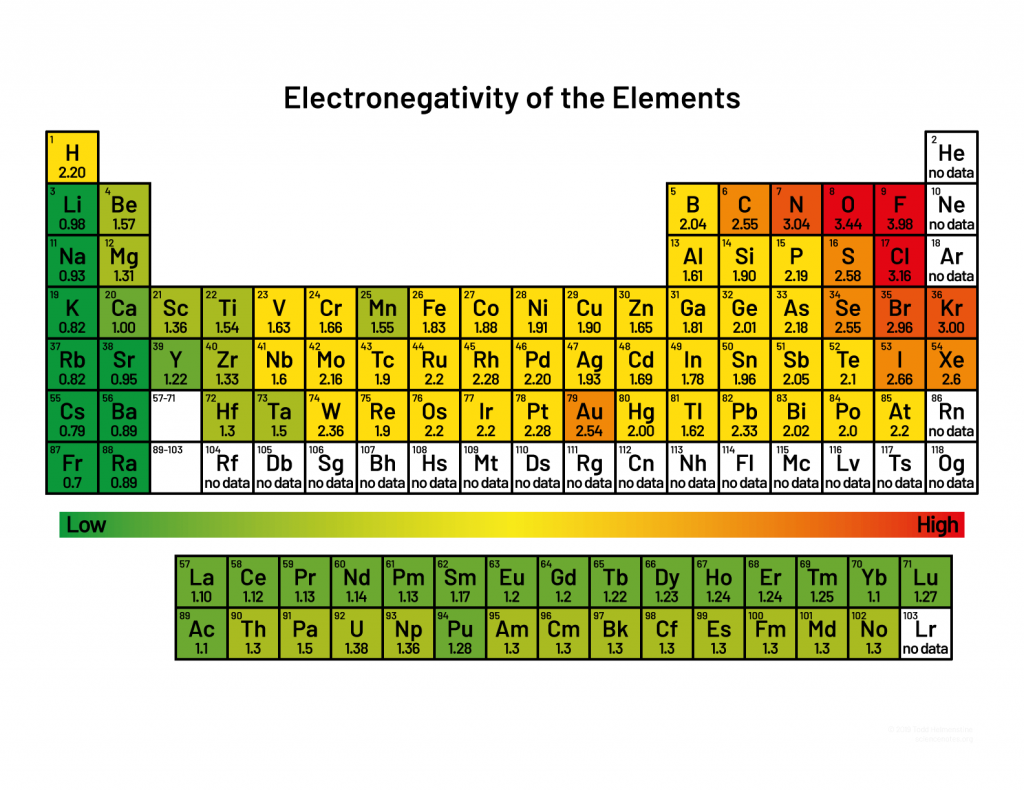
electronegativity trend
how well an element can attract electrons
halogens are the most electronegative because they have 7 valence electrons; noble gases are not electronegative
