GCSE WJEC Chemistry- bonding
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
why is diamond very hard
strong covalent bond
hydrogels
cross linked polymer structure influtes with solvent
why graphite has very high MP+BP
strong covalent bonds
ionic compounds MP+BP
high
simple molecular/ giant covalent bond
non-metal X2
nano silver use
anti-bac sprays → sterlising
nano titanium dioxide use
sunscreen (blocks UV) + self-cleaning glass (hydroliphic- water <3)
thermochromic paint use
thermometer
shape memory polymer
can gain original shape when heated
shape memory alloy
pseudo- elasticity, shape retenition memory
ionic bond
metal + non-metal
shape memory alloy example
braces
photochromic paint example
sunglasses
shape memory polymer example
self- repairing car body
photo- chromic paint
paint that changes with light
thermo-chromic paint
paint that changes with heat
2 hydrogel uses
nappies + artificial muscles
why metals conduct electricity
outer electrons free to move + carry a charge
when does ionic compound conduct electricity
when dissolved in water/ molten
metallic bond
metal X2
covalently bonded structure w 60 carbon atoms + football shape
buckminsterfullerene
does diamond or graphite conduct electricity
graphite
why does graphite conduct electricity
4th outer shell electron on carbon atom is free to move + carry charge
why cant diamond conduct electricity
no free electrons to carry a charge
is diamond or graphite soft
graphite
does simple molecular conduct electricity
no
2 properties of carbon nanotube
conducts electricity + very small
why metals have high MP + BP
difficult to separate ions due to strong forces of attraction
why simple molecular have low MP+BP
only weak forces of attraction holding them together
nano- particle size
1-100 nm
