AP Biology Vocab
1/108
Earn XP
Description and Tags
all the small lil things you should know that are not incredibly easy
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Heat of Vaporization
The amount of energy(joules/calories) needed to vaporize one gram or mole of a substance from a liquid to a gas at constant temp and pressure.
Evaporative Cooling
Occurs when the highest kinetic energy molecules of a liquid evaporate, reducing the average kinetic energy(temperature) of the remaining liquid
Hydration shell
A sphere of water(H2O molecules) that surrounds dissolved ions, orienting themselves to match the ions charge(+ or -)
Hydrophilic/Hydrophobic
Hydrophilic refers to a molecules affinity for water as a polar molecule, and is therefore attracted to water
Hydrophobic is the opposite, as a non polar molecule, it is repelled by water
Cohesion
The ability for like molecules(not just water) to stick together due to intermolecular forces(like hydrogen bonding for water)
Adhesion
The ability for molecules to stick to different surfaces like water’s ability to stick to other polar surfaces due to its already polar nature.
Specific Heat Capacity
Specific heat capacity is the amount of energy (in joules or calories) required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
Solvent
Substance that is being dissolved in the solute
For example: NaCl(salt) is the solvent when placed in water
Solute
Substance dissolving the solvent
For example: Water is the solute when NaCl is placed in water
Dalton
The approximate weight of 1 proton or 1 neutron(unit of mass)
Hydrocarbons
Carbon atoms bonded with Hydrogen
Isomers
Molecules with the same chemical formulas but with different atomic orientations, resulting in multiple distinct molecules
For example: Glucose & Fructose are both written as C6H12O6 but are different 
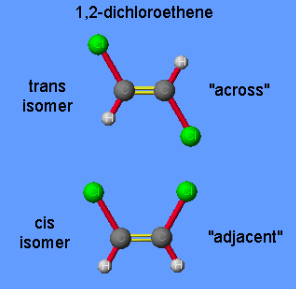
Trans-isomers
Cis-isomers
Both are variations of the same molecule
atoms/groups are on opposite sides of each other on a ring structure
Differentiating factor = atoms/groups are adjacent to each other
These occur around a rigid structure, typically a double bond or a ring
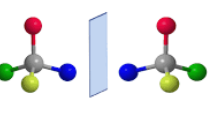
Enantiomers
Molecules with the same molecular formula but are structurally mirror images of each other
Polymers
LONG Molecular chains made up of repeating monomers(smaller units) which can be composed of multiple kinds of monomers
Monomers
A single molecular unit that has the ability to covalently bond with other monomers(not just its own type of monomer) to form a larger chain called a polymer
Macromolecules
Massive molecules(usually polymers) made from other organic molecules that are fundamental to life
These include: Carbohydrates, Proteins and Nucleic Acids
Monosaccharides
A single simple carbohydrate that is usually some form of CH2O
Serve as monomers for other more complex carbs
Examples include Fructose and Glucose
Disaccharides
A more complex carbohydrate that is formed through 2 monosaccharides bonding through a glycosidic linkage(a covalent bond) through a dehydration reaction
For example: Maltose is formed through two molecules of glucose
Polysaccharides
The most complex form of carbohydrate, formed through hundreds to thousands of monosaccharides joined by glycosidic linkages
Often used as fuel storage, and are hydrolyzed when needed or are used as structural protections
Enzymes
Specialized large protein molecules(a macromolecule) that speed up chemical processes by catalyzing the process
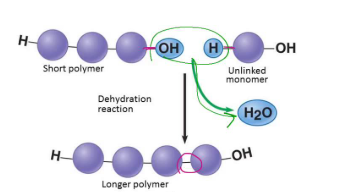
Dehydration Reaction
A reaction that bonds two monomers together through the loss of a -OH from one and a -H from another in order to create a polymer and a H2O
A “build” mechanism
Forms macromolecules
