7.1: Quantifying Reactants and products
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Overview
Information Given by Chemical Equations
Mole-Mole Relationship
Mass Calculations
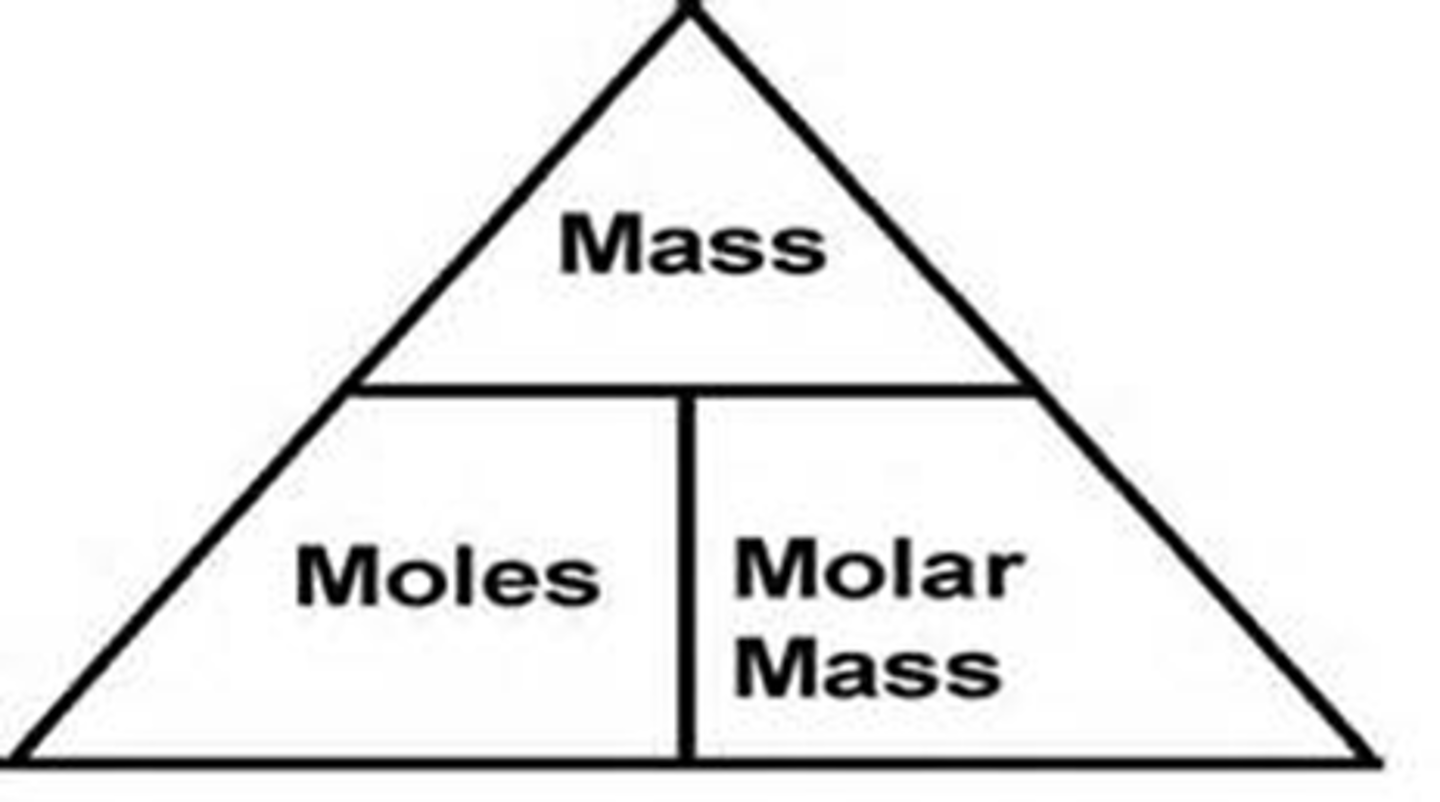
Information Given by Chemical Equations
A balanced chemical equation gives relative numbers (or moles) of reactant and product molecules that participate in a chemical reaction.
The coefficients of a balanced equation give the relative numbers of molecules.
