chymotrypsin
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Chymotrypsin
Hydrolyzes dietary proteins into small peptides and amino acids for absorption by the small intestine
N terminal half
Carboxyl component
C terminal half
Amino component
Optimal activity of chymotrypsin
At pH 8
Specificity of chymotrypsin
Cleaves preferentially on the carboxylic terminal side of large hydrophobic side chains (Phe, Met, Ile, Trp, Tyr)
Specificity pocket of chymotrypsin
Hydrophobic therefore prefers hydrophobic side chains
Catalytic triad of chymotrypsin
Asp 102, His 57, Ser 195
Asp 102
Plays a roles in orienting His 57 side chain through hydrogen bonding and electrostatic effects (in its deprotonated form, -ve)
His 57
Acts as a general acid/base catalyst to initially withdraw a proton from the Ser 195 side chain to generate a nucleoside
See 195
Acts as a nucleotide to attach a substrate
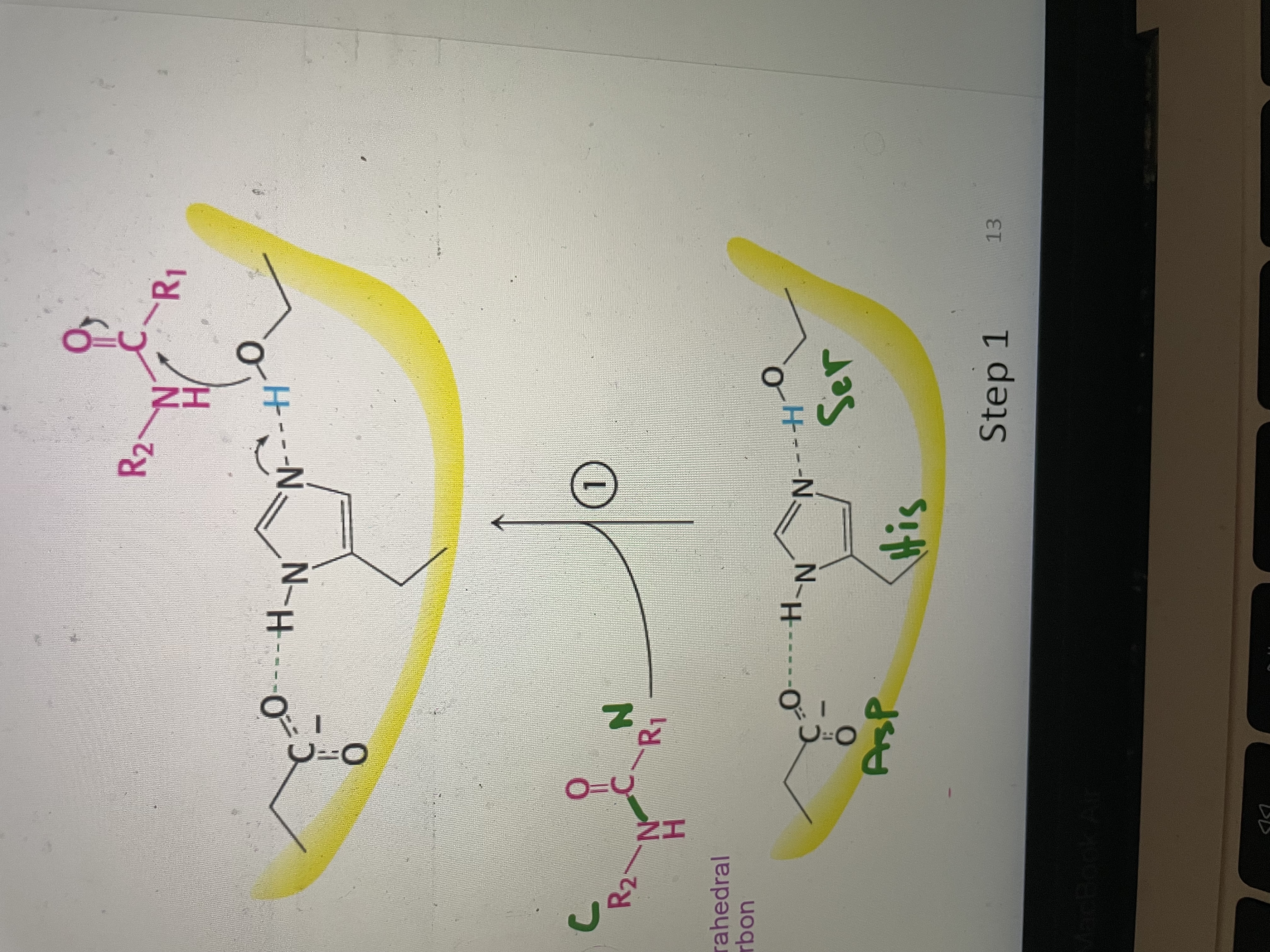