use of nano particle problems
dont know long term effects- could cause lung inflammation + cancer
2 examples of giant covalent substances
diamond + graphite
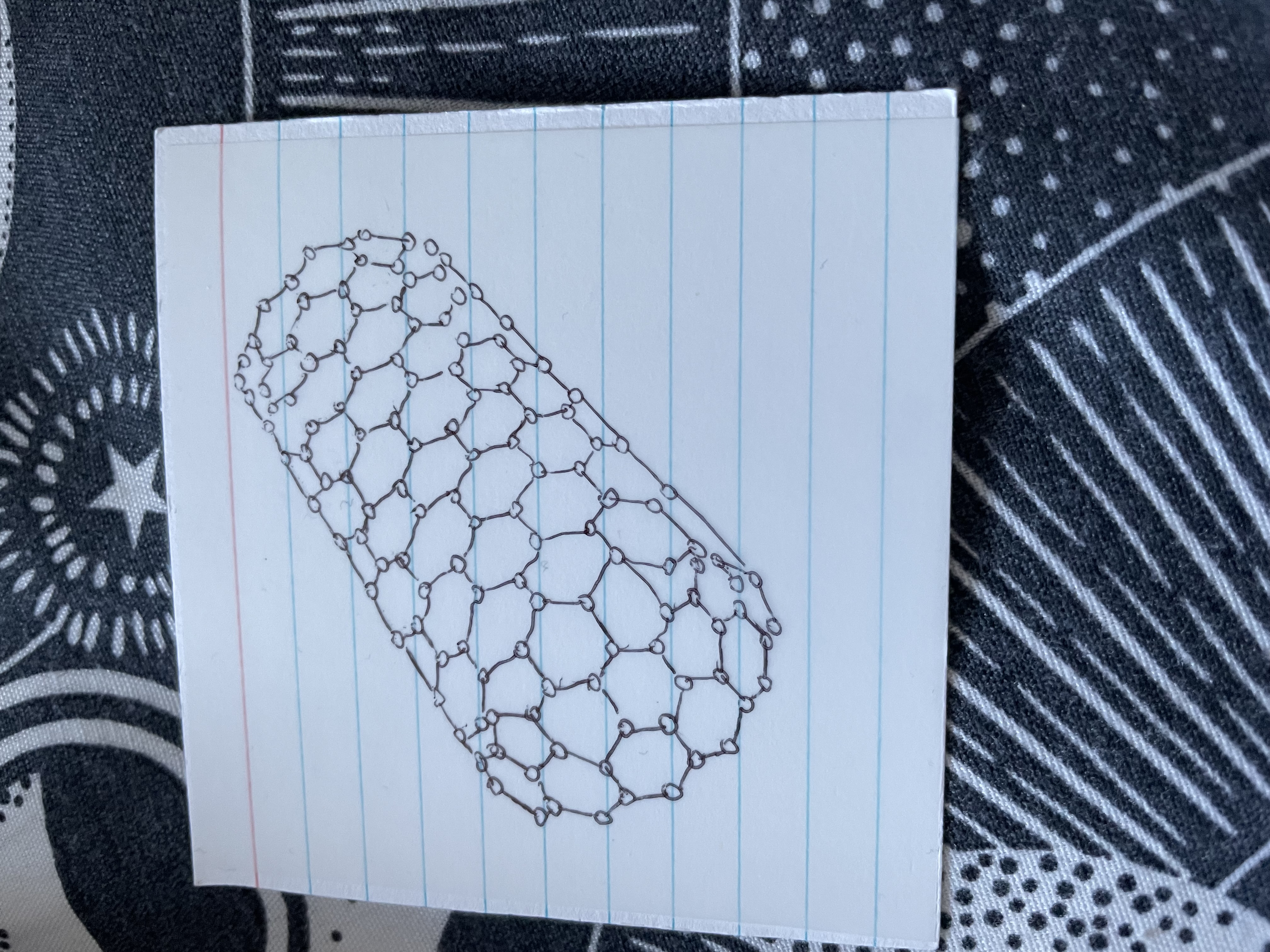
Name this
Carbon nanotube
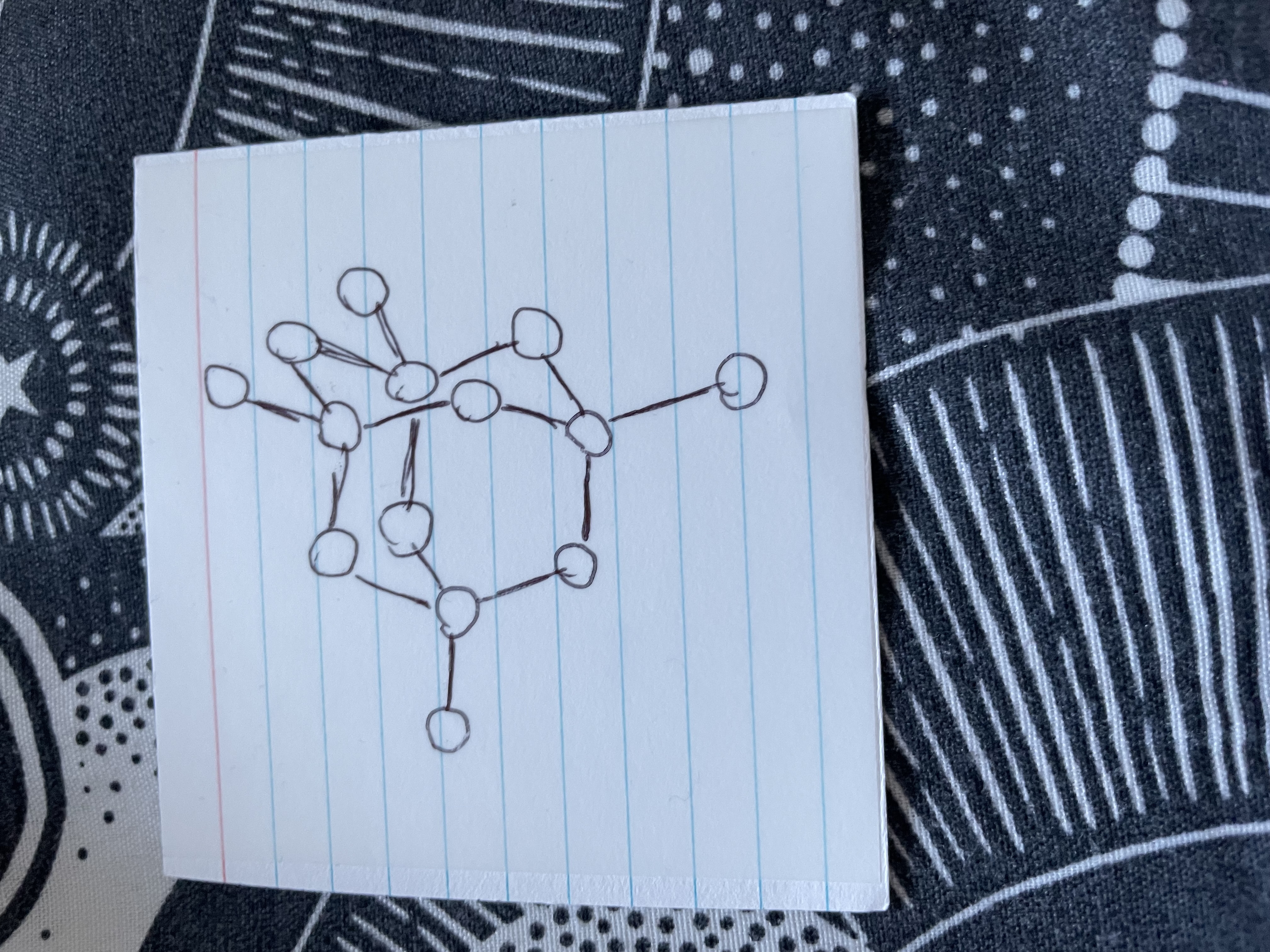
Name this
Diamond
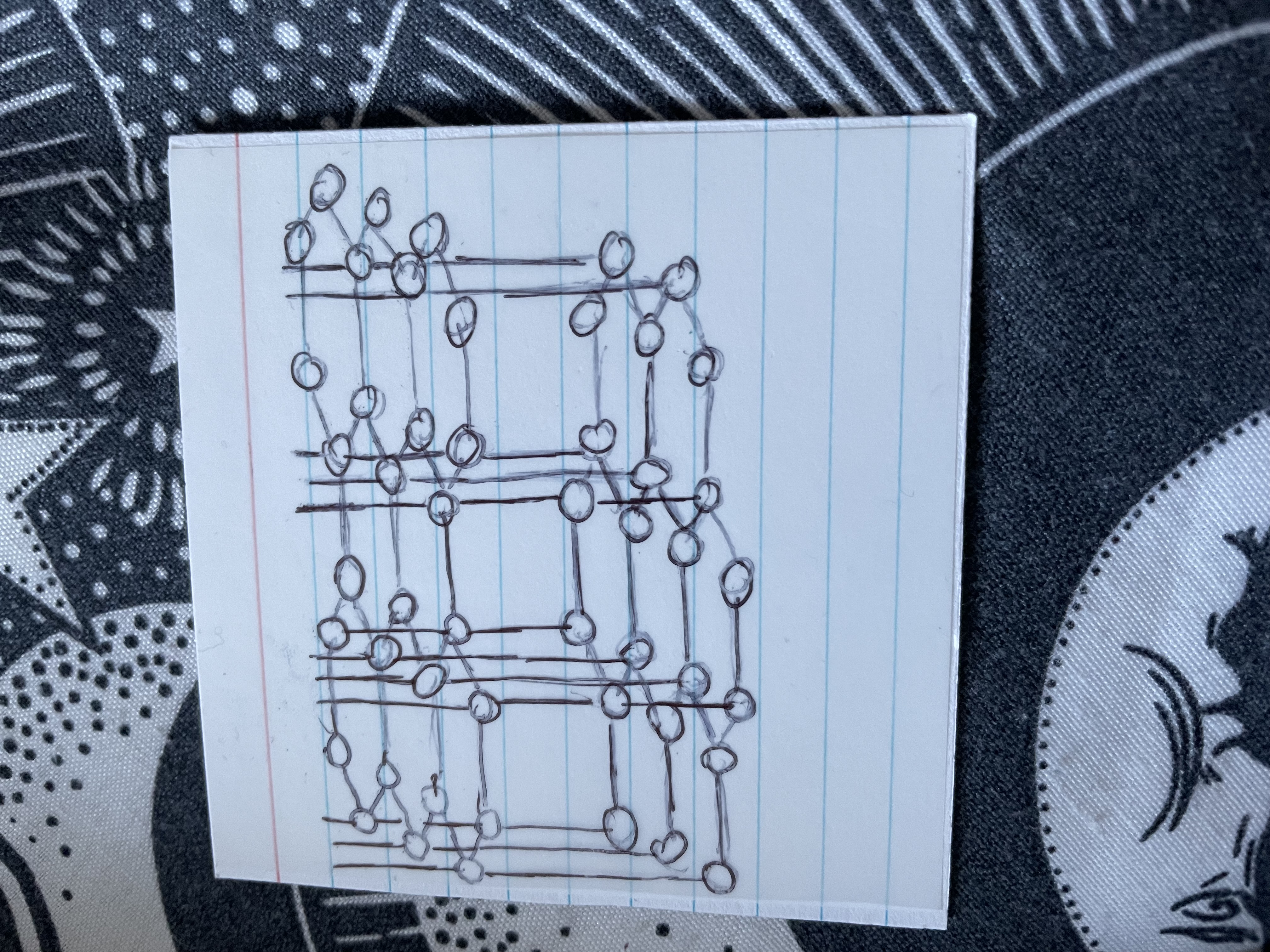
Name this
Graphite
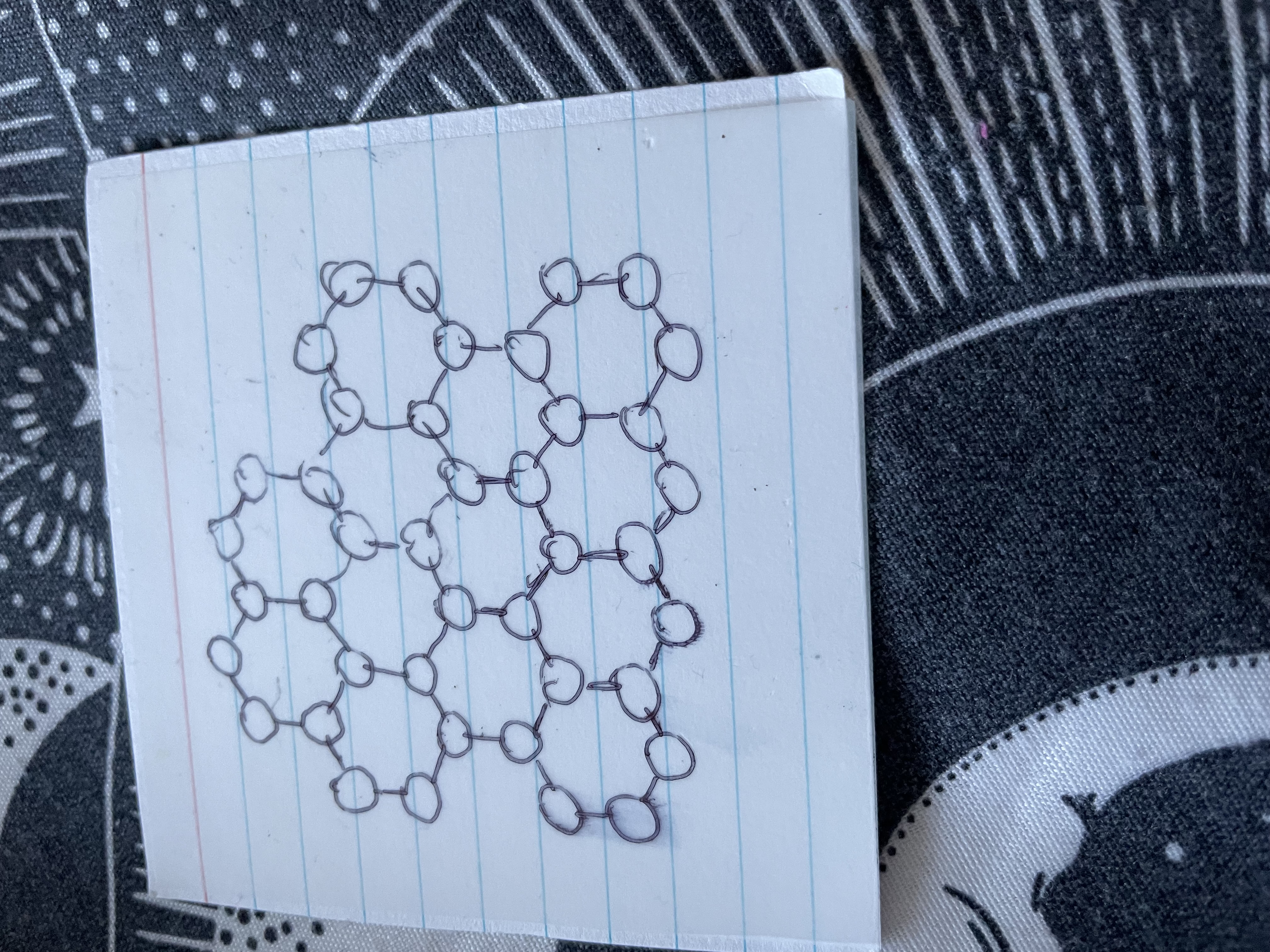
Name this
Graphine
What bonding exists in CO₂
covalent
Two differences between structures of diamond and graphite
graphite has layers but diamond doesn’t + graphite has free electrons, but diamond doesn’t