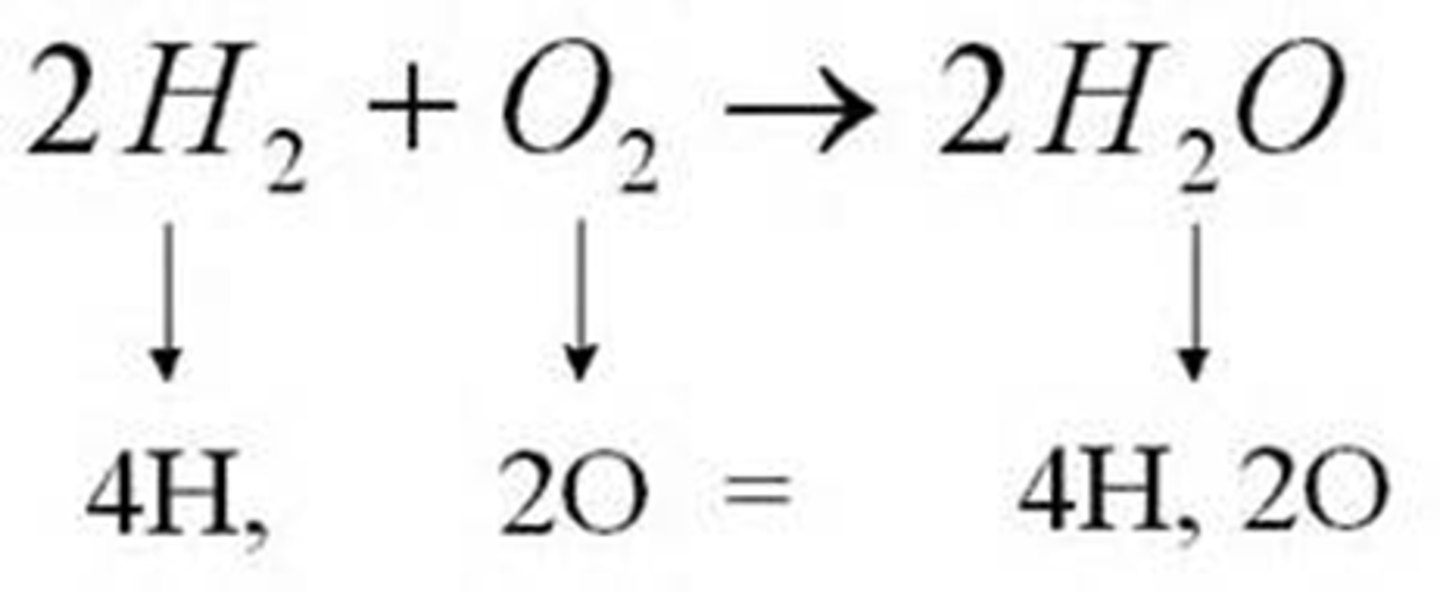
Describe the reaction: C2H5OH + 3O2 ---> 2CO2 + 3H2O
C2H5OH + 3O2 ---> 2CO2 + 3H2O
The equation is balanced.
All atoms present in the reactions are accounted for in the products.
1 molecule of ethanol reacts with 3 molecules of oxygen to produce 2 molecules of carbon dioxide and 3 molecules of water.
1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water
A balanced equation can predict the moles of product that a given number of _____ of reactants will yield.
moles
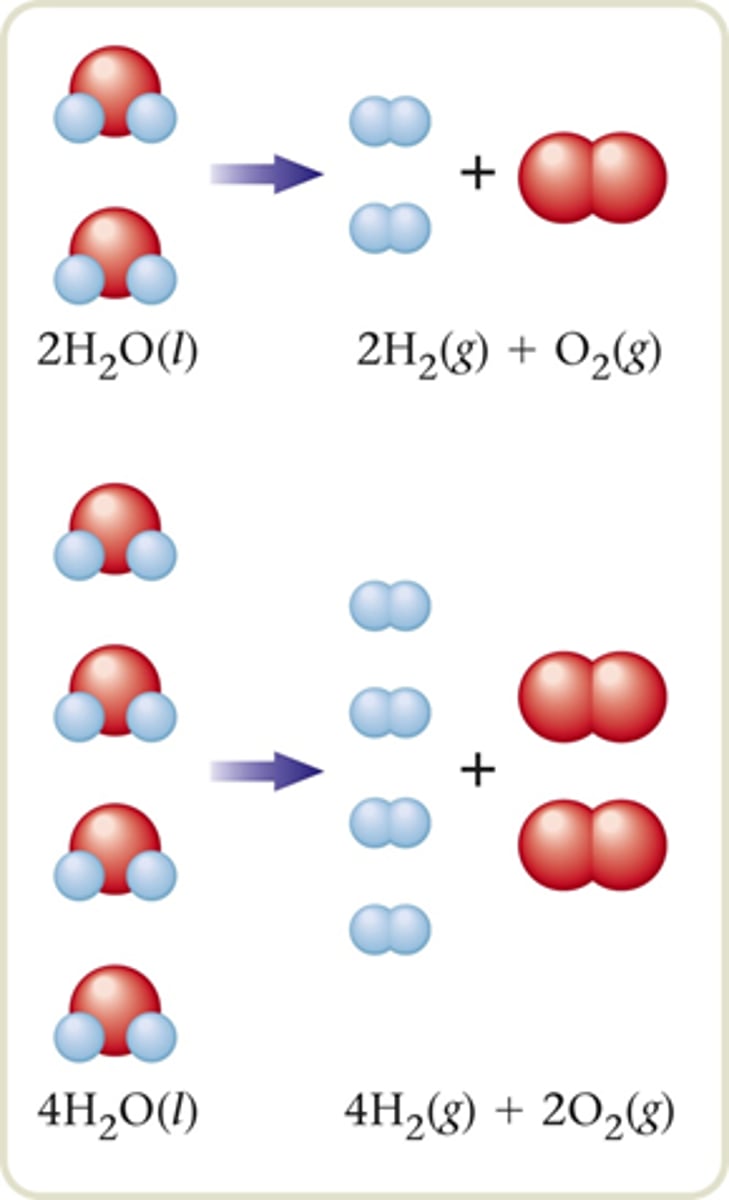
Describe the number of moles that will react and the number of moles that will be produced from the following: 2H2O(l) → 2H2(g) + O2(g)
2 mol of H2O yields 2 mol of H2 and 1 mol of O2.
