week 11(/12) : adrenal glands
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
69 Terms
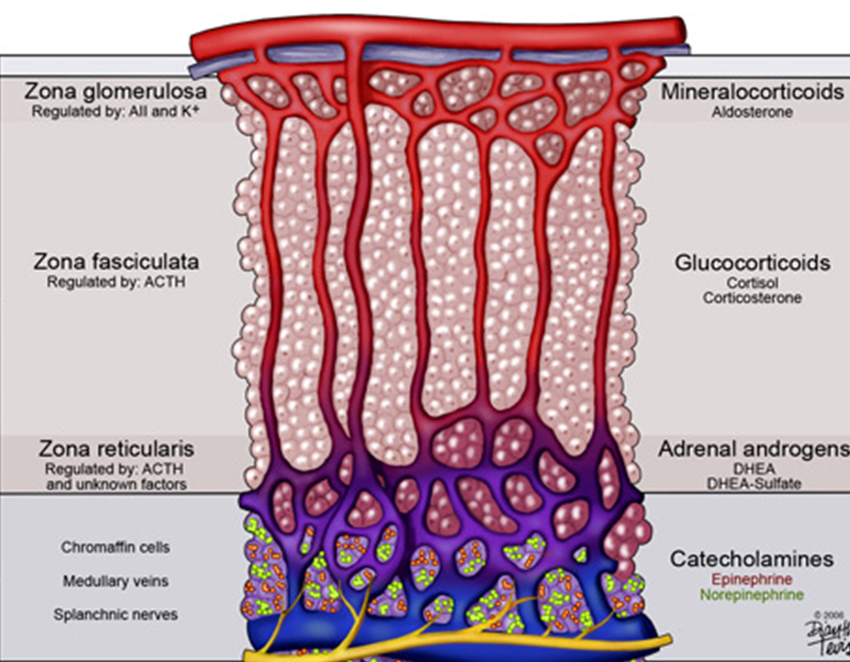
What are the three cortical zones of the adrenal gland and the hormones they produce?
Zona glomerulosa – aldosterone (mineralocorticoid) → regulated by angiotensin II & K⁺.
Zona fasciculata – cortisol (glucocorticoid) → regulated by ACTH.
Zona reticularis – adrenal androgens (DHEA, DHEA‑S) → mainly ACTH‑driven.