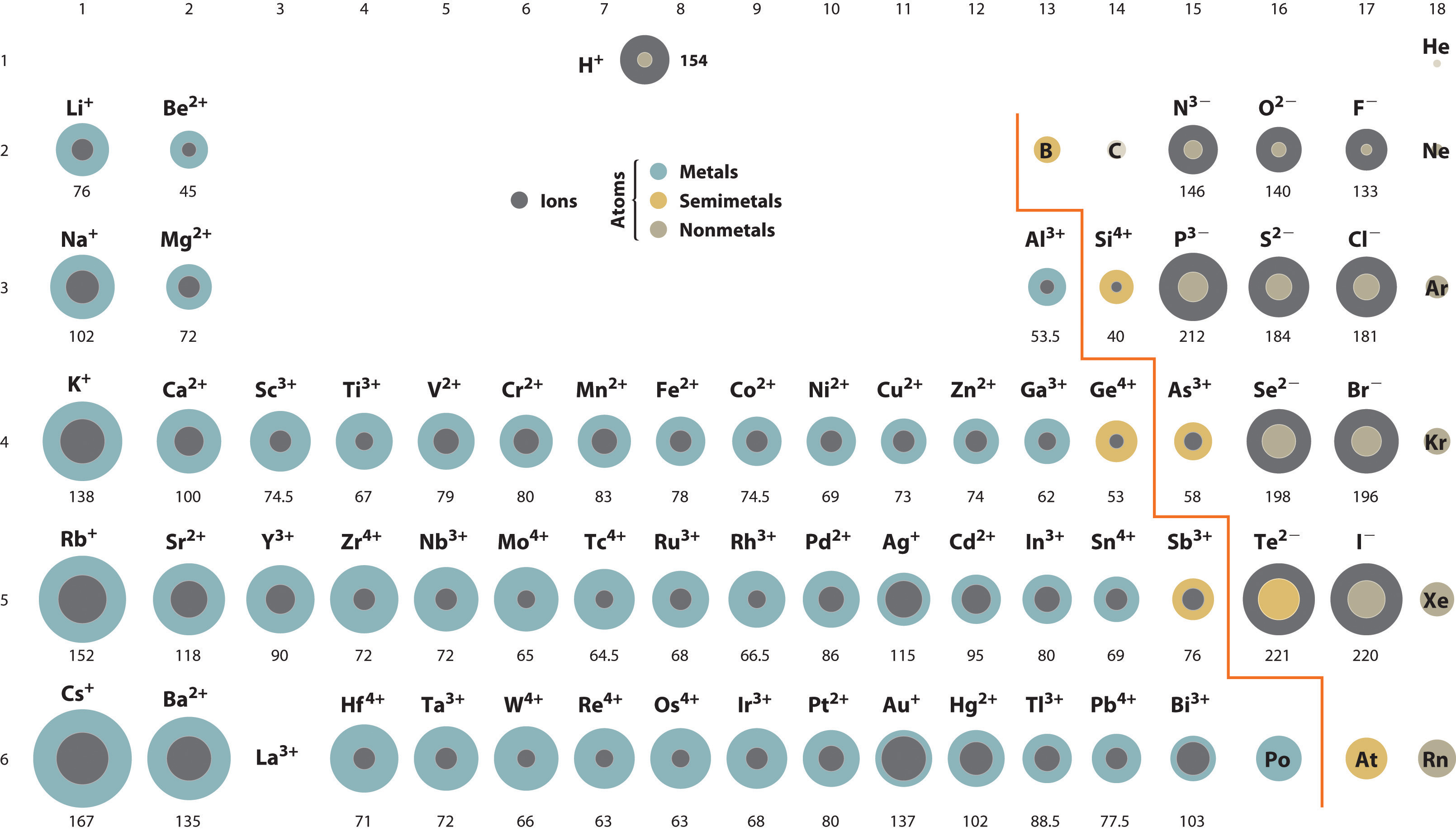
ions atomic radius
ion: element w/ a charge
largest to smallest: anions (-) » neutral » cations (+)
increases down a column
atomic radii
½ distance between nuclei
