patho exam 1
1/235
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
236 Terms
pathology
The causes of
disease and the changes in
cells, tissues, and organs that
are associated with
development of disease
etiology
the origin of a
disease, including the
underlying causes and
modifying factors
pathogenesis
steps in
disease development
homeostasis
steady state of
steady internal physical and
chemical conditions
adaptation
cell adjusts and survives in new steady state
reversible injury
homeostasis is restored
irreversible injury
leads to cell death; permanent
oxidative stress
Harms membranes, proteins, DNA.
Normally controlled by enzymes (catalase, glutathione peroxidase).
endoplasmic reticulum stress
• This process is imperfect, and some misfolded
polypeptides are generated
Misfolded proteins in the ER activate the
unfolded protein response (an adaptive
response) via sensors like IRE1
• UPR increases chaperone expression, reduces
protein synthesis, and increases protein
buildup of misfolded proteins.
Mild = adaptive (helps refold/remove proteins).
Severe = triggers apoptosis.
ER stress injuries
Misfolded proteins can accumulate inside cells due to two main reasons: an increase in their production or a decrease in the body's ability to eliminate them. Factors contributing to this problem include:
- Genetic mutations in the proteins, or in the unfolded protein response (UPR) pathway
- Aging
- Viral infections
- Changes in intracellular pH and redox state
- Conditions like hypoxia and ischemia
These misfolded proteins can lead to disease in several ways: they can result in a shortage of essential proteins (loss of function), trigger programmed cell death (apoptosis), or acquire harmful properties (gain of function). For example, mutations in proteins can cause disorders like cystic fibrosis.
ubiquitin proteasome system
cell’s garbage disposal / recycling system.
It removes proteins that are damaged, misfolded, or no longer needed.
ubiquitin ligases attach to ubiquitin to the protein that needs to be destroyed
distribution of ca homeostasis
too much Ca²⁺ inside → activates enzymes that damage membranes, cytoskeleton, etc.
cellular adaptation to stress
Reversible changes in the number, size, phenotype, metabolic
activity, or functions of cells in response to changes in their
environment
physiologic adaptations
the responses of cells to normal stimulation by
hormones or endogenous chemical mediators, or to the demands of
mechanical stress
ex: enlargement of ovary during pregnancy
pathological adaptations
the responses to stress that allow cells to
modulate their structure and function and thus escape injury, but at the
expense of normal function
ex: hypertrophy of the heart in response to increased orkload
hypertrophy
Increased cell size leads to increased organ size, but no increase
in cell number
• Can progress to cell injury if the stress is not relieved or if it
exceeds the adaptive capacity of the tissue
hyperplasia
Increase in number of cells in an organ; increased proliferation
• Hyperplasia can be physiologic or pathologic
Physiologic hyperplasia
Hormonal: proliferation of the glandular epithelium of the female breast
at puberty and during pregnancy
• Compensatory: residual tissue grows after removal or loss of part of an
organ
pathologic hyperplasia
Endometrial hyperplasia: increased uterine epithelial proliferation due to
increased estrogenic stimulation
• Benign prostatic hyperplasia: disruption of androgens and estrogens
leads to hyperplasia in the prostate
atrophy
Reduced size of an organ or tissue caused by reduction in the
size and number of cells due to both pathologic and physiologic
causes
Atrophy results from a combination of decreased protein
synthesis and increased protein degradation (UPS)
• Atrophy is often accompanied by increased autophagy
metaplasia
A change in which one adult cell type is replaced by
another adult cell type
• Typically arises from reprogramming of stem cells rather
than phenotypic change of differentiated cells
High risk of malignant transformation: If the stimulus that
induces metaplasia persists, this can lead to malignant
transformation and the development of cancer
mechanisms of cell injury and cell death
Causes: low oxygen, toxins, infections, physical/chemical damage.
Reversible injury: swelling, fatty change (esp. liver).
Irreversible injury: cannot restore mitochondria or DNA; membranes and proteins destroyed.
Key damage points:
Mitochondria → less ATP, more ROS → apoptosis.
Membranes → leaks, swelling, enzyme release.
