Protien structures
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
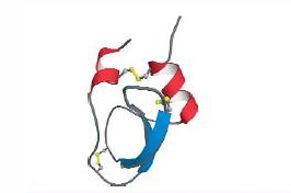
The structure of the small protein
bovine pancreatic trypsin inhibitor, BPTI, has 3 disulfide bridges.
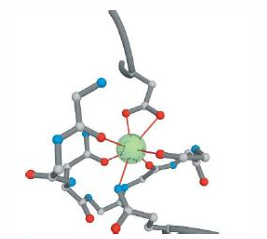
Stabilization by coordinate
covalent bonds
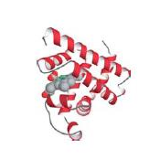
myoglobin
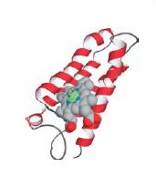
Cytochrome C having a heme group is
attached to the protein in two places: by a
coordinate covalent bond between the sulfur of
a methionine residue and the heme iron, and by
covalent attachment of the porphyrin cofactor
to the protein.
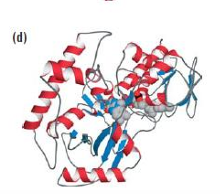
Polyamine oxidase having PQQ as a cofactor formed by the
reaction of two side chains with each other,
leaving it attached to the protein.
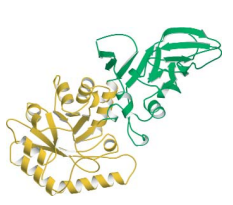
Alanine racemase, TIM barrel interrupted by insertion of another domain
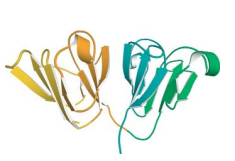
gamma crystallin
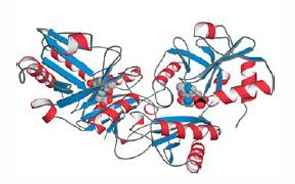
D-amino acid aminotransferase
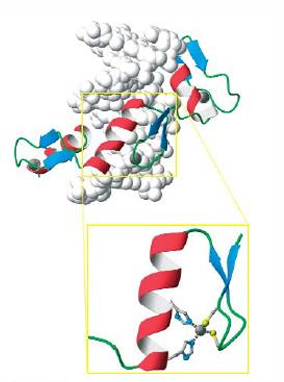
Zinc finger motif
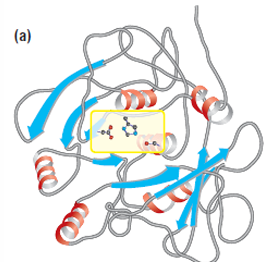
Catalytic triad
subtilisin
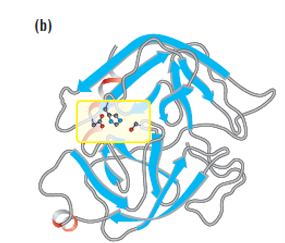
Catalytic triad
Chymotrypsin
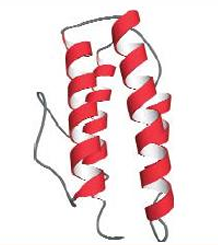
Myohemerythrin, a four helical bundle domain(helix bundles serve as O2 transportt, nucleic acid binding, and ETC. Myohemerythrin stores oxygen in marine worms.
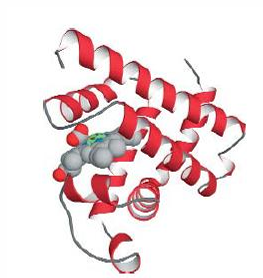
Myoglobin, composed of a single globin fold domain(globin folds consist of a bag of eight alpha helices, this motif leads to hydrophobic pocket formation in which large hydrophobic organic and organometallic groups an bind)
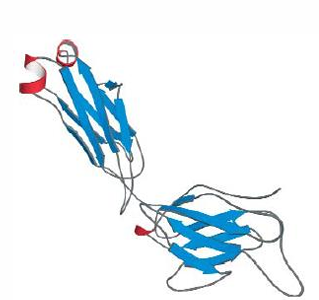
Immunoglobulin, an only beta sheet domain, connected by tight turns and irregular loops.
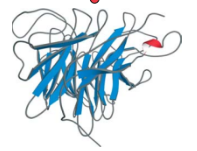
Neuraminidase beta-propeller domain, a four subunit domain made of repeating up and down beta motifs. found in the influenza virus.