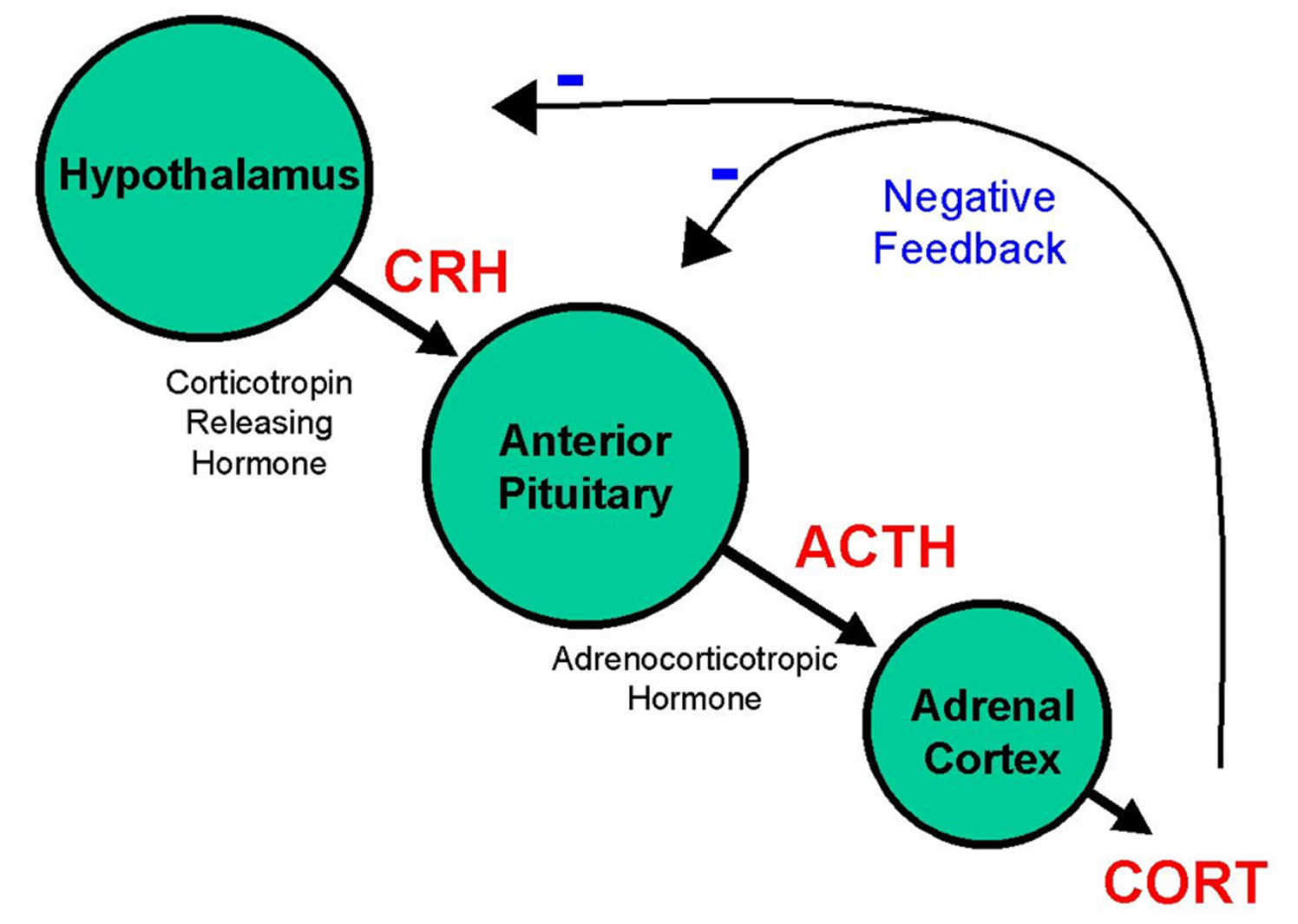
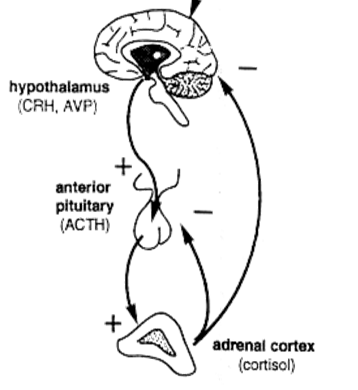
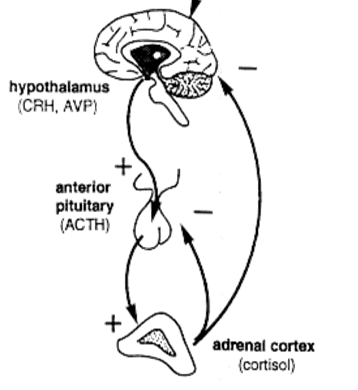
How does the HPA‑axis normally regulate cortisol?
CRH (hypothalamus) → ACTH (pituitary) → cortisol (adrenal). ↑ cortisol feeds back negatively on both CRH and ACTH, creating a diurnal rhythm (peak early‑morning, lowest at midnight).
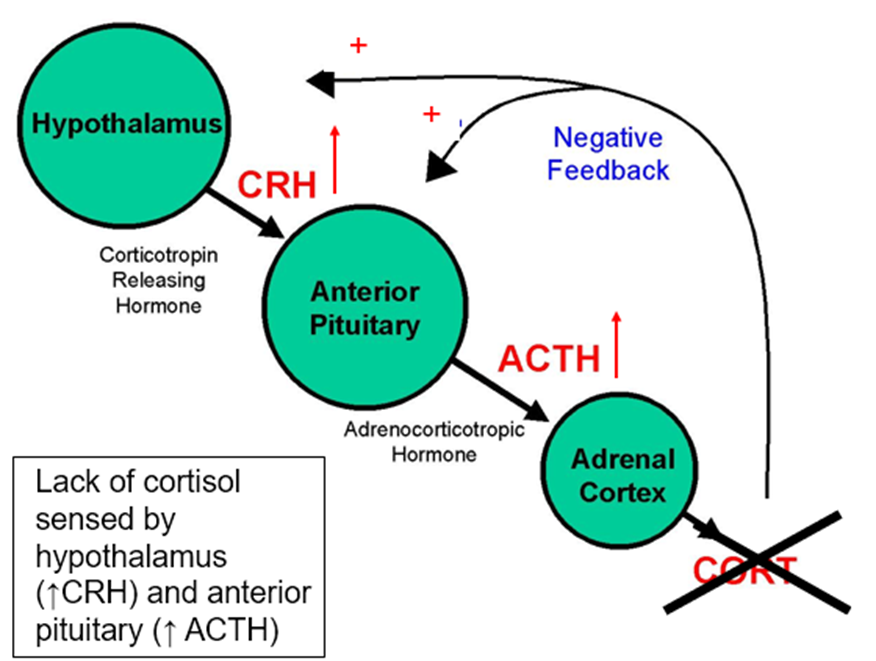
What happens to the HPA‑axis in primary adrenal insufficiency?
