Unit 5: Chemical Reactions
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
Synthesis React
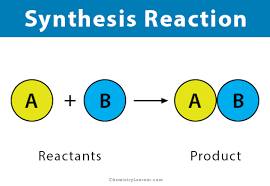
ion
Reactants
Reactants are two element
Neither of the reactants are aqueous
Products
Use the charges to determine the formula
If the compound is ionic → solid
If the compound is molecular → solid, liquid, or gas
Decomposition

Reactants
Compound
Product
Two elements
Identify it element is diatomic
Use periodic table to find state of matter
Balanced equation
Combustion

Reactants
An element or hydrocarbon reacts with O2(g)
Products
If reactant is element, use rules of synthesis
If reactant is hydrocarbon (CxHy), the products are always H2O(g) and CO2(g)
Balancing trick: its often that that the coefficient is a full number (ex:7.5), so multiply the whole ratio by x2
Single Replacement
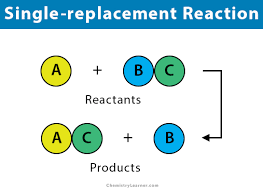
Only occurs when the free element is more reactive than the element it replaces
Metal replaces metal and hydrogen
Non-metal replaces non-metals
Hydrogen replaces metal
The compounds on the product or reactant side are always aqueous
Double Replacement
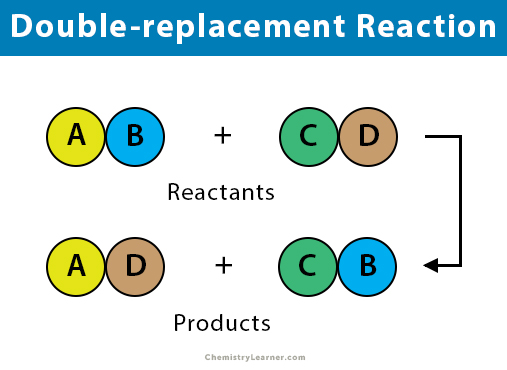
When two aqueous compounds exchange ions
→ If the reactant compound is unsoluble (solid) then it cant break apart into to
Double replacement reaction will occur if
Both reactants are aqueous
At lest on product is a solid, gas, or water
If both products are aqueous then no reaction occurs