Hydrolysis Reaction
The process of H2O pushing apart two bonded monomers apart, one gaining a hydroxyl group and the other gaining a H
A “break” mechanism
Digestion that breaks complex carbs into simpler sugars
Ester Linkage
A covalent bond formed between a glycerol and fatty acids through a dehydration reaction, creating a triglycerol
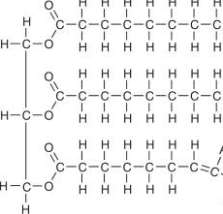
Carbohydrates
One of the 4 main biological macromolecules and ranges from small simple sugars (monosaccharides) to big complex carbohydrates(polysaccharides)
Functions: Providing sugar(energy) to cells and serving as structural components
Lipids
Composed of mostly hydrocarbons, is characterized by its Hydrophobic nature allowing them to group up together in water
Includes: Fats, Phospholipids, Waxes and Steroids
Fats
Is a lipid
Formed through an ester linkage with glycerol and fatty acids, the resulting triglycerides are able to store tons of energy
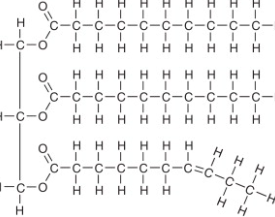
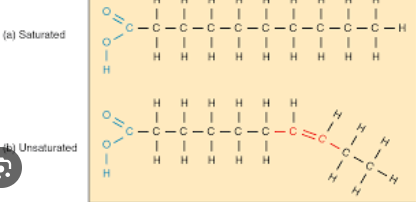
Saturated Fat
Refers to when the fatty acid lacks double bonds, allowing them to pack tightly to each other. Is seen in animal fat and stores lots of energy
Unsaturated Fat
Refers to when the structure of the hydrocarbon chains in a fatty acid contains double bonds(altering their typically straight structure), preventing them from packing tight. Most commonly seen in oils
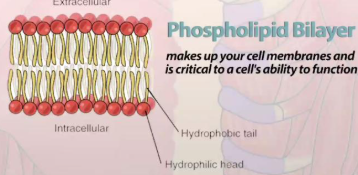
Phospholipids
A type of lipid, is typically seen as the cellular bilayer(membrane) since its dual(ampipathic) hydrophobic & hydrophilic nature forms a natural shield 
Waxes
A type of lipid characterized by an alcohol group bonded(via ester linkages) to a long fatty acid chain
Super duper hydrophobic and is therefore seen in waterproofing!(like ducks put it on their feathers so they stay waterproof)
Steroids
A type of lipid characterized by its 4 carbon skeleton rings
They differ through the varying positions of their functional groups(Ex. hydroxyl)
Cholesterol and the sex hormones are common examples
Trioses
a monosaccharide with 3 carbons formed in a straight line
Hexoses
a monosaccharide with 6 carbons formed MOST likely in a ring but occasionally in a straight line as well
Most common
Glycosidic Linkage
A covalent bond formed through a dehydration reaction between two monosachharides to form disaccharides or polysaccharides
Have 2 forms: α(alpha) and β(beta)
Alpha linkages are all the same
Beta linkages alternate
Enzymes that hydrolyze(break down) these linkages are unable to do both(alpha and beta). This is why we poop, our enzymes can only deal with one, and the other goes out as waste
Aldoses & Ketoses
Aldoses= monosaccharides with an aldehyde group (-CHO)
Ketoses= monosaccharides with a ketone group (C=O)
Glycerol
An alcohol molecule with a 3 carbon backbone with a -OH group on every Carbon
This structure allows it to form ester linkages to other molecules like fatty acids
Fatty Acids
Long hydrocarbon chains with a carboxyl group (-COOH) at one end.
This carboxyl group allows them to form ester linkages with glycerol (or other alcohols) through a dehydration reaction, creating lipids such as triglycerides and phospholipids.
Bilayer
Also called the cellular membrane, is a layer around the cell made of phospholipids that controls what comes in and out of the cell while providing protection.
Polypeptide
A molecule formed through the formation of amino acids bonded via peptide bonds
Polypeptides are linear chains of amino acids and are only called proteins when folded into 3 dimensional shapes
Peptide bond
A bond formed between amino acids
Is a dehydration reaction
To be more specific: a covalent bond formed between the carboxyl group of one amino acid and the amino group of another amino acid.