Loss of cortisol removes negative feedback → CRH ↑ → ACTH ↑↑.
The excess ACTH also contains α‑MSH → hyperpigmentation.
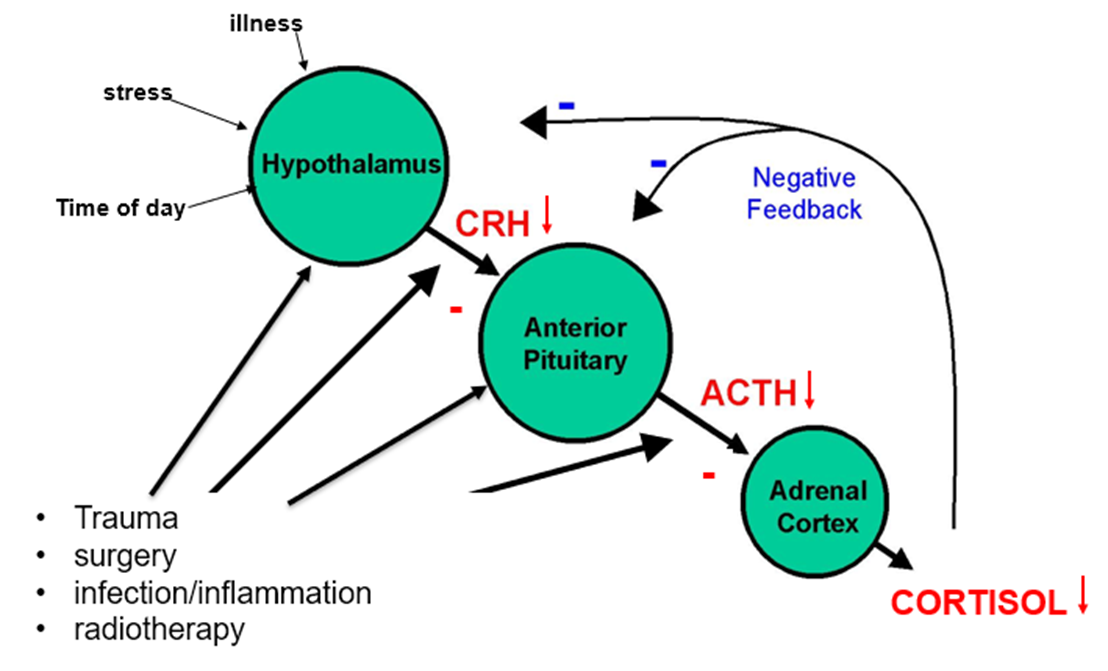
What happens to the HPA‑axis in secondary adrenal insufficiency?
Pituitary/hypothalamic failure → ACTH ↓ (low‑normal) → CRH ↓ (if pituitary disease). Cortisol ↓ but aldosterone stays normal because RAAS is intact.
Name two ACTH‑dependent and two ACTH‑independent causes of Cushing’s syndrome.
ACTH‑dependent:
pituitary adenoma (Cushing’s disease)
ectopic ACTH‑secreting tumor (e.g., small‑cell lung cancer).
ACTH‑independent:
adrenal adenoma
adrenal carcinoma
nodular hyperplasia.
Cushing’s Disease= pituitary
Cushing’s Syndrome= excess cortisol, e.g. in adrenal adenoma
List metabolic, cardiovascular, and musculoskeletal consequences of chronic cortisol excess.
Metabolic: central obesity, glucose intolerance/diabetes, hyperlipidemia (high levels of lipids (fats) in the blood), dyslipidaemia (abnormal levels of lipids (fats) in the blood)
Cardiovascular: hypertension, increased thrombotic risk, accelerated atherosclerosis.
Musculoskeletal: proximal myopathy (Hips and shoulders), osteoporosis, pathologic fractures.