Peptide hydrolysis part 1
Substrate binds to active site
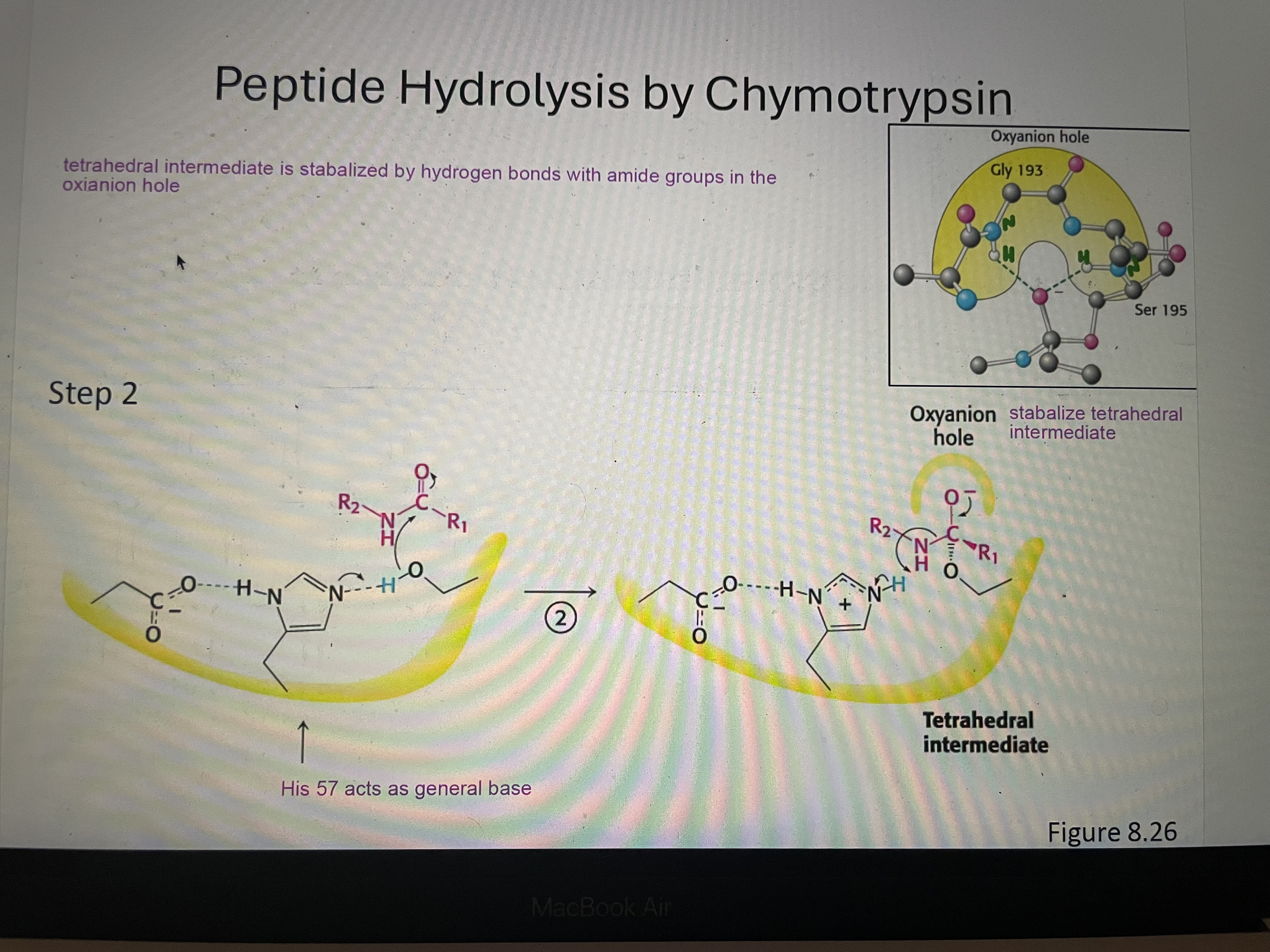
Peptide hydrolysis part 2
Tetrahedral intermediate is stabilized by hydrogen bonds with amide group in the oxianion hole. His 57 acts as general base (give electrons)
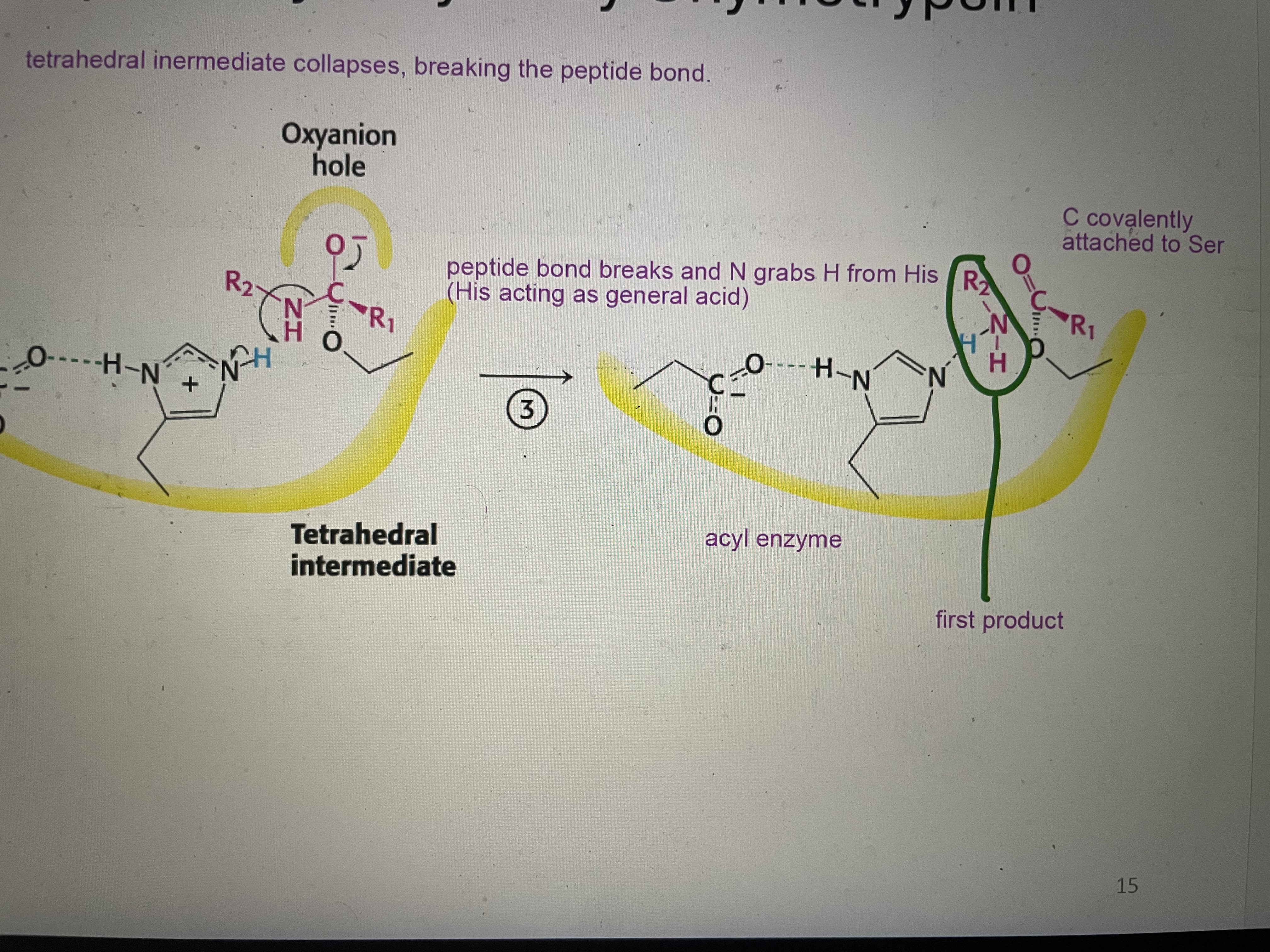
Peptide hydrolysis part 3