Proteins
Incredibly specialized and complex molecules made up of one or more polypeptides
Is 3-D in shape(polypeptides are only linear)
Have tons of diversity in their workplace, with some of their functions:
Enzymes
Structural
Transport
Signaling
Defense
Movement
Storage
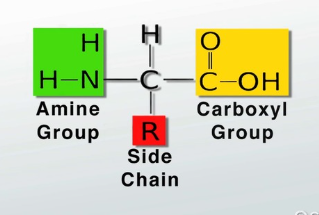
Amino Acids
(Hydrophobic)
(Hydrophilic)
(Acids and Bases)
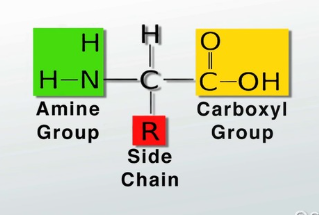
Amino acids are the building blocks of proteins.
Each amino acid consists of one central(alpha) carbon surrounded by:
An amino group(like NH2),
A Carboxyl group
A H
A R side group(molecular groups bonded to the amino acid that determine its properties)
An amino acid can be acidic, basic polar(hydrophilic), or hydrophobic based on the properties of the R side group
Disulfide Bridges
Covalent bond formed in polypeptides(can be the same polypeptide or in different ones) between the sulfhydryl groups within the polypeptide.
These sulfhydryl groups are only existent in the amino acid cysteine
Most importantly, these bonds fold the polypeptide into a protein with a 3-D structure
Denaturation
The unfolding of a protein's three dimensional shape through stress placed on the protein.
This leads to the complete destruction of that proteins function
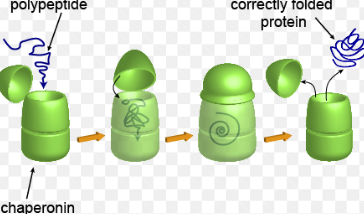
Chaperonins
Chaperonins- Cylindrical tubes that house a protein and provides it with optimal conditions to form
They are also a type of protein themselves
Proteins: Primary Structure
The primary structure of a protein consists of a linear chain of its amino acids
Only peptide bonds between the amino acids are prevalent at this stage
Act as the blueprint for the rest of the protein to follow as it forms
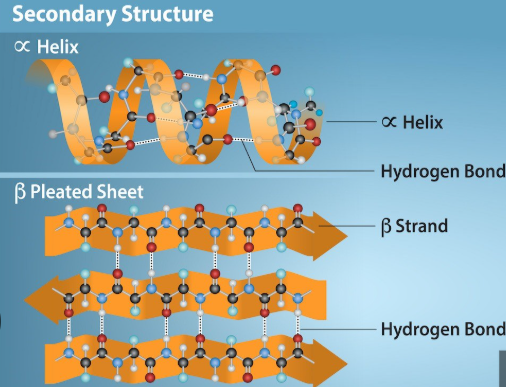
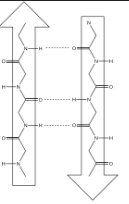
Proteins: Secondary Structure
The secondary structure is where the initial folding of the polypeptide occurs
This folding occurs between the carboxyl O of one and the H of the other(not R groups) and is formed/stabilized by hydrogen bonds(H and O = hydrogen bonding!)