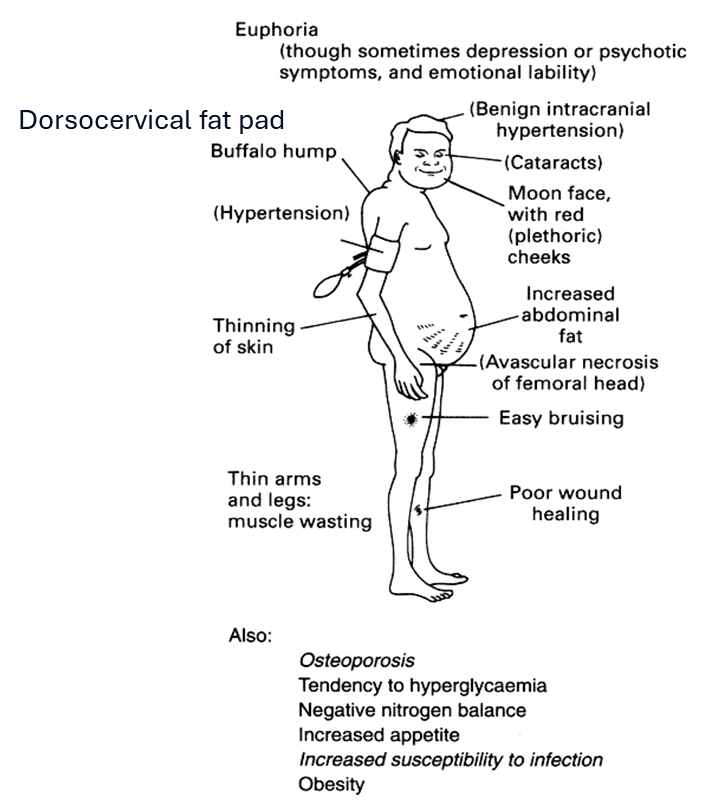
What are three dermatologic and two neuro‑psychiatric signs of Cushing’s syndrome/ excess cortisol?
Dermatologic: thin skin, easy bruising, violaceous striae, poor wound healing.
Neuro‑psychiatric: depression, cognitive impairment/emotional lability.
How does excess cortisol affect the reproductive system?
Menstrual irregularities, decreased libido, infertility (via gonadotropin suppression).
What laboratory pattern defines primary adrenal insufficiency?
↓ cortisol, ↓ aldosterone, hyponatremia(Na), hyper‑K⁺ (Hyperkalemia), markedly ↑ ACTH, hyperpigmentation.
What laboratory pattern defines secondary adrenal insufficiency?
↓ cortisol, normal aldosterone, normal Na⁺/K⁺, low/normal ACTH, no hyperpigmentation.
Where do ADH and Oxytocin originate?
ADH and Oxytocin are Synthesized in the Hypothalamus and Released From the Posterior Pituitary
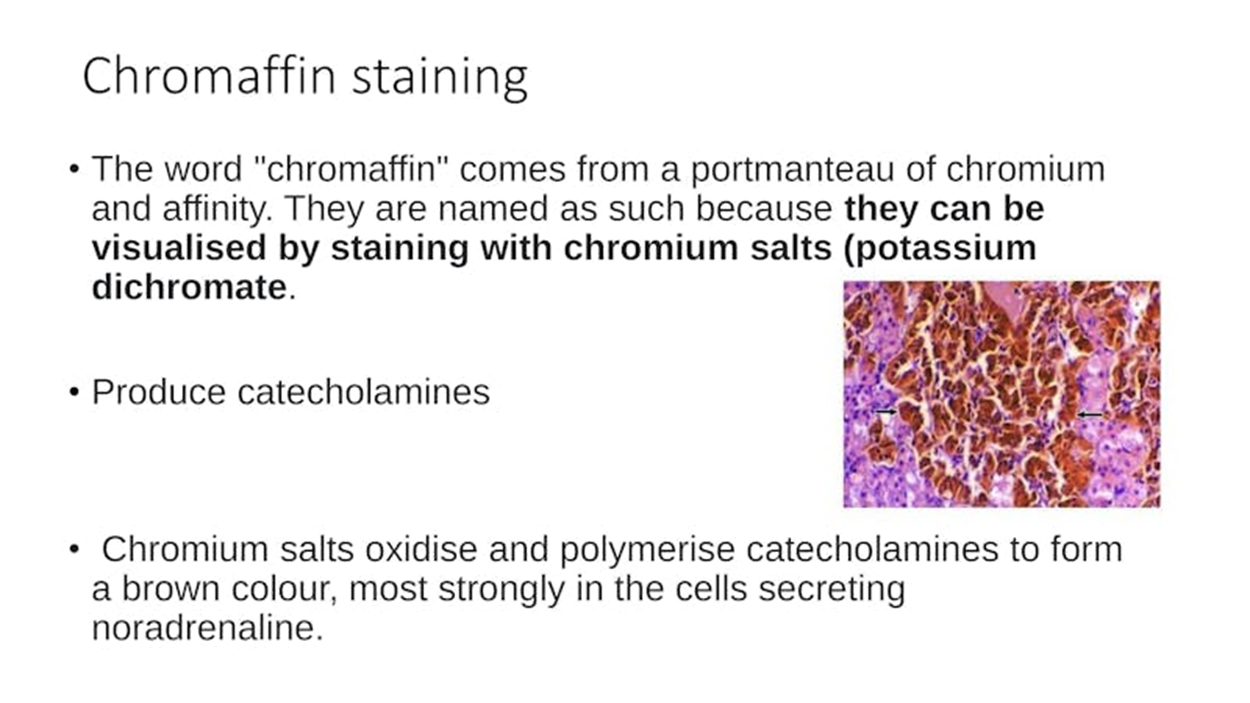
What does the chromaffin reaction stain?
