Quiz 9- Chapter 20
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
You see an absorption at 1680 cm-1 in the IR spectrum of a compound. What kind of functional group is present?
An amide
Where do the carbonyl signals appear in the 13C NMR spectrum of carboxylic acid derivatives?
180–160 ppm
Why doesn't nucleophilic acyl substitution stop at the tetrahedral intermediate?
Reforming the carbonyl is energetically favorable.
Why is an amide less reactive to nucleophilic acyl substitution than an acid chloride?
Nitrogen donates more electron density into the carbonyl.
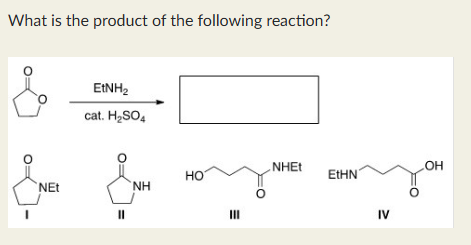
III
How can you convert a carboxylic acid into an ester?
Both heat with an alcohol and catalytic acid and deprotonate with a base and react with an alkyl halide.
How can you convert a carboxylic acid into an acid chloride?
React with thionyl chloride (SOCl2).
What is the direct product of the base-promoted hydrolysis of an ester?
A carboxylic acid salt
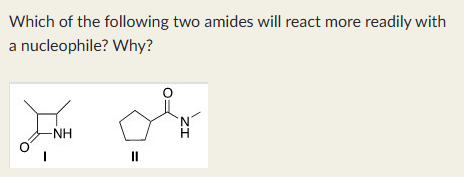
I, because it is more strained.
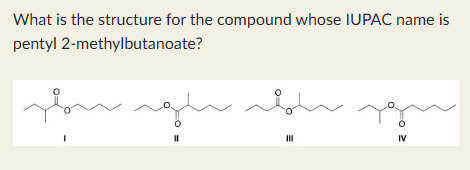
I

Phenyl propanoate
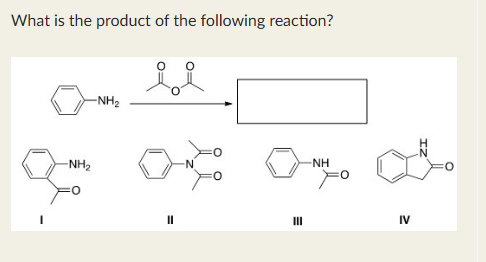
III
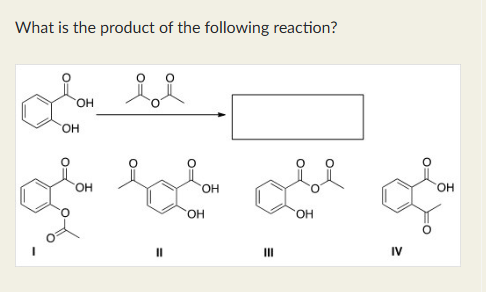
I
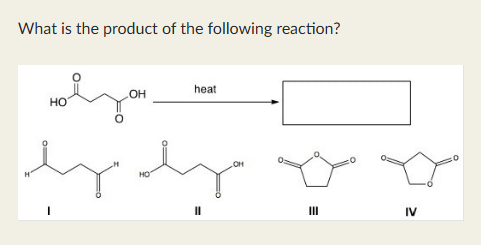
III
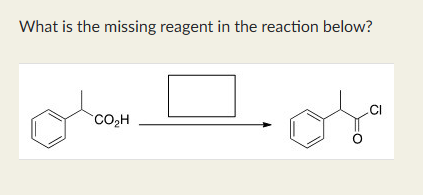
SOCl2
Which of the following reaction conditions can be used to synthesize an ester (RCOOR')?
RCOOH + R'OH + H+
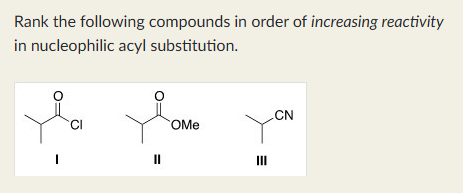
III < II < I
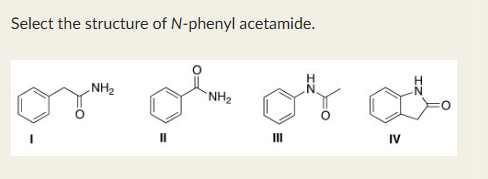
III
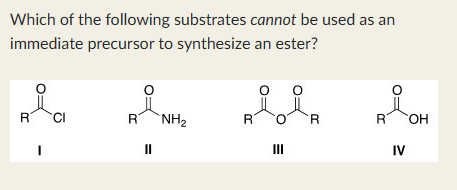
II
Which of the following statements about amides is true?
Amides are hydrolyzed in acid or base to form carboxylic acids or carboxylate anions.
All of the following contain sp2 hybridized atoms in their functional group except
a nitrile.
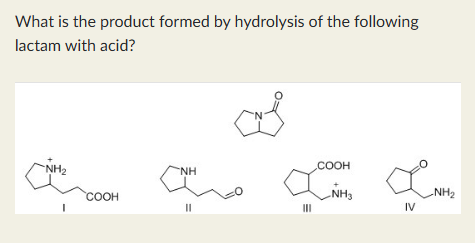
I
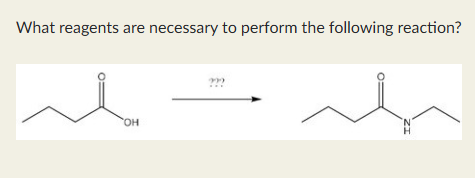
CH3CH2NH2, DCC
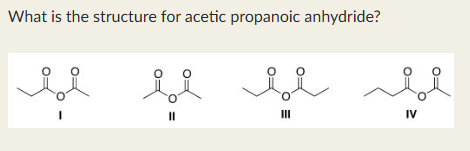
I
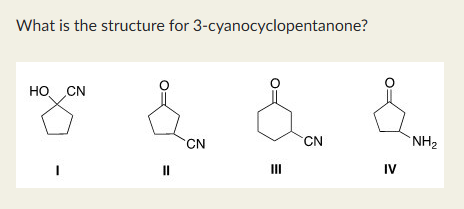
II