DNA → if badly damaged, p53 triggers apoptosis.
cellular swelling
Increased cell size and swollen organelles
• Accumulation of degenerated organelles and
lipids within injured cells
• Gross morphology: Pallor, turgor, increased
organ weight
• Microscopic changes: hydroponic change,
vacuolar degeneration
• Commonly seen when cells are injured by
hypoxia and other causes that deplete ATP
fatty changes
Lipid vacuoles in cytoplasm
• Common in organs involved in lipid
metabolism (liver)
mitochondrial dysfunction and damage
Decreased ATP generation and depletion of ATP in cells leads to:
• Reduced activity of plasma membrane ATP-dependent sodium pumps leads to
cellular swelling and dilation of ER
• Compensatory increase in anaerobic glycolysis leads to lactic acid
accumulation, decreased intracellular pH, and decreased activity of many
cellular enzymes
• Prolonged ATP depletion leads to structural disruption of the protein
synthetic apparatus
• Detachment of ribosomes from rough ER, dissociation of polysomes, reduction
in protein synthesis
Mitochondrial membranes
formation of mitochondrial
permeability transition pore
Plasma membranes:
loss of
osmotic balance, influx of fluids
and ions, loss of cellular contents
Lysosomal membranes
leakage
of enzymes into cytosol
Dystrophic calcification
in dead/damaged tissue.
Extracellular Deposits: Pathologic
Calcification
the result of abnormal deposition of calcium salts; fine white
granules or clumps and gritty deposits
Dystrophic calcification
deposition of crystalline calcium phosphate
in membrane-bound vesicles
Dna damage
mutations in mitochondrial and nuclear DNA
accumulate with age and cause the following
Cell aging
Certain environmental stresses, such as
calorie restriction, alter signaling pathways that influence aging,
including insulin-like growth factor (IGF-1) and molecular target of
rapamycin (mTOR) signaling
Telomeres = “caps” at chromosome ends that shorten with age.
Persistent inflammation:
accumulation of damaged cellular
components can activate the pathways that cause low-level
inflammation
difference between apoptosis and necrosis cell death
mictochondrial intrinsic pathway (apotosis)
Most physiologic and
pathologic situations
programmed, neat, no inflammation
extrinsic pathwway apoptosis
Controlled by death
receptors (TNF
receptor family and
Fas)
• When ligand binds,
receptors cross-link via
death domain and bind
adapter proteins
• Leads to the activation
of caspase cascade
caspase cascade
Degradation of cellular
proteins and nuclear
fragmentation
• Apoptotic cells recruit
phagocytes that
clearance of apoptotic
bodies
• No inflammatory
response
autophagy
“Self-eating:” lysosomal
digestion of a cell’s own
components
• Recycling mechanism during
nutrient deprivation
• Organelles and portions of
cytosol are sequestered in
membrane-bound
compartments
• Autophagosome fuses with
lysosome, where enzymes
digest cellular components
Metastatic calcification
in normal tissue due to high blood calcium.
necrosis
accidently, messy, causes inflammation (cell death)
hypoxia
low o2
ischemia
no blood flow, worse than hypoxia because its faster cell death
restoring blood flow causes even more damage
direct toxins are direct, indirect need conversion
primary lympahtic organs
bone marrow, thymus, fetal liver (maintain immune system)
secondary lymphatic organs
spleen, lymph nodes, lymph ducts, tonsils, adenoids
lymphatic vesesels
-part of the circulatory system; network of vessels
–carry clear fluid (lymph) towards the heart
–transports immune cells (lymphocytes; MP)transports immune cells (lymphocytes; MP
first line of immune defense
physical barriers (skin/epithelial
barriers; mucous membranes) and chemical barriers
second line of immune defense
Innate immune system
nonspecific immediate response (phagocytic cells,
inflammatory response, antimicrobial proteins,
complement, natural killer/NK cells)
third line of immune defense
Adaptive immune system specific
slower response; provides improved recognition;
immunological memory- faster recognition & stronger
secondary response (B and T lymphocytes)
innate immune system
– Phagocytes: monocytes, macrophages (MP),
neutrophils
– Mast cells, eosinophils, basophils
– Natural killer (NK) cells, innate lymphoid cells (ILC)
body’s rapid non specific first line of defense, immediate but general protection against broad range
adaptive immunity
Adaptive Immunity
– Specific antigen receptors
– B cells (humoral immunity)
– T cells (cell mediated immunity)
– NKT cells (cell mediated immunity)
fat microphage is going to turn on immune response