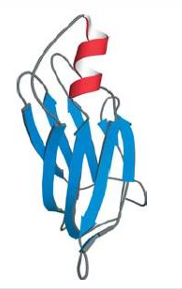
Pre-albumin, example of an antiparallel beta domain made up of greek key motifs. Only a subunit out of 2 is shown.
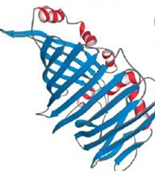
Bacteriochlorophyll A protien, consisting of an up and down, antiparallel beta sandwich, a motif known as jelly roll.
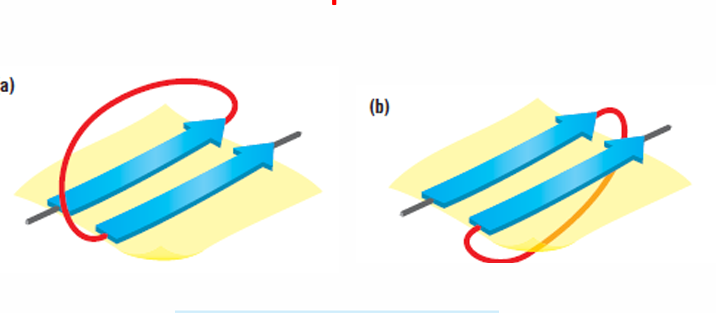
parallel strands joined by alpha helices=beta-alpha-beta-alpha unit. Crossover might be either right-handed or left-handed. more biased towards the right. The same applies if the connection is a loop, not a helix.
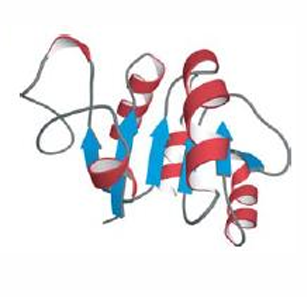
An alpha beta domain. Aspartate semi-aldehyde dehydrogenase. Connecting segments are usually alpha helices.
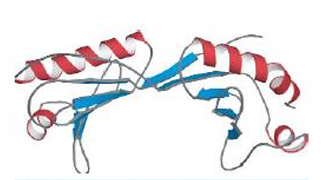
Alpha-beta saddle. Segregated alpha helices and beta sheets. Sometimes the layer of helices form recognition sites, here, this is the example of the TATA binding protien;helices makes contact with the DNA and binds to major groove, and the sheets form the saddle, binds in minor groove of the DNA bending it signficantly.
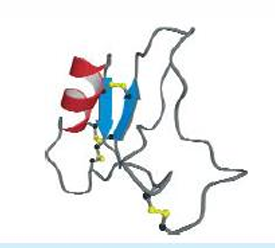
Disulfide linked protien. Toxin, stable to proteolytic digestion and heat denaturation. Stabilized by 4 disulfide bridges.(disulfide link—→not 2ndary), but too small to have a hydrophobic core, has minimal 2ndary structures.
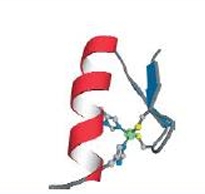
zinc finger motif or domain, stablilized by coordination of 2 histidines and 2 cysteines to a zinc ion. no zinc—>unfolded. too small to have a hydrophobic core. very abundant.
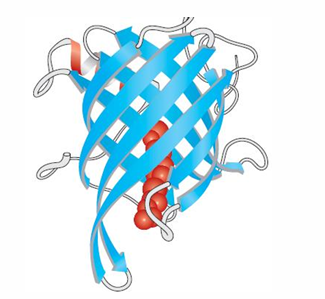
Beta barrel
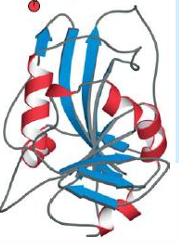
Dihydrofolate reductase
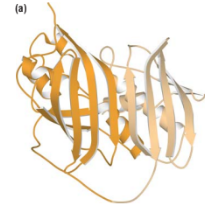
Thioesterase
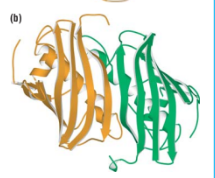
Thioester dehydrase
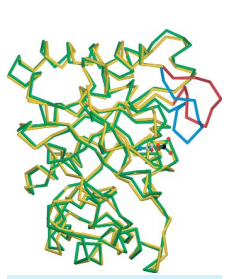
triosephosphate isomerase
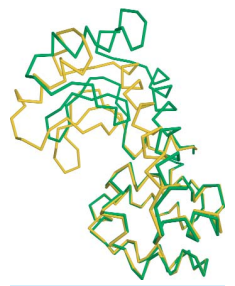
T4 lysozyme
Aspartate aminotransferase