4 mol of H2O yields 4 mol of H2 and 2 mol of O2.
Mole Ratio
The mole ratio allows us to convert from moles of one substance in a balanced equation to moles of a second substance in the equation.
Example
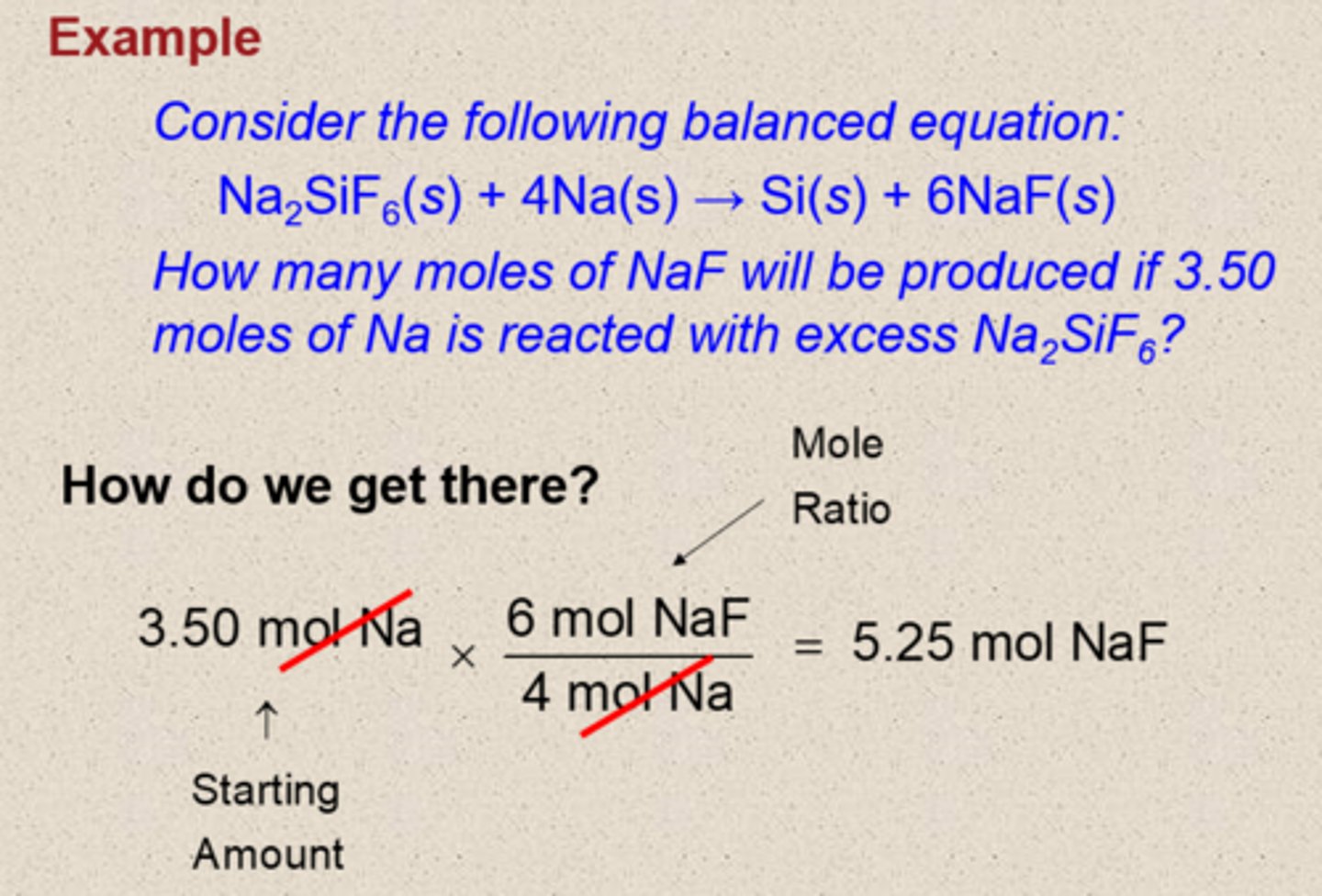
Example
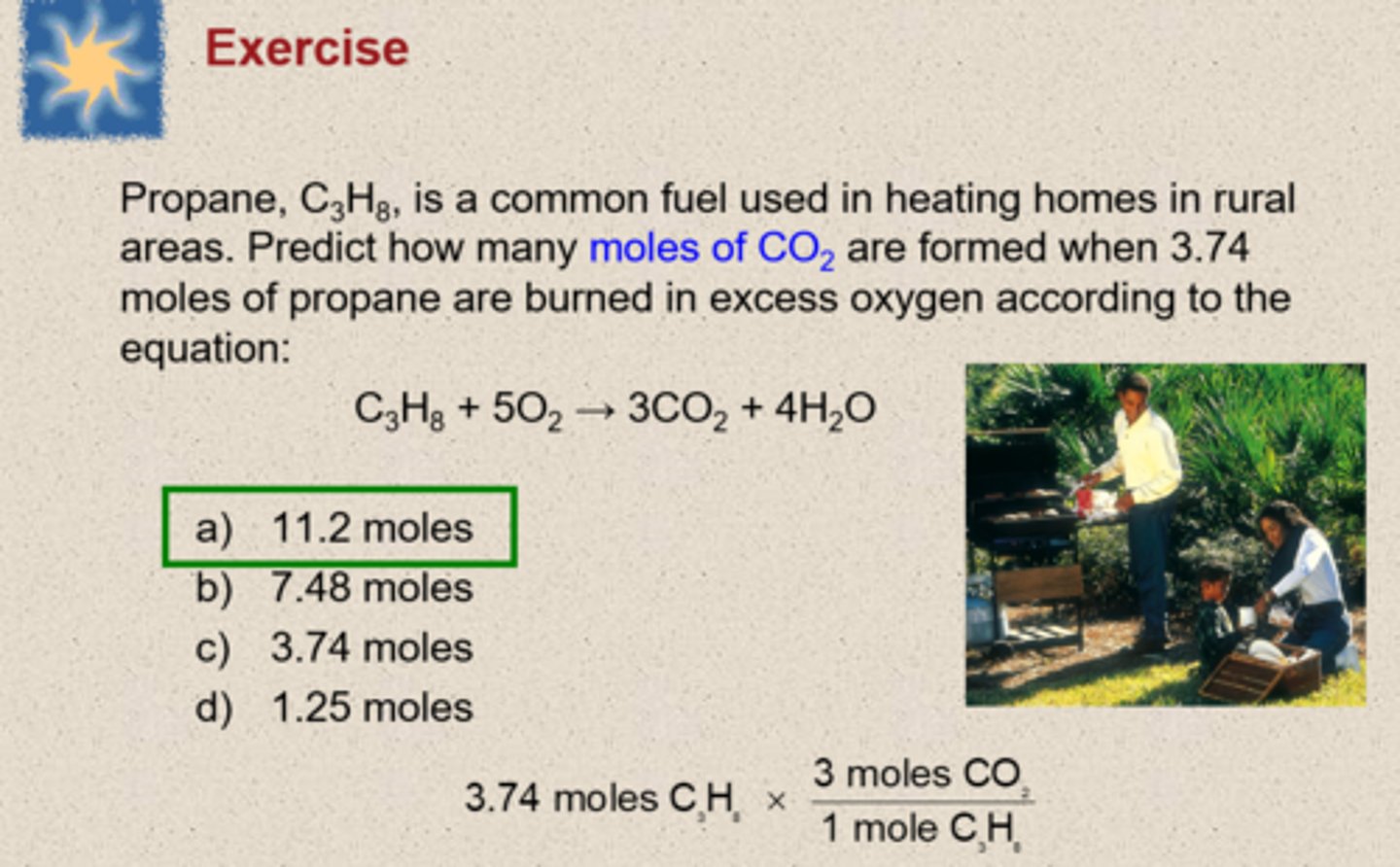
Steps for Calculating the Masses of Reactants and Products in Chemical Reactions
Steps for Calculating the Masses of Reactants and Products in Chemical Reactions