treg cells shut down immune response
innate immunity
every animal has this, first line of defense and conserved mechanism of host defense against infection
Distinguishes self from non-self perfectly
Defects in innate immunity are very rare and almost
always lethal
nonspecific defense
does not require specific antigen receptors or prior exposure/learning
provides barriers to prevent the spread of infection
identifies and eliminates pathogens and other foreign bodies
initiates an inflammatory response
activates specific immune responses
neutrophils
present in blood (60-70%) of WBC
present in tissues at relatively low levels, increase following injury or infection
short span 9-12 hours
functions:
first cell at the site of infection/injury
ingest and kill microbes and foreign materials; activation involves PAMPS and DAMPS binding to PRRs
need to be called in by the tissue resident macrophages (actual real responders)
mononuclear phagocytes
blood- monocytes (1-6%) WBC
tissues- macrophages
resident and inflammatory macrophages
resident macrophages
located throughout the body; largest populations in liver and lung most embryonic origin
inflammatory macrophages
respond to injury and infection, bone marrow derived
macrophage functions
identify and eliminate pathogens and other foreign materials
non-specific recognition system, PPRS activated by PAMPS or DAMPS
regular inflammatory responses, promote angiogenesis, and initiate wound healing
maintain homeostasis, lipid, and iron metabolism
major secretory activity
activate adaptive immunity antigen processing and presentation
tumor surveillance and cytotoxicity
m1 macrophages
cytotoxic/ proinflammatory
produce ros, rns, il-12, tnfa, chemokines
m2 macrophages
anti inflammatory/ wound repair
produce il-10, tgfb, arg, vegf, egf
subsets m2a, m2b, m2c, m2d
macrophage activation
as environmental cues and regulators change in response to pathophys conditions, marcophages readily modify their phenotype (phenotypic switching)
mixed phenotype macrophages co exist w m1 and m2 macrophgaes
m1 to m2 reprogramming is key to the resolution of inflammation and wound repair
inflammation
dybamic response of a vascularized tissue to injury or infection
physiologic protective responses
serves to bring defense and healing mechanisms to the site of injury
fiunctions
neutralizes or destroy offending agent
restrict tissue damage to smallest possible area
alerts body and prepares injured tissue for repair and healing
acute inflammatory response
short term responses (typically lasts days)
initial response: proinflammatory/ cytotoxic
cellular response: proinflammatory neutrophils and m1 macrophages accumulate at injured site to release mediators to destroy pathogens and recruit additional inflam cells
secondary response anti inflammatory/ wound repair
cellular response: proresolution m2 macrophages accumulate and release mediators that suppress inflammation and promote wound repair
resolution of acute inflammation
neutrophils undergo apoptosis
resp;vong mediations released and stimulate m2 macrophage efferocytosis (act of macrophages phagocyting and removing dead neutrophils)
m2 macrophage release mediations that down regular inflammation and initian wound repair and angiogensis
inflammation fails to resolve
chronic inflammation- persistence of inflammatory neutrophils and macrophages, perpetuation of tissue injury, fibrosis/ scarring
foreign body response- frustrated phagocytosis, macrophages wall of injurious agent
granulomas
cancer
chemotaxis
migration to injured or infected site mediated by chemotactic factors
phagocytosis
ingestion of foreign substances, receptor mediated, active process, requries energy
metabolic destruction
intracellular digestion'; killing
oxygen independent: antimicrobial proteins, cationic proteins, lysozyme, acid hydrolases
oxygen dependent: myeloperoxidase, reactive oxygen species, reactive nitrogen species
foreign body reaction
if macrophages cant destroy the object they wall it off to protect the body
fusion of macrophages into giant cells along w other immune cells, formation of a fibrous capsule around the foreign body
outcome of fbr depends on size of foreign body, structure and surface characteristics
pathologic granulomas can form, usually smaller substaces, chronic inflammatory response
secretory functions of macrophages
2nd most potent secretory cell in body
enzymes capable of degrading extracellular matrix proteins
products involved in host degense
regulatory proteins
excessive release of mediatiors by overactive macrophages
can damage normal tissues
macrophage immune functions