Tetrahedral intermediate collapse breaking the peptide bond and N grabs H from His. C is covalently attached to Ser
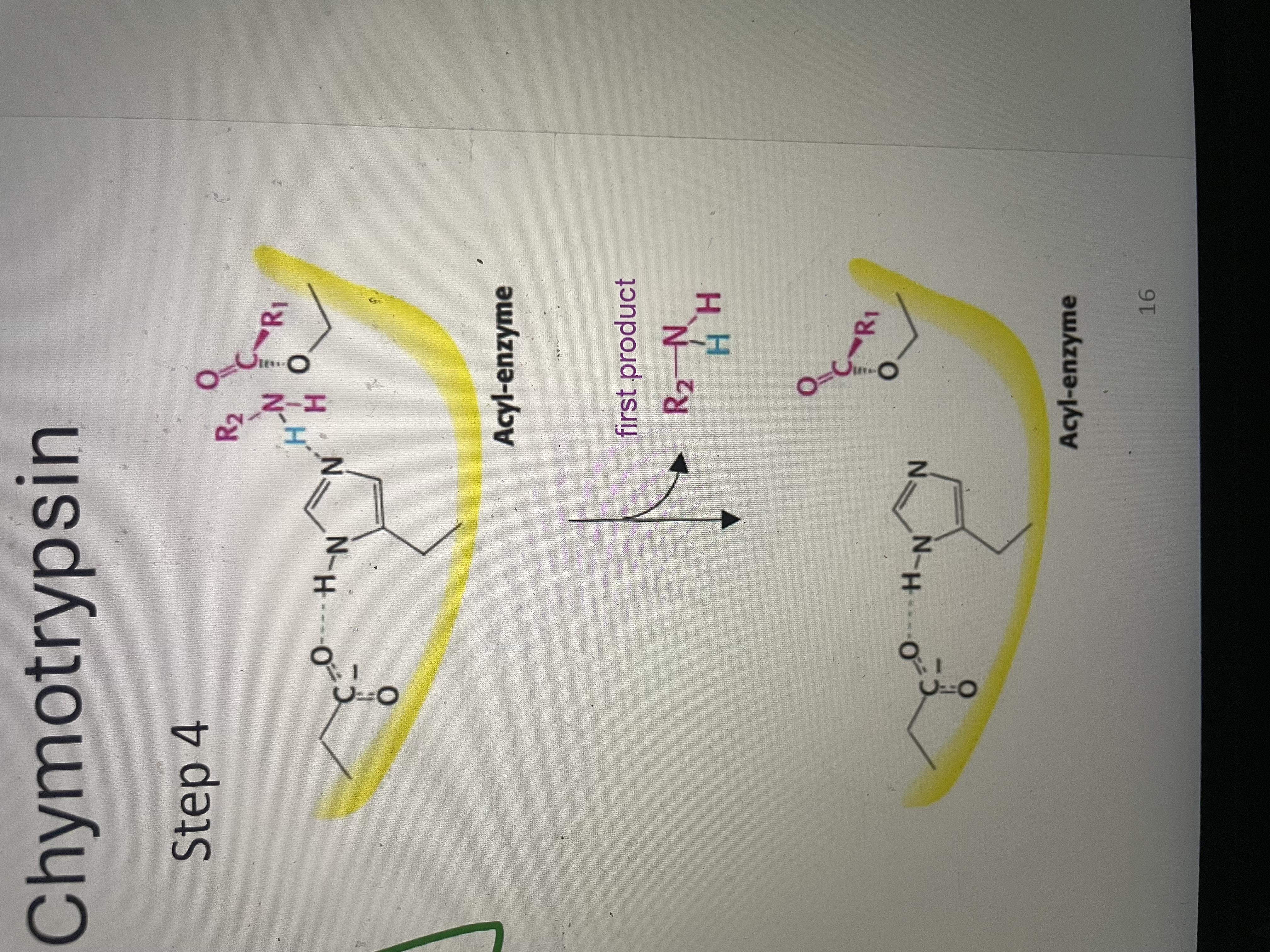
Peptide hydrolysis part 4
First product is released - amino component. Enzyme is now acyl enzyme with carboxyl component still covalently attached to Ser