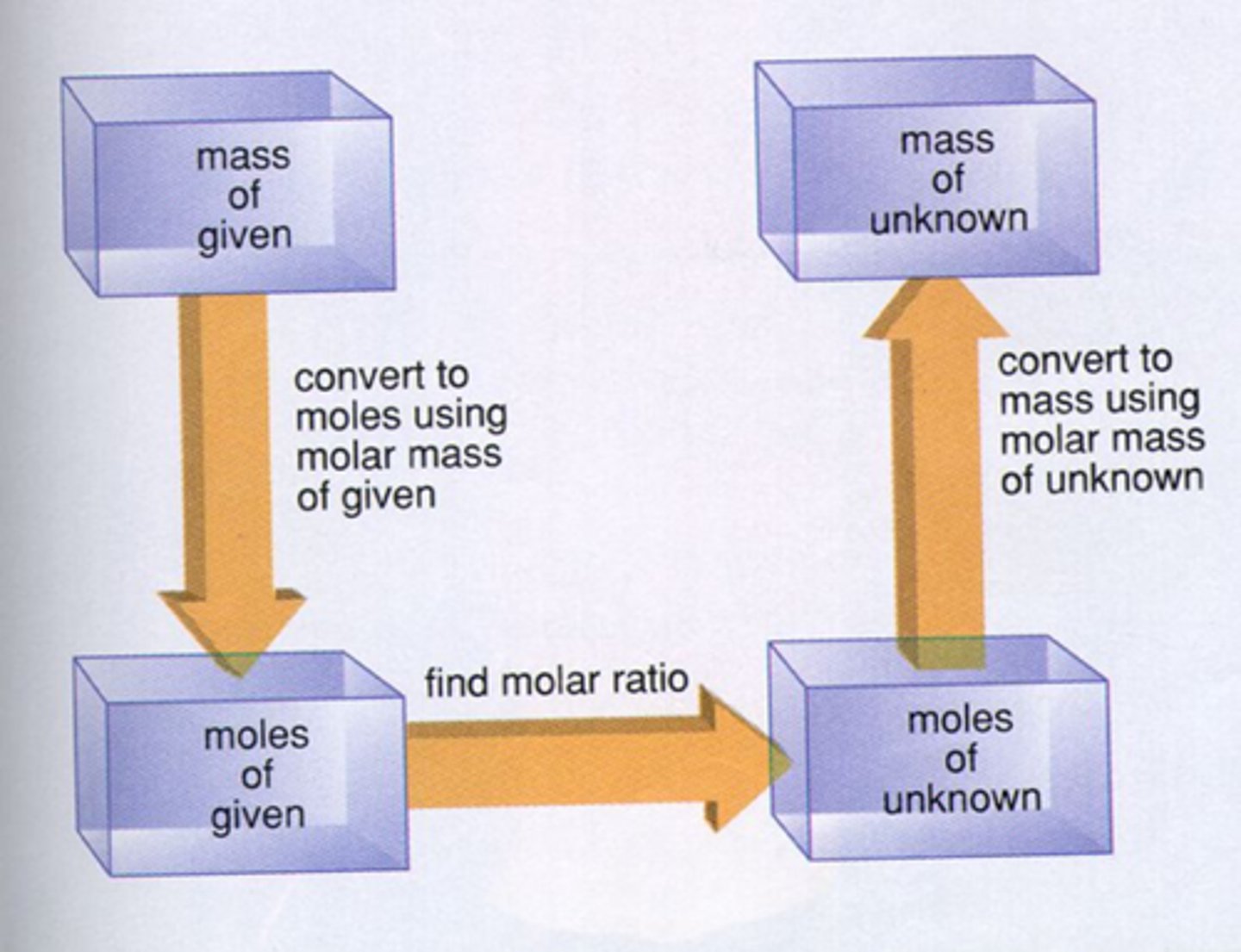
1. Balance the equation for the reaction.
2. Convert the masses of reactants or products to moles.
3. Use the mole ratio to calculate the number of moles
of the desired reactant or product.
4. Convert from moles back to masses
Stoichiometry
Stoichiometry is the process of using balanced chemical equation to determine the relative masses of reactants and products involved in a reaction.