cells of the adrenal medulla
What is the Short Synacthen (ACTH) test result in primary vs. secondary disease?
Primary: low baseline and low post‑ACTH cortisol (adrenal cannot respond).
Secondary: low baseline but appropriate rise after ACTH (adrenal still functional).
What are the acute‑crisis signs of adrenal insufficiency?
Severe fatigue, hypotension/shock, nausea/vomiting, abdominal pain, hyponatremia, possible hyper‑K⁺ (primary)
How is iatrogenic Cushing’s different from endogenous Cushing’s?
Caused by prolonged high‑dose exogenous glucocorticoids → suppresses ACTH → adrenal cortical atrophy. Patients lose stress‑response capacity; abrupt withdrawal precipitates adrenal crisis.
Iatrogenic Cushing's is from external steroid medications (most common), while endogenous Cushing's comes from the body overproducing cortisol
What is the diagnostic test for cortisol excess (Cushing’s) and its interpretation?
1 mg overnight dexamethasone suppression: failure to suppress cortisol (<1.8 µg/dL) suggests Cushing’s. Follow with ACTH level to determine ACTH‑dependence.
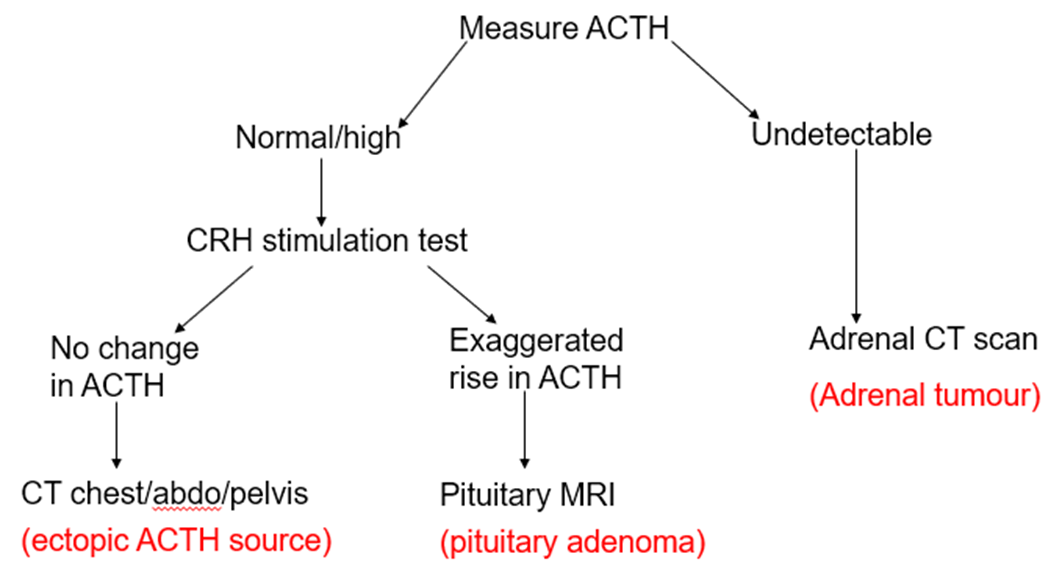
After confirming cortisol excess, what imaging steps are taken based on ACTH?
ACTH‑high → pituitary MRI (Cushing’s disease) or CT chest/abdomen for ectopic source.
ACTH‑low → adrenal CT/MRI to look for adenoma or carcinoma
What are the definitive treatment options for Cushing’s syndrome?
Surgical removal of the source (trans‑sphenoidal pituitary adenomectomy, laparoscopic adrenalectomy, or excision of ectopic tumor)
Medical therapy (ketoconazole, metyrapone, osilodrostat) when surgery contraindicated or as bridge
Which glucocorticoid is preferred for physiologic replacement and why?
Hydrocortisone – short half‑life, mimics natural cortisol kinetics, allows split dosing to match diurnal rhythm.
What is the standard adult hydrocortisone regimen for Addison’s disease?