There are two commonly seen structures as a result of this folding:
Alpha-helix (α-helix) as a spiral or a coil
Beta-pleated sheet (β-pleated sheet) as a sheet like structure
Proteins: Tertiary Structure
The tertiary structure is when a singular polypeptide chain attains its complex 3-D form
It does so through the folds that occur via many interactions and attractions such as:
H-bonding
Disulfide Bridges
Hydrophobic interactions(non-polar parts are pushed into the core of the molecule)
Van Der Waals(IMFS)
Ionic Bonds(between the negatively and positively charged parts of the R groups)
These interactions are often a driven by the R side groups of the amino acids
Tertiary structures can be proteins on their own
Proteins: Quaternary Structure
Quaternary structures emerge when 2 or more polypeptides come together to form an even more complex protein
The attractions between these polypeptides are the same forces as the ones within tertiary structures
Examples are collagen and hemoglobin
Polynucleotides
A long chain of many nucleotides(polymer) linked together through phosphodiester bonds
Make up the nucleic acids like DNA & RNA
Antiparallel
When two molecular strands are running side by side but in opposite directions
The most famous example is DNA, with its two strands going in opposite directions
This is critical for the replication process of DNA as the two strands are complementary
Nucleotides
A monomer consisting of three parts:
A nitrogenous base(adenine, guanine, thymine, cytosine, uracil)
A five-carbon sugar (ribose in RNA, deoxyribose in DNA)
One or more phosphate groups
These monomers are linked via phosphodiester bonds to form polynucleotides, the polymer that makes up nucleic acids
DNA
Is a nucleic acid
Found in the nucleus of Eukaryotic cells, DNA(or deoxyribonucleic acid) holds the genetic code for life
It exists as a double helix and its main function is to allow cells to duplicate accurately as well as transcribing RNA, which drives protein synthesis
RNA
Is a nucleic acid
Is transcribed from DNA(meaning it is based on DNA’s language into its own RNA language)
Main function is the synthesis of proteins(is quite complicated)
Exists as a single strand
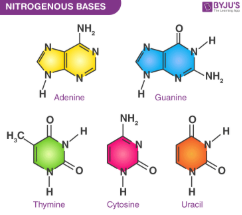
Nitrogenous base
A slightly basic molecule and consist of Adenine, Guanince, Thymine, Cytosine and Uracil(in RNA instead of T)
Are rings containing Carbon and Nitrogen
Their specific sequence within Nucleotides determines the genetic code
Complement each other(crucial in DNA’s replication)
Guanine and Cytosine
Adenine and Thymine/Uracil
Pentose
A 5 carbon sugar
anything with -ose is usually a sugar
Another part of nucleotides
a monosaccharide with 5 carbons formed in either a ring or in a straight line (linear)
Phosphate Group
A functional group: phosphorus atom bonding with 4 oxygen atoms
In a nucleotide, this group is bonded to the 5’ carbon
Important for ATP(adenosine triphosphate)
Purines
Nitrogenous bases characterized by a double-ring structure
Include Adenine and Guanine(complementary)
Pyrimidines
Nitrogenous bases characterized by a single ring structure. Includes Cytosine, Thymine and Uracil in RNA
Functional groups: Hydroxyl
-OH
Is polar
Functional groups: Carbonyl

Determines whether a sugar is a ketone or an aldose
Functional groups: Carboxyl
-COOH
acts like an acid
Functional groups: Amine
-NH2
Acts like a base
Functional groups: Sulfhydryl
-SH
Can react with itself to stabilize proteins
Functional groups: Phosphate
-PO3
Contributes negative charge allowing molecules to react with H2O
Functional groups: Methyl
-CH3
Impacts gene expression
Phosphodiester Bonds
Dehydration reaction created between nucleotides to form polynucleotides(like Dna and Rna)
Nucleus:
Made out of what parts?
What do each of these parts do?
Nucleus is the general statement of these parts:
Nuclear Envelope, a double membrane structure perforated with nuclear pores that decide what comes in and out the nucleus
Chromatin, DNA strands(uncoiled form) wrapped around stabilizing proteins called histones. When preparing to replicate, the chromatin condenses into chromosones
Nucleolus, the dense middle section of the nucleus, is where RNA is synthesized and is also where the initial structures of ribosomes are formed
Ribosomes:
Initially formation where?
Have what role based on where they are?
Ribosomes have their initial(and partial) formation within the nucleolus
Free Ribosomes in the cytoplasm synthesize ALL the proteins required to operate within the cytoplasm, such as enzymatic proteins(these proteins will stay within the cell indefinitely)
Bound Ribosomes in the rough ER will produce proteins that will either be destined for the bloodstream(secretion), cell membrane, or various organelles of the cell like the Golgi Apparatus or Lysosomes.
Endoplasmic Reticulum?
What are the different types and what differentiates them?
The Endoplasmic reticulum is a wide network of membranes with various functions:
The rough ER has ribosomes in it, giving it a rough appearance.