Stoichiometry Examples
Chemical Quantities Overview
The Concept of Limiting Reactants
Calculations Involving Limiting Reactant
Percent Yield
Stoichiometric Mixture
contains the relative amounts of reactants that matches the numbers in the balanced equation.
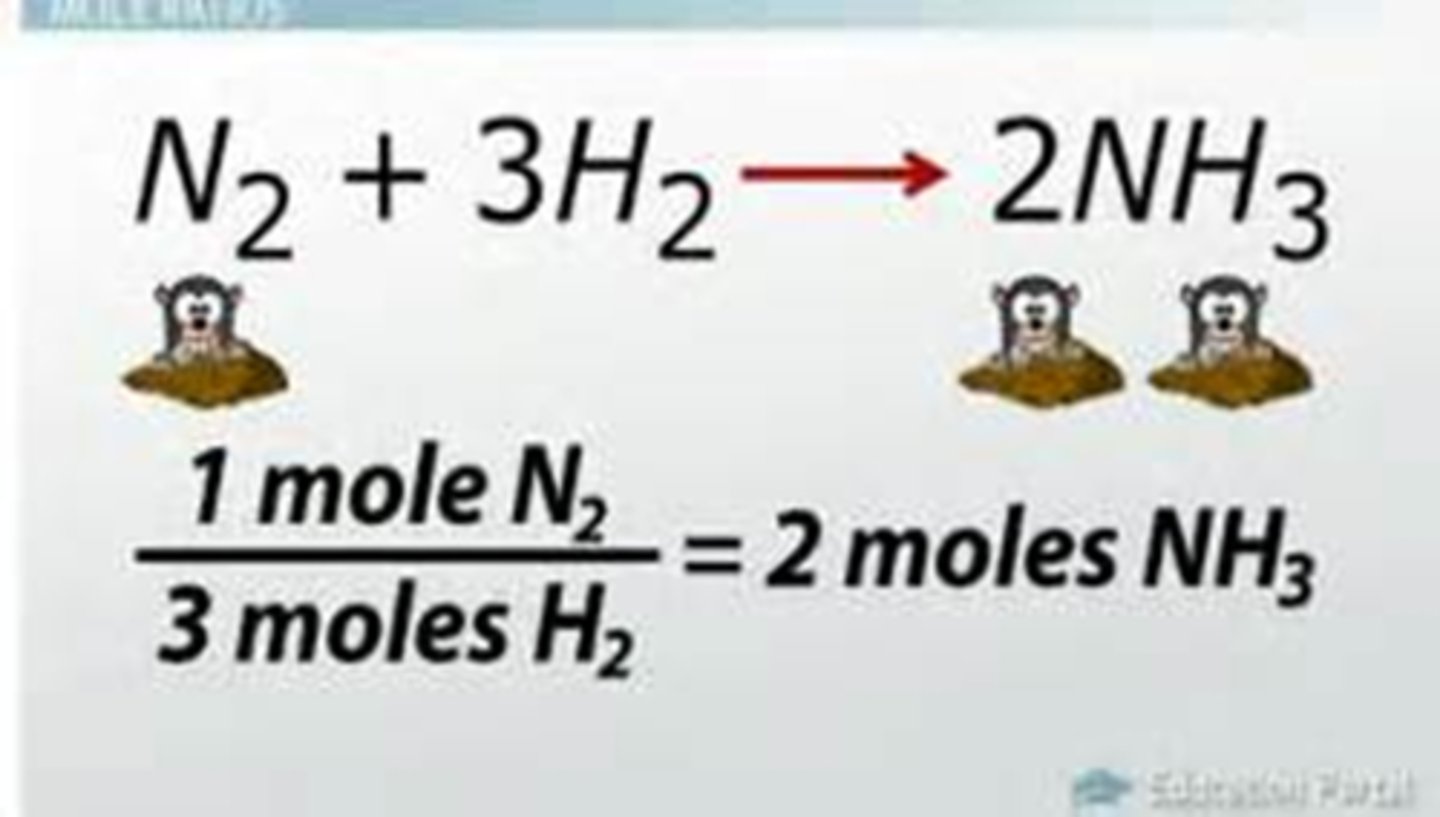
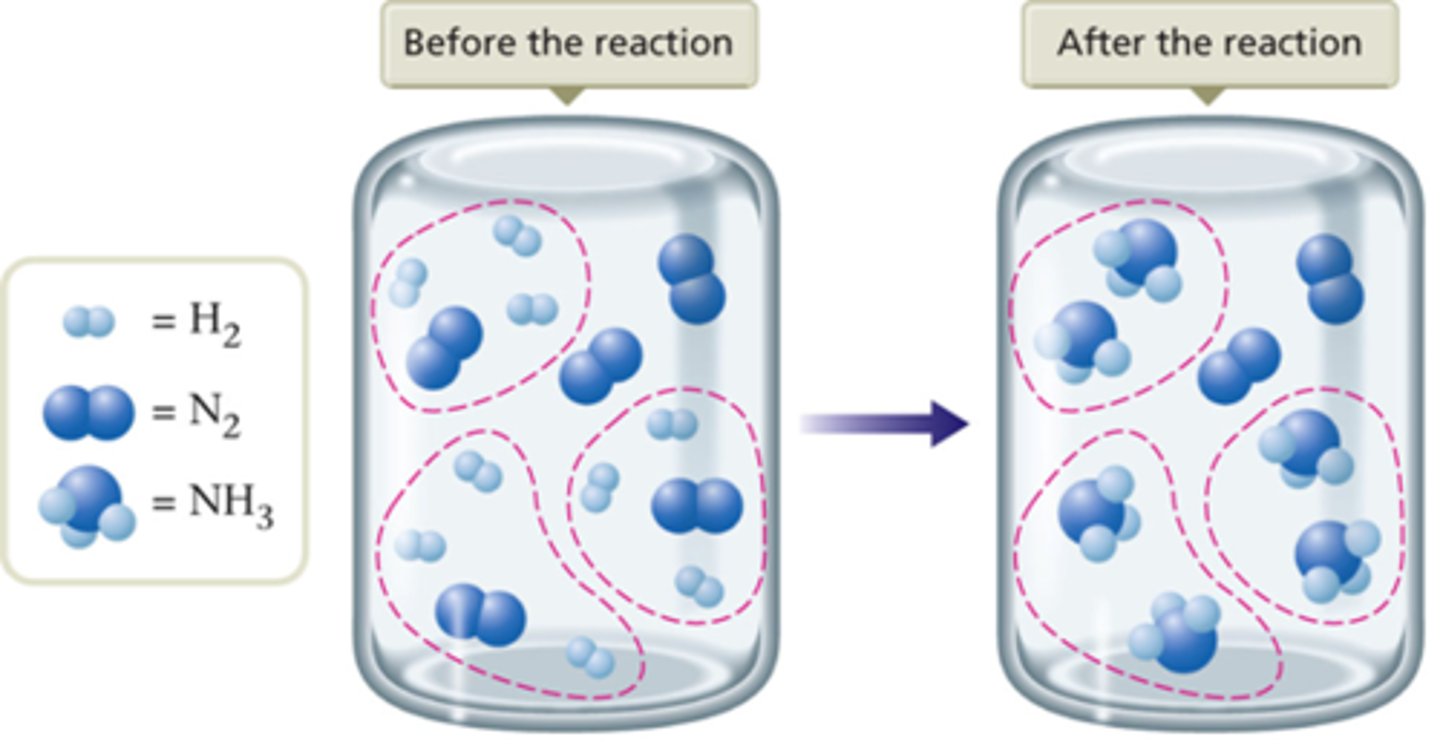
Example: N2(g) + 3H2(g) 2NH3(g)
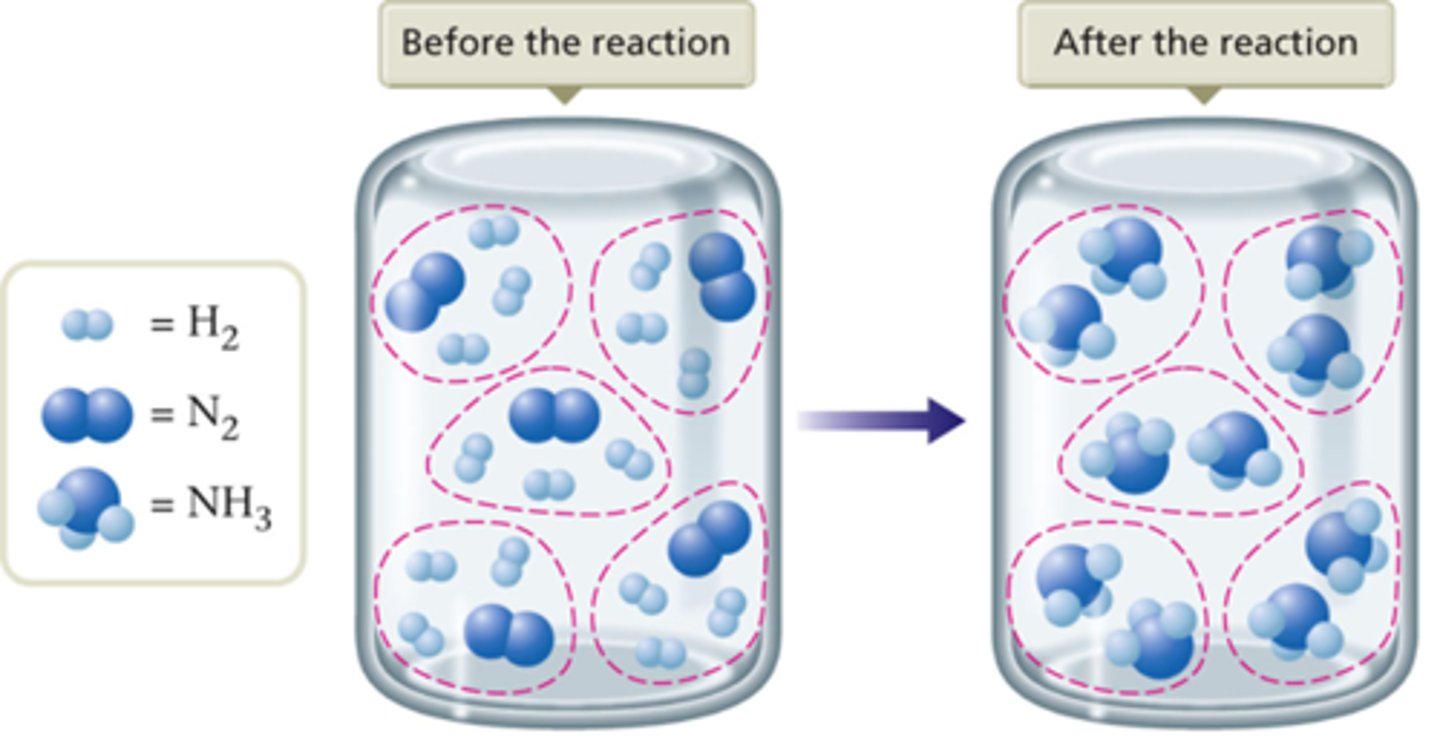
Limiting Reactant
Limiting reactant is the reactant that runs out first and thus limits the amounts of products that can form.
What is the limiting reactant of the following equation?
N2(g) + 3H2(g) ---> 2NH3(g)
H2
What is the limiting reactant of the following equation?
CH4 + H2O ---> 3H2 + CO
H2O is used up first, leaving 2 unused CH4 molecules unreacted.
The amount of products that can form is limited by water.
Water is limiting reactant.
Methane is in excess.
Steps for Solving Stoichiometry Problems with Limiting Reactants
1. Write and balance the equation of the reaction.
2. Convert the known masses of reactants to moles.
Using the numbers of moles of reactants and the appropriate mole ratios, determine which is limiting
3. Use the amount of the limiting reactant and the appropriate mole ratios, compute the number of moles of desired product.