15‑30 mg/day in divided doses: 10‑20 mg on waking, 5‑10 mg around 2‑3 PM.
When might prednisolone be used instead of hydrocortisone for Addison’s disease?
For once‑daily dosing convenience or when a longer‑acting agent is preferred (3‑4 mg PO daily).
Outline the “Sick‑Day Rules” for adrenal‑insufficient patients.
1⃣ Double oral glucocorticoid dose for fever/illness.
2⃣ If unable to take PO (prednisolone) → 100 mg IM/IV hydrocortisone.
3⃣ Seek emergency care for vomiting, severe illness, or trauma.
4⃣ Wear a medical‑alert ID at all times.
How should the dosing for Addison’s disease be adjusted for major stress (e.g., surgery)?
Give 100 mg IV/IM hydrocortisone bolus, then 50 mg q6 h IV (or 200 mg/day divided) until stress resolves.
What is the role of fludrocortisone and when is it added?
Replaces mineralocorticoid in primary adrenal insufficiency (0.05‑0.1 mg daily). Not needed in secondary disease because aldosterone is preserved.
List monitoring parameters for patients on replacement therapy.
Clinical: energy, weight, BP.
Biochemical: serum Na⁺/K⁺ (primary), fasting glucose.
Other: frequency of “sick‑day” dosing, growth (peds), pregnancy status.
What is the first‑line acute treatment for adrenal crisis?
IV hydrocortisone 100 mg bolus, then 50 mg q6 h, plus aggressive isotonic saline resuscitation (often with dextrose if hypoglycemic).
Define primary adrenal insufficiency.
Destruction of the adrenal cortex → loss of cortisol and aldosterone (e.g., autoimmune Addison’s).
Define secondary adrenal insufficiency.
Pituitary or hypothalamic failure → deficient ACTH → isolated cortisol deficiency; aldosterone production remains intact via RAAS.
Summarize the key lab differences between primary and secondary disease.
Primary: ACTH ↑↑, aldosterone ↓, Na⁺ ↓, K⁺ ↑, hyperpigmentation.
Secondary: ACTH ↓/low‑normal, aldosterone normal, electrolytes normal, no hyperpigmentation.
What clinical sign are indicative for primary insufficiency?
Hyperpigmentation of skin and mucosa (due to excess ACTH/α‑MSH).
Why is fludrocortisone unnecessary in secondary insufficiency?
Aldosterone secretion is preserved because the renin‑angiotensin system is still functional.
What is the treatment for primary vs. secondary insufficiency?
Primary: Hydrocortisone + fludrocortisone + patient education.
Secondary: Hydrocortisone alone + education; monitor electrolytes.
What are the two main parts of the adrenal gland?
Outer cortex (steroid hormones) and inner medulla (catecholamines).
(eg. dopamine, noradrenaline, and adrenaline)
What is the blood supply of the adrenal gland?
Superior, middle, and inferior suprarenal arteries; centripetal blood flow from capsule to medulla.
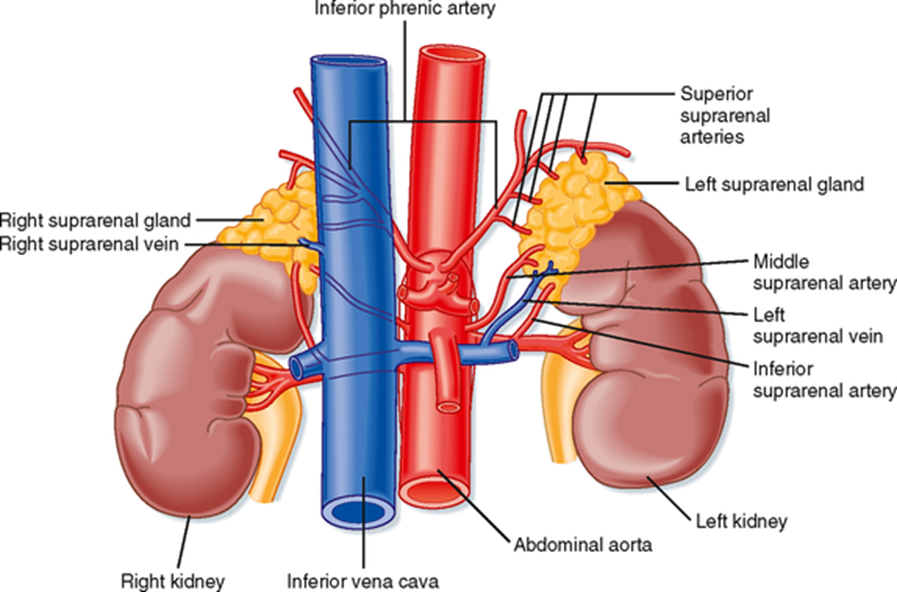
What is the nerve supply of the adrenal gland?