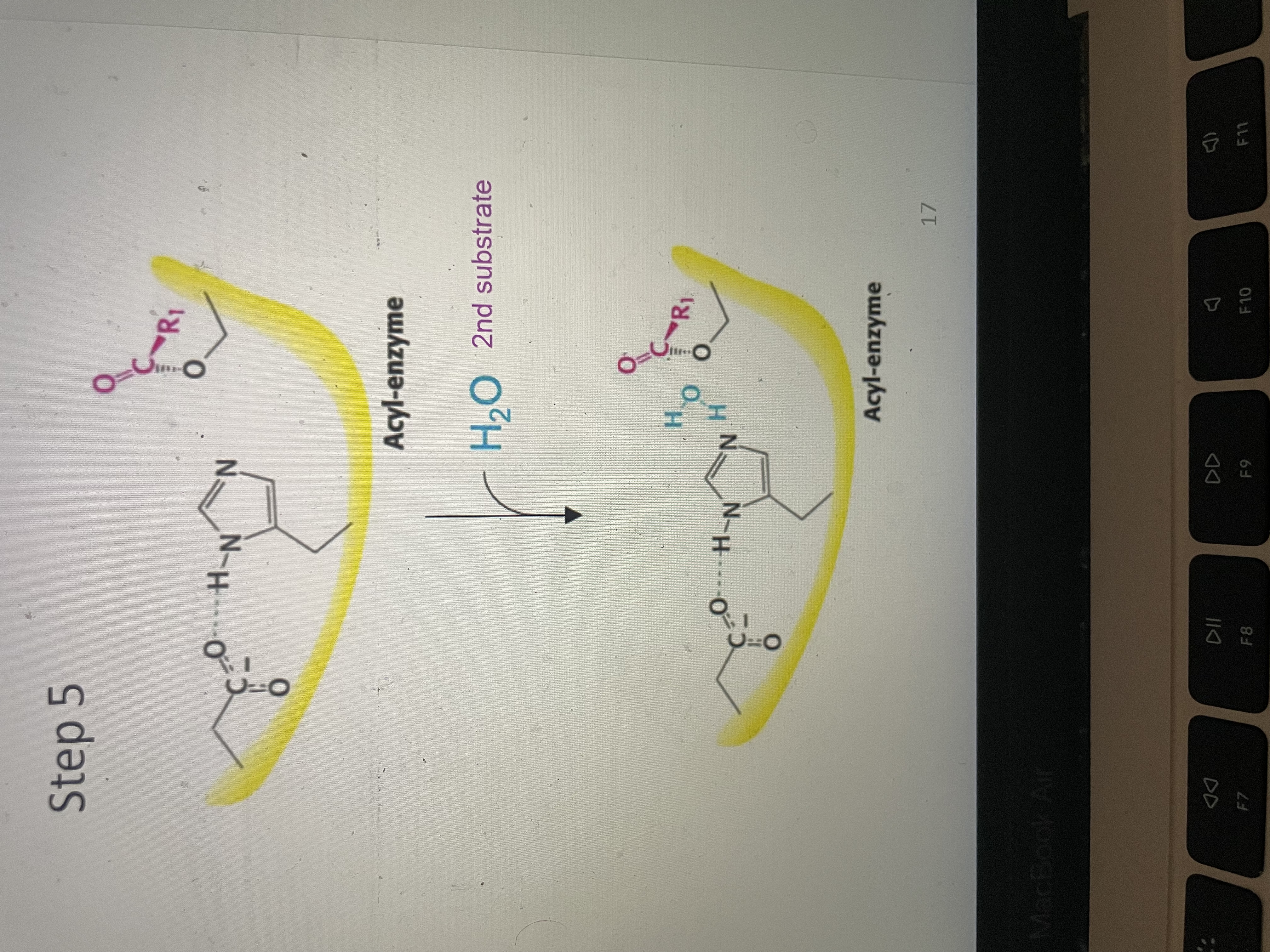
Peptide hydrolysis part 5
Second substrate (water) binds to acyl enzyme
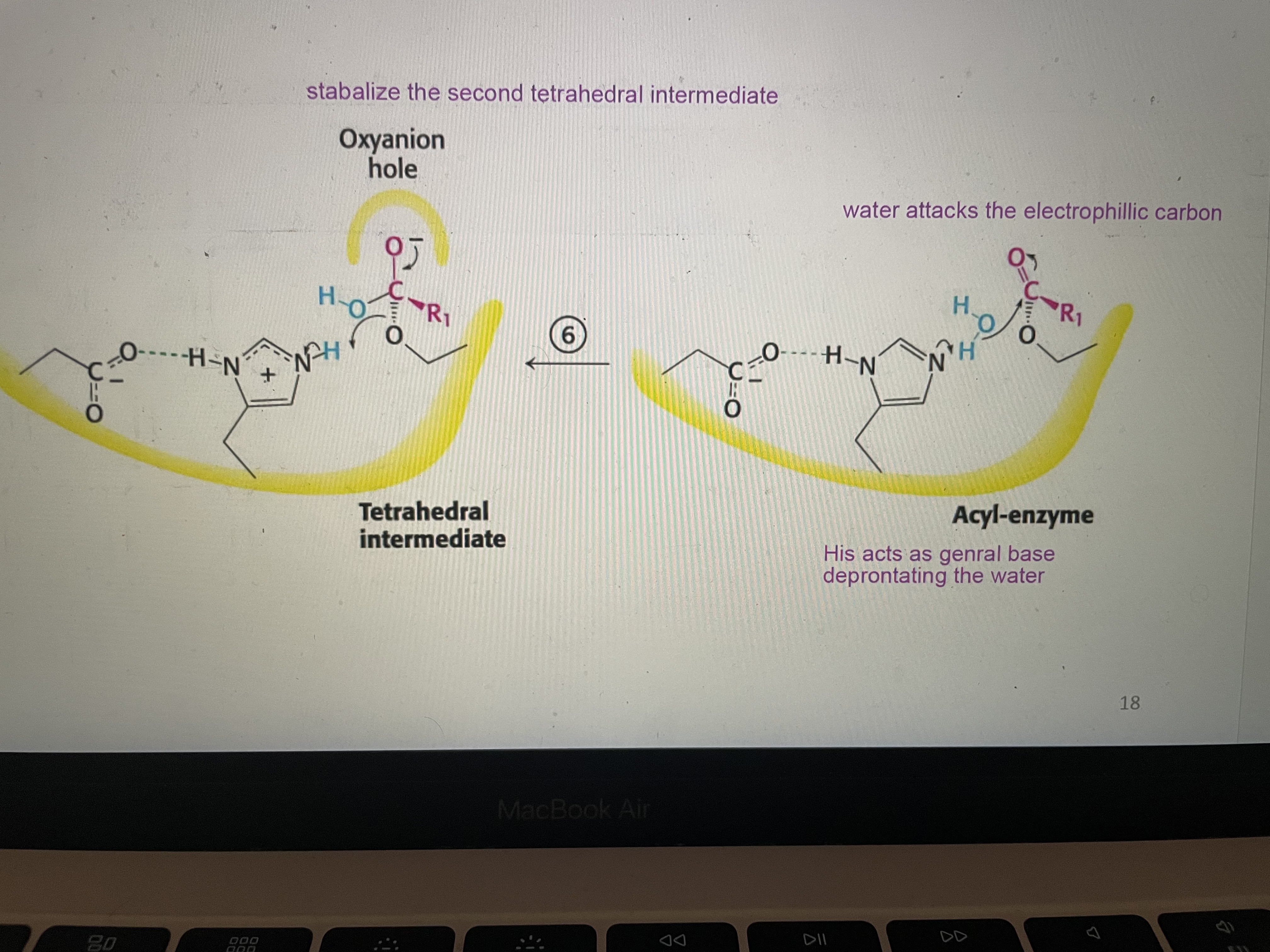
Peptide hydrolysis part 6
His 57 deprotonates the water to generate a nucleophile, acting as a general base. Water attacks the electrophillic carbon forming