closest to Cs, Fr, Rn has higher atomic radii

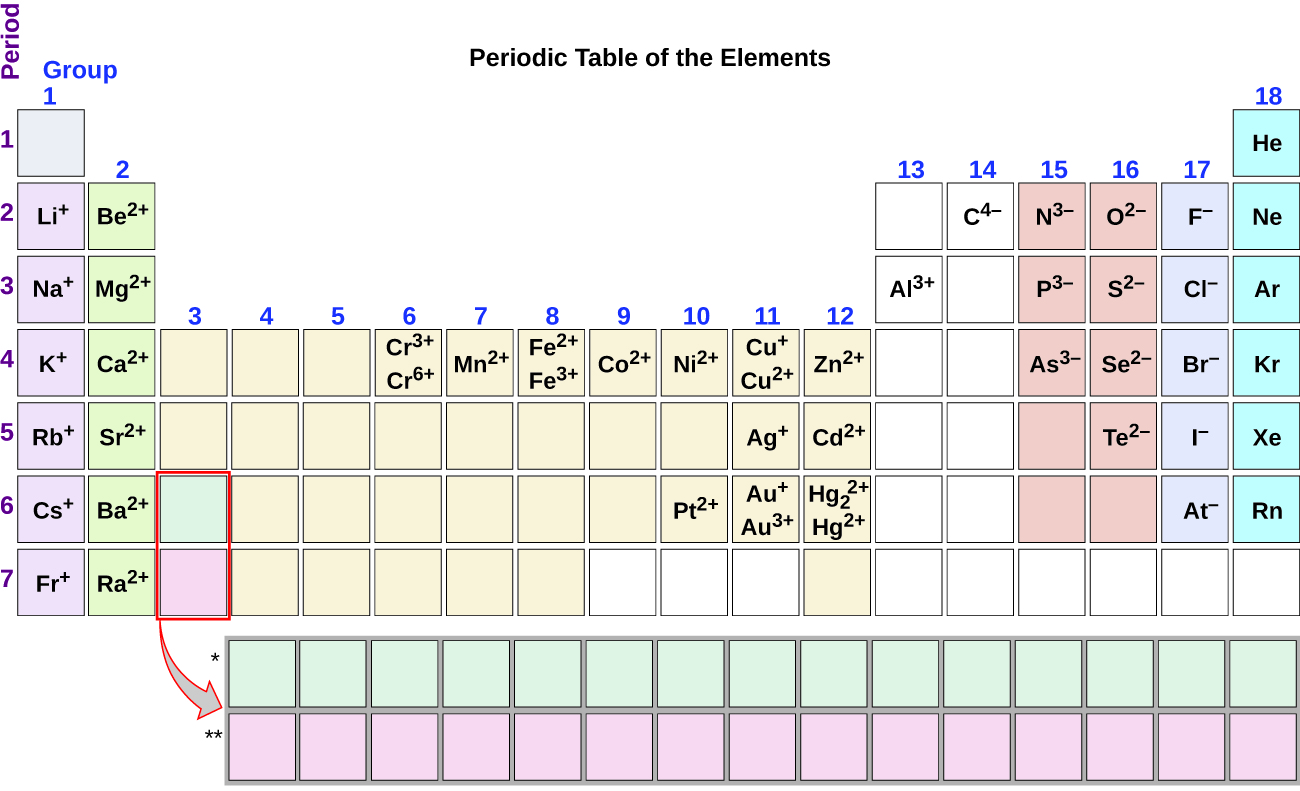
valence electrons trends

ionic charge trends
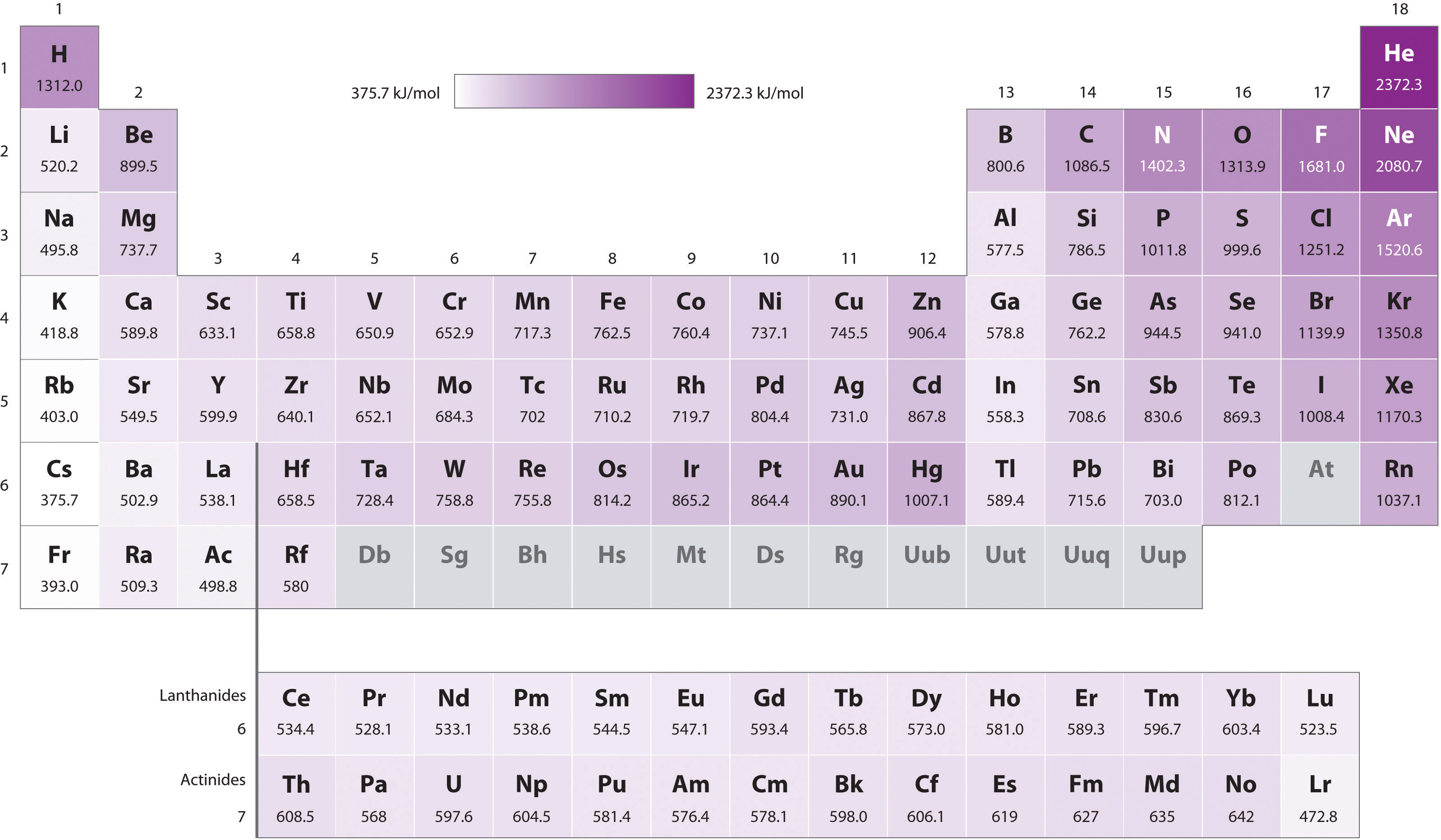
first ionization energy trends
how to find energy
E = BE + KE
E = hc/λ