Coeliac plexus and thoracic splanchnic nerves
(innervate chromaffin cells of the medulla)
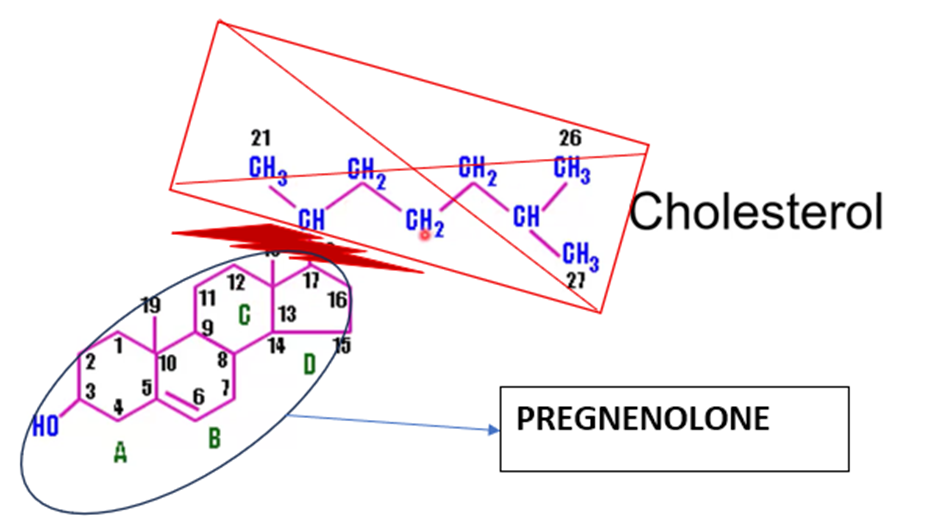
What is the substrate for all steroid hormones?
Cholesterol (from LDL uptake or de novo synthesis via HMG‑CoA reductase).
What is the rate‑limiting step in steroid biosynthesis?
Transport of cholesterol into mitochondria by StAR protein.
What is the first enzymatic step in steroid biosynthesis?
Cholesterol side‑chain cleavage → pregnenolone (enzyme: CYP11A1, mitochondrial P450).
Where are steroidogenic enzymes located?
(responsible for the biosynthesis of steroid hormones)
Mitochondria and smooth endoplasmic reticulum.
How is aldosterone production regulated?
Renin‑angiotensin system (RAS) and plasma potassium.
Renin → angiotensin I → angiotensin II (via ACE).
Angiotensin II stimulates aldosterone secretion.
What type of receptors do corticosteroids act on?
Nuclear receptors (transcription factors).
What is the structure of steroid receptors?
Six domains: N‑terminal, DNA‑binding domain, hinge region, ligand‑binding domain, C‑terminal.
How do corticosteroids exert their effects?
Steroid crosses membrane → binds receptor → receptor‑ligand complex enters nucleus → binds DNA → stimulates transcription → new proteins mediate effects.
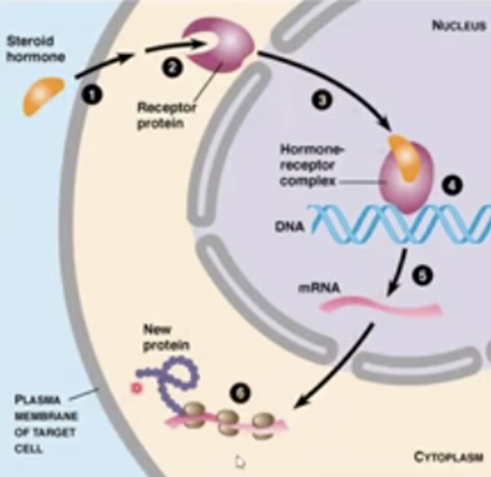
What is pre‑receptor regulation of mineralocorticoid receptors?
11β‑hydroxysteroid dehydrogenase type 2 (11β-HSD2) converts
cortisol → cortisone (inactive),
preventing cortisol from occupying mineralocorticoid receptors.
What are the main effects of cortisol (glucocorticoid)?
Stimulates gluconeogenesis in liver.
Mobilises fat (lipolysis).
Suppresses insulin release.
Increases protein breakdown in muscle.
Reduces bone formation.
Suppresses immune system.
Influences memory and learning.
What are the main effects of aldosterone (mineralocorticoid)?
Acts on distal tubule and collecting duct.
↑ Sodium reabsorption and water retention.