Is responsible for the synthesis of proteins, which are then secreted into the bloodstream or into other organelles
Makes a whole bunch of membrane for all the other organelles
The smooth ER with 0 ribosomes drives metabolic processes
Is responsible for detoxifying toxic materials within the cell
Synthesis of lipids(like steroids)
Metabolizes carbs
Stores Calcium Ions
Golgi Apparatus
Two faces?
Also produces ___________
The Golgi Apparatus is like the warehouse shipper of the cell, it receives membranes(called vesicles) from other organelles. For example, the Golgi Apparatus receives proteins from the ER and after modifying and packaging them, it is sent out to a specific destination.
The Golgi Apparatus looks like a bunch of membranous sacs(called cisternae)
When “receiving” something, it goes to its cis face, where the vesicle fuses with the cis face
The trans face then packages them and sends them out
The Golgi is also responsible for the production of macromolecules like polysaccharides
Lysosomes
Two processes?
Lysosomes are membranous sacs that contain powerful enzymatic proteins
When something comes into the cell, it is encapsulated in a membrane called a food vacuole. The Lysosome then fuses with the food vacuole, releasing all its enzymatic proteins within, breaking the substance down(hydrolysis). This process is called phagocytosis
The Lysosome also has the ability to recycle materials within a cell, allowing a cell to continuously renew itself. This process is called autophagy
Inside the Lysosome is a very acidic environment
An example is the macrophage, a human white blood cell that helps destroy foreign things in our bloodstream
Vacuoles
What are they?
What are the different types?
A vacuole is a large, membrane-bound sac found in the cytoplasm of a cell. Its function is to hold various materials, including water, food, and waste products.
Large central Vacuole
Large Central Vacuole (in plants): This is a prominent vacuole that stores water, nutrients, and waste products. It is crucial for maintaining turgor pressure, which is the internal pressure that keeps a plant cell rigid and helps the plant stand upright.
Contractile vacuole
Contractile Vacuole (in some protists): These vacuoles are found in freshwater protists like Paramecium. They expand as water flows into them and then contract to pump excess water out of the cell. Their primary role is osmoregulation (maintaining water balance) to prevent the cell from bursting.
Food Vacuole
What process is it associated with?
Food Vacuoles (in some protists and animals): These temporary vacuoles are formed during phagocytosis to store food particles. They fuse with lysosomes, which release digestive enzymes to break down the food for the cell to use.
Cytoskeleton
A network of fibers that functions as both structural support and communication within the cell
Is made of microtubules and microfilaments
Microtubules
Shape the cell, guide organelle movement, and separate chromosones. Cilia & Flagella have microtubules within them
Microfilaments
Super thin part of the cytoskeleton
Structure: They provide structural support for the cell, particularly in maintaining its shape and resisting tension.
Movement: Microfilaments are involved in many forms of cell movement, including muscle contraction, amoeboid movement, and the formation of pseudopods.
Cytoplasmic Streaming: In plant and animal cells, they are responsible for the circular flow of the cytoplasm, which helps distribute nutrients and organelles.
Endosymbiont Theory
A proven scientific theory that states that both the mitochondria and chloroplasts(good for producing energy) originated from bacteria with the similar function
Mitochondrian
creates what byproduct?
cellular _______
Is responsible for cellular respiration, generating most of a cells ATP which is used as a source of energy
Chloroplast
creates what byproduct?
Found in plant cells(green) and are present in all photosynthetic cells.
Creates glucose as a product of photosynthesis
Extracellular Matrix
Is a complex network of macromolecules secreted by cells(like glycoproteins & proteoglycans) and provides stability in tissues while also holding and influencing cells within the tissues.
Amphipathic
Means to be both hydrophobic and hydrophilic, most likely because of their two different ends(like a phospholipid)
Fluid Mosaic Model

This model describes the membrane as a fluid structure with its parts like phospholipids or proteins moving about.
These proteins are depicted as floating within the bilayer
Membrane fluidity
Is caused by what?
How do external stressors affect it?
Role of cholestrol?
Membrane fluidity describes the fluid nature of the membrane as loose hydrogen bonds hold it together, allowing its lipids and proteins to have some degree of freedom in their movements.