the second tetrahedral intermediate that is stabalized by hydrogen bonds in the oxianion hole
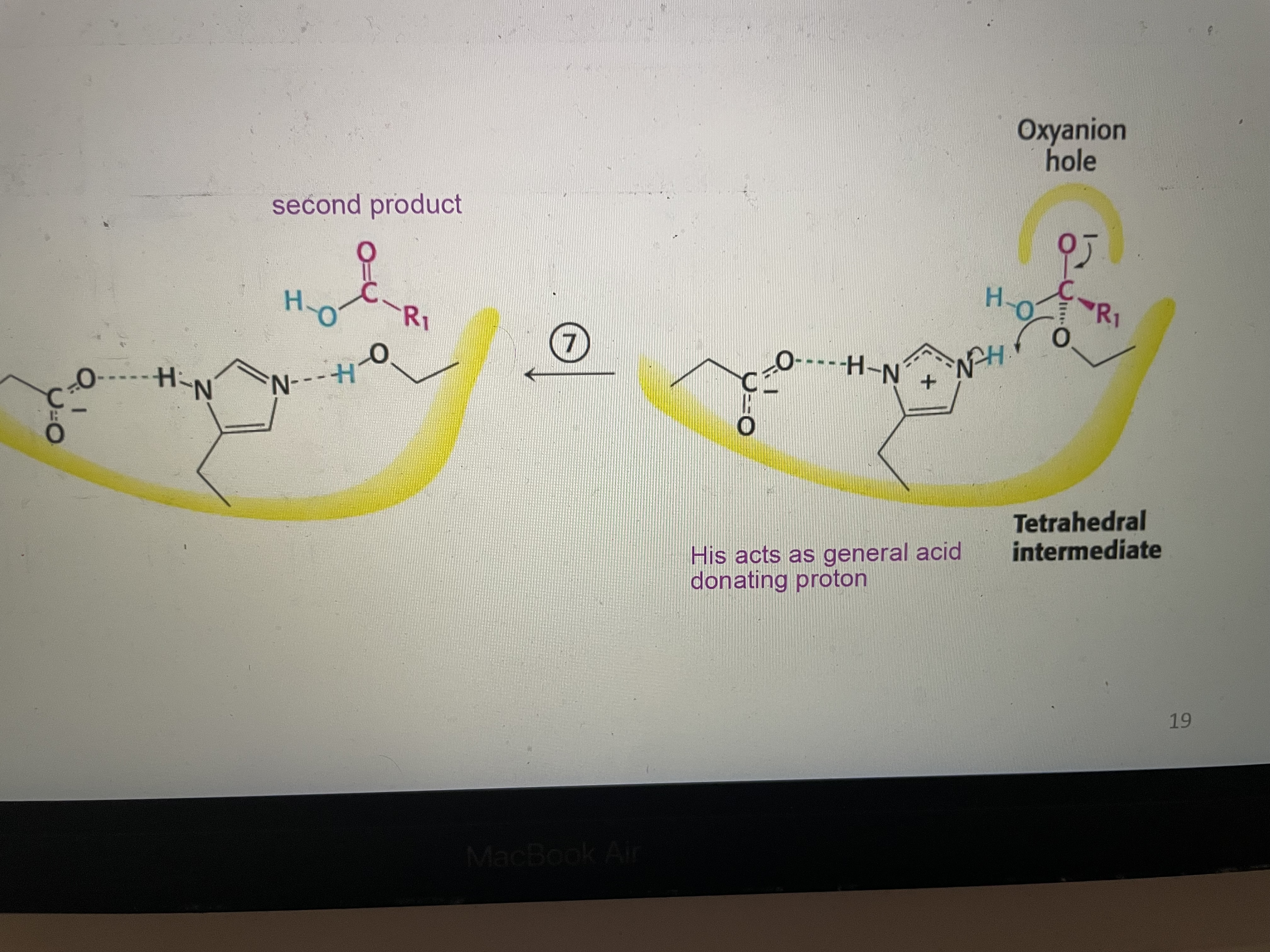
Peptide hydrolysis part 7
Seconds tetrahedral intermediate collapses. His acts as general acid. Second product (carboxyl component) is released and catalytic triad is reformed.