↑ Potassium and hydrogen excretion.
Regulates epithelial sodium channels (ENaC):increases ENaC (epithelial sodium channel) permeability
Also acts on gut, salivary glands, sweat glands, heart, vasculature.
What is primary aldosteronism?
Excess aldosterone → sodium retention, potassium excretion, water retention
= hypertension, hypokalaemia, alkalosis.
Causes: aldosterone‑producing adenoma, bilateral adrenal hyperplasia.
What rare genetic defects affect steroid biosynthesis?
Mutations in CYP11B1, CYP17A1, CYP21A2 → congenital adrenal hyperplasia.
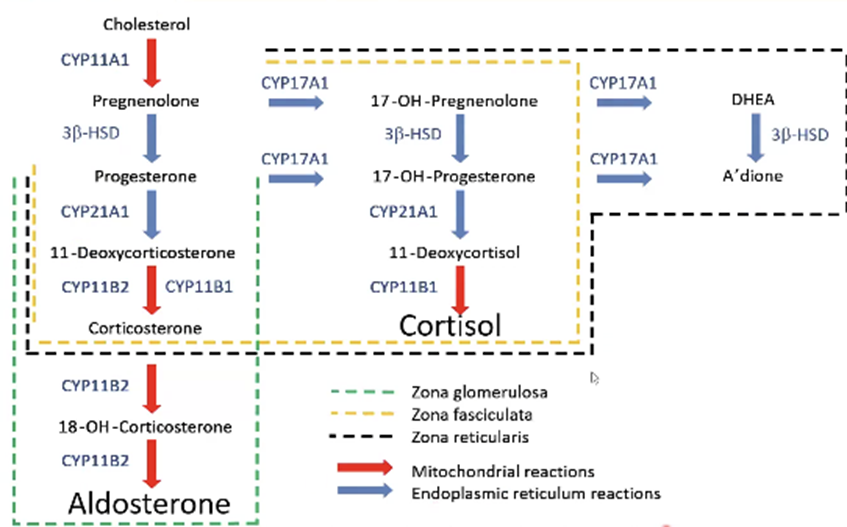
What unusual clinical phenomenon can mimic mineralocorticoid excess?
Excess liquorice ingestion → inhibits 11β‑HSD2 → cortisol activates mineralocorticoid receptors → hypertension.
What triggers ADH release?
↑ Plasma osmolality → hypothalamus → posterior pituitary → ADH secretion.
How does aldosterone increase plasma volume?
↑ Sodium reabsorption in distal nephron → water follows → ↑ plasma volume & BP.
What is pseudo-hyponatraemia?
false lab results of low sodium due to high lipids/proteins.
Name 3 causes of euvolemic hyponatraemia.
SIADH, excess water intake, impaired renal excretion.
SIADH: condition in which the body makes too much antidiuretic hormone (ADH)
What are severe symptoms of hyponatraemia?
Seizures, coma, reduced GCS (glasgow coma scale)
Why must hyponatraemia correction be slow?
To avoid osmotic demyelination syndrome (the protective myelin sheath around nerve cells is destroyed)
First step in managing hyponatraemia?
Assess patient’s volume status.
Most common cause of hypernatraemia in elderly?
Dehydration
What condition should be suspected if hypernatraemia + polyuria + urine/plasma osmolality ratio <1?
Diabetes insipidus.
How is hypovolemic hypernatraemia treated?
correction of volume deficit with normal saline
correction of water deficit with 0.45% saline, 5% dextrose
Normal plasma osmolarity range?
275–295 mmol/kg, tightly regulated within 1–2%.
Normal sodium range?
135–145 mmol/L.
How do you confirm pseudo‑hyponatraemia?
Compare measured vs calculated osmolarity; if they match → true hyponatraemia, if not → check lipids/proteins.
Does sodium need direct treatment in hyperglycemia‑induced hyponatraemia?
No — treat glucose, sodium normalizes.
What should urine osmolarity be in hypernatremia?
>800 mmol/kg (ADH stimulation).
If urine osmolarity < plasma osmolarity in hypernatremia, what does this suggest?
Primary renal water loss → diabetes insipidus (cranial or nephrogenic).
Urine osmolarity <100 mmol/kg in hyponatraemia suggests what?
Excess water intake (rare).
Hyponatraemia + glucose 69.7 mmol/L + bicarb <5 → diagnosis?
Diabetic ketoacidosis (DKA).
Hypernatremia + polyuria + urine/plasma osmolarity ratio <1 → diagnosis?
Diabetes insipidus.
What insensible water losses occur daily?
~850 mL via perspiration & respiration (↑ in fever, burns, hospital environment).