antigen processing and presentation
tumor cytotoxicity
tumor surveillance
macrophage antigen processing
phagocytosis of antigen
partial degradation or unfolding
binding to mhc 2 proteins
re expression of processed antigen on cell surface with mhc 2
presentation to t helper cells
nk cells
type of cytotoxic lymphocyte
part of innate immune system
apoptosis mediated by small granules of perforin and granzyme
response is independent of mhs and antibodies= faster response < 3 days
innate lymphoid cells
derived from clp
part of innate immune system
no specific antigen recptor
regulate homeostasis and inflammation
humoral immunity
Mediated by B lymphocytes which produce
antibodies or immunoglobulins (Ig) in
response to antigen challenge
Five Classes: physical, chemical and
antigenic differences
antibodies
glycoproteins; selective, highly
specific; found in y-globulin fraction of
serum (humoral=blood)
igM
primary immune response (7%); Type III
hypersensitivity reaction; immune complexes;hypersensitivity reaction; immune complexes;
B cell receptorB cell receptor
pentamer
igG
secondary immune response, B memory
cells (70%)cells (70%
igA
external secretions, produced locally
against bacteria and viruses (15%)against bacteria and viruses (15%
monomeric, becomes multimeric in the endothelium and acquires a glycoprotein that proteins it against digestion
igE
Type I hypersensitivity reactions, minute
amountsamounts
igD
umbilical cord blood, primitive recognition
or regulation; B cell receptoror regulation; B cell receptor
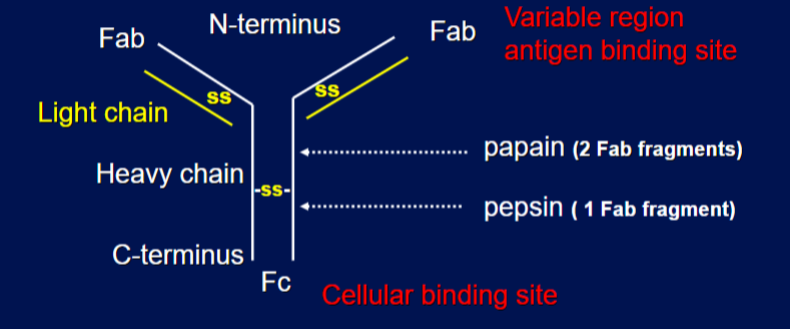
antibody structure
Y-shaped, protein molecule that binds to a specific antigen to neutralize or mark it for destruction. It consists of two identical heavy chains and two identical light chains connected by disulfide bonds. top is variable region antigen binding site and bottom is cellular binding state
secondary immune response
igM and igG
– Shorter lag
– Higher levels of specific IgG produced
– Steady state level persists longer
– IgG predominates
– Quantitative difference between primary
secondary immune response due to an increase
the number of potentially reactive B cells
primary immune responses to antigen are
transient (passive)
b cells
will only recognize particular antigens to activate , will be making monoclonal antibodies, only one role
monoclonal antibodies
revolutionized immunology; involves development
of single antibody secreting immortalized cell
monoclonal antibodies benefit
monoclonal antibodies- no two antisera identical
reproducible- identical antibody more specifc and reliable
unlimited quantities- permanent cell line, grows indefinitely and produces very large amount
antigen need not to be pure or characterized
antigen
substance recognized as foreign
hapten: small molecule that binds to antibdoy does not elicit immune response
immunogen: foreign substance that binds antigen and elicts immune response
carrier: large protein
hapten + carrier= immunogen
t-independent antigens
– Complex carbohydrates
– Do not require processing
– Can directly interact with B cells
– No memory
t-dependent antigens
Require macrophages or other APC
– Require T-helper cells
– Require major histocompatibility antigens
– Mostly proteins
antibody-antigen interactions
not covalent, ionic, or perm binding
occurs in the variable region of the antibody molecule
cell mediated immunity
Mediated by t lymphocytes which release soluble mediators
important in host defense against viruses etc, transplant rejection and tumor surveillance
t cells
derived from precursor cells in bone marrow, mature in thymus, become educated
t cell education
learning to distinguish between self and non-self
controlled by mhc (self marker) proteins
specific for a given polypeptide chain to become activated
antigen recognition by t cells
specific t cell receptor
are MHC RESTRICTED- only recognize antigen together with major MHC protein
t helper cells recognize processed antigen and MHC II (self)
Cytotoxic TA cells recognize processed antigen and MHC I (non-self, altered self)
successful immune response also requires costimulatory and or coinhibitory molecules
immune checkpoints
MHC
large cluster of genes coding for proteins essential in regulation of immune cell function; destruction of non self
class 1 MHC
expressed on all somatic cells; classic “transplantation antigens”
class II MHC
Expressed on immune cells, immune associated antigens, important in immune regulation
class III MHC
complement
plasma proteins that play a role in lysing cells that are foreign or attractant compounds
not species specific