5. Convert from moles of product to grams of product, using molar mass (if this is required by problem).
Examples
Percent Yield
An important indicator of the efficiency of a particular labratory or industrial reaction.
Theoretical yeld
The theoretical yield is the maximum amount of a given product that can be formed when the limiting reactant is completely consumed.
Actual yield
The actual yield(amount actually produced) of a reaction is usually less than the theoretical yield
Percent yield
The percent yield is the actual amount of a given product as the percentage of the theoretical yield.
Formula
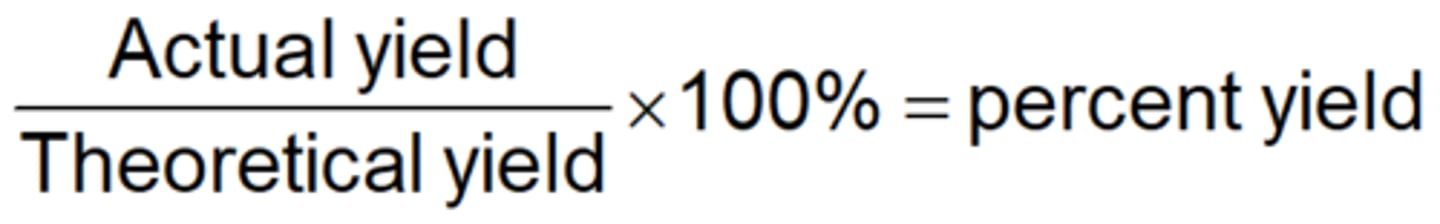
Example: We determined that 8.33 g C should be produced. 7.23 g of C is what was actually made in the lab. What is the percent yield of the reaction?
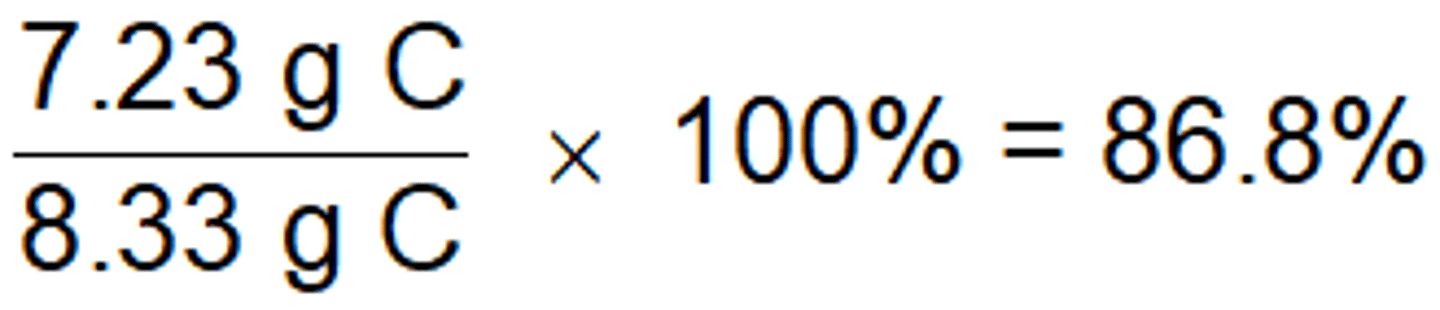
Example

To calculate amounts of reactants or products, you need a balanced chemical equation, a periodic table, and one known quantity.
1. Balance the given chemical equation.
2. Find the appropriate mole ratio from the balanced equation (product-to-reactant, reactant-to-product, reactant-to-reactant).
3. Convert the given quantity from units (molecules, mass, volume) to moles.
4. If you are calculating products from reactants, you may need to determine which reactant may be limiting.
5. Use the given molar quantity and the mole ratio to find moles of the unknown quantity.
6. Convert the moles of the unknown quantity to the desired units (molecules, mass, volume).
When aluminum oxidizes in air, it forms aluminum oxide (Al2O3):
4Al (s) + 3O2 (g) → 2Al2O3 (s)
If a 54 g sheet of aluminum foil oxidizes completely in excess oxygen, how many grams of aluminum oxide will be formed?
First determine if the equation is balanced.
~ Yes it is
Find the appropriate mole ratio from the balanced equation.
~ 4 : 2, or 2 : 1
Determine the molar mass of Al
~ (Periodic Table Needed) 26.982 or 27 g/mol
Determine the number of moles of the reactant that you were given.
~ ( Get rid of gAl in equation) 54 gAL* 1molAL/ 27 gAl= 2 MolAl
Determine the number of moles of the product that you will get.
~ ( Get rid of molAl) 2 molAl* 1Mol Al2O3/ 2 molAl= 1molAl2O3
You have 1 mol of Al2O3
Convert moles of product to mass. The molar mass of Al2O3 equals 102 g/molAl2O3=102 g/mol.
~ (Get rid of molAl2O3) 1molAl2O3* 102g Al2O3/ 1 molAl2O3= 102g of Al2O3