Stressors like an increase in temperature will increase the fluidity of the membrane
Cholestrol acts as a fluidity buffer within the membrane
Membrane proteins overview:
Two major groups?
While phospholipids make up the main form of the cellular bilayer, its proteins are what dictate much of the membrane’s functions.
For example, there are over 50 kinds of different membranous proteins within a red blood cell
The two main groups are: Integral Proteins & Peripheral Proteins
These membrane proteins can be connected to various structural supports such as the cytoskeleton within the cell or the extracellular matrix outside the cell, allowing the cells themselves to have a stronger framework
However they have A LOT of functions(and there is another flashcard about that)
Integral Proteins
Are embedded within the bilayer. They either span the full width of the bilayer with either end poking out from each side, or are partially embedded, but still in the bilayer.
Most span the full width and are called: Transmembrane proteins
Peripheral Proteins
Are under the bilayer(within the cell), and are loosely bounded there
Membrane Proteins Functions:
(6 major kinds)
Transport- Some transmembrane proteins can have Hydrophilic channels within them to shuttle a specific kind of solute while others use ATP energy to actively transport substances
Enzymatic Activity- Allows the membrane to carry out metabolic processes(sometimes these proteins group up as well to perform sequenced processes)
Signal Transduction- Proteins exposed to the outside can have receptors to receive signaling molecules to relay the message
Cell-Cell recognition- Some Glycoproteins serve as identifiers and are recognized by other proteins in other cell membranes
Intercellular binding- Membrane proteins can hook themselves to various lengths and are more long lasting than the process in cell-cell recognition
Attachment to structural supports- Membrane proteins can attach to the cytoskeleton or the extracellular matrix to stabilize the cell and overall strucure of the area
Role of Glycoproteins within the cell membrane
Since glycoproteins(proteins with carbs) are so diverse, they act as identifiers - markers to distinguish one cell from one another
Selective permeability
Defines the cell membrane’s ability to “choose” or allow certain substances through the membrane while keeping undesirable ones out/in
Non-polar substances like CO2 and O2 cross the lipid bilayer more easily while polar molecules like glucose and even water have a harder time crossing.
The membrane is only one gateway though, and molecules can pass through via membrane transport proteins
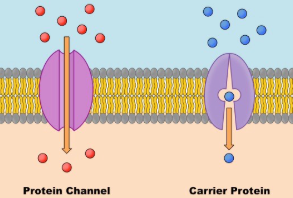
Transport Proteins
Since the cell membrane itself is made of lipids, which are non-polar & therefore don’t allow polar molecules to pass easily, transport proteins like aquaporins aid in the transport of substances into the cell.
Furthermore, there are two kinds of transport proteins: Channel proteins and Carrier proteins
Channel proteins provide a Hydrophilic channels within their membrane spanning structure to essentially bypass the membrane(all passive transport)
Carrier proteins hold on to their “passengers” and modify their own shape to pass release their passengers into the cell(passive & active Transport)
Aquaporins(channel), specifically, are incredibly important to bring water into the cell and are responsible for most of the supply a cell gets
Passive Transport(diffusion)
Diffusion is essentially transport of a substance with no energy investment
They go down to what is called the concentration gradient(a substance will diffuse from where its most concentrated to least concentrated)
Osmosis definition:
Water moves from an area of higher to lower free water concentration(lower to higher solute concentration)
This is essentially because solutes(like NaCl) are polar as ions broken down in water, and the H2O forms hydration shells around them, compacting into a smaller space, leaving more space for more water to fill.
Isotonic
A state of a cell where there is no net movement of water across the plasma membrane(like equilibrium)
Hypertonic
A state of the cell where more water is expelled rather than brought in, causing the cell to shrivel and shrink.
And this will happen due to more solute outside the cell causing osmosis to occur
Hypotonic
A state of the cell where more water is brought in rather than expelled, causing cells to either expand, or in serious cases lyse which is essentially the cell exploding due to severe bloating(like my stomach)
Osmoregulation
Evolutionary adaptations within cells living in hypertonic or hypotonic conditions to survive(such as a contractile vacuole to expel water in a hypotonic environment)