Test Review 2
0.0(0)
Card Sorting
1/113
Earn XP
Description and Tags
Bio-241 Microbiology
Last updated 2:47 PM on 8/5/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
1
New cards
Define metabolism
The sum of energy and chemical changes in a cell
2
New cards
Define energy
a property of objects which can be transferred to other objects or converted into different forms, but cannot be created or destroyed
3
New cards
What is the difference between potential and kinetic energy?
Energy associated with the motion of an object is called kinetic energy.
Energy stored in an object due to its position is called potential energy.
Energy stored in an object due to its position is called potential energy.
4
New cards
List the 5 types of energy
Chemical
thermal
kinetic
potential
Solar
thermal
kinetic
potential
Solar
5
New cards
Define calorie
(unit of heat)
6
New cards
How are calories measured?
Measure by the amount of heat needed to raise 1 gram or 1 milimeters of water 1 °C
7
New cards
What is the relationship between a kilocalorie and a calorie?
1 kcal = 1000 calories
8
New cards
Define chemical change
Molecules are reaaranged to form a new molecule
9
New cards
State the Law of Conservation of Matter
Matter can not be created or destroyed. The same amount of matter exists before and after any physical or chemical change.
10
New cards
What factors increase collisions of substances during chemical reactions?
Heat is the main factor that increase collisions of substances during chemical reactions. However, also in an increase in the concentration of the reactants cause a increase in collisions.
11
New cards
What are the two divisions of metabolism?
Catabolism and anabolism
12
New cards
What is the difference between catabolism and anabolism?
Catabolism is to destroy or decompose large molecules to smaller ones. Where’s Anabolism is to build up and create smaller molecules into bigger ones.
13
New cards
What is the difference between a hydrogen ion and the hydrogen atom?
A hydrogen atom has one electron and one proton. A hydrogen ion is created when a hydrogen atom loses an electron
14
New cards
Identify examples of catabolism and anabolism
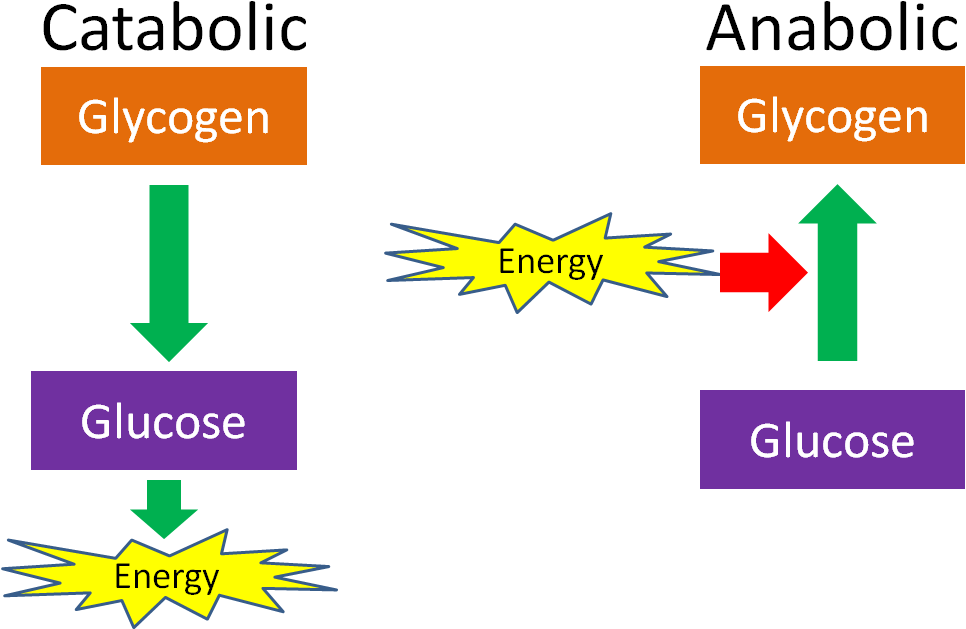
15
New cards
**What are electron carriers ?**
**Molecules that carry 2 electrons and either 1 or 2 hydrogen ions**
16
New cards
List the 3 types of electron carriers found in most cells.
NAD (Nicotinamid adenine dinucleotide)
NADP (Nicotinamid adenine dinucleotide Phosphate)
FAD ( flawin)
NADP (Nicotinamid adenine dinucleotide Phosphate)
FAD ( flawin)
17
New cards
What is the difference between the oxidized and reduced form of each electron carrier?
NAD- is not carrying the 2 electrons or a single hydrogen ion
NADM- carries 2 electrons and a single hydrogen ion
FAD - is not carrying the 2 electrons or 2 hydrogen ions
FADM: carries 2 electrons and a hydrogen ion
NADM- carries 2 electrons and a single hydrogen ion
FAD - is not carrying the 2 electrons or 2 hydrogen ions
FADM: carries 2 electrons and a hydrogen ion
18
New cards
List 4 characteristics of enzymes
==Organic molecules== – most enzymes are made of proteins, but some are made of ribonucleic acid
==Catalysts== – substances that speed up a chemical reaction but are not changed permanently in the chemical reaction
==Lowering the activation energy== – energy required to disassemble molecules so that they can recombine to form new molecules
denoted by the suffix “ase”
==Catalysts== – substances that speed up a chemical reaction but are not changed permanently in the chemical reaction
==Lowering the activation energy== – energy required to disassemble molecules so that they can recombine to form new molecules
denoted by the suffix “ase”
19
New cards
What is the difference between a simple enzyme and a conjugated enzyme
Simple enzymes – only made of protein
Conjugated enzymes – only made of protein and some other nonprotein molecules
-Holoenzymes – protein part + nonprotein part
-Apoenzymes (Cofactor) – nonprotein part (Metal ion or coenzyme)
Conjugated enzymes – only made of protein and some other nonprotein molecules
-Holoenzymes – protein part + nonprotein part
-Apoenzymes (Cofactor) – nonprotein part (Metal ion or coenzyme)
20
New cards
List 2 types of cofactors found in biochemical systems.
Metal ion – magnesium, copper, iron, etc.
Coenzyme – organic molecule (eg. Vitamins
Coenzyme – organic molecule (eg. Vitamins
21
New cards
Identify the two parts of a conjugated enzyme
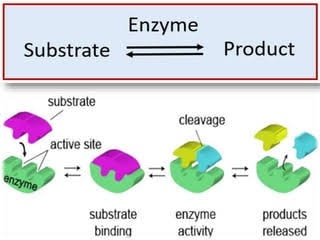
22
New cards
What is the relationship between the substrate and the active site of an enzyme?
The substrate is a substance acted upon by the enzyme and the active site is place on the enzyme where the substrate combines.
23
New cards
Define active site
Active site – place on the enzyme where the substrate combines
24
New cards
Define substrate.
S = Substrate – substance acted upon by the enzyme
25
New cards
What is the function of a catalyst
a substance that speeds up a chemical reaction
26
New cards
What is the fate of E and S in the enzymatic equation, E + S -> ES -> E + P?
One substrate can be broken into 2 products (catabolism) or two substrates can combine to form 1 product (anabolism)
A catalyst is a substance (as an enzyme) that enables a chemical reaction to proceed at a usually faster rate under different conditions (as at a lower temperature) than otherwise possible.
Catalase converting hydrogen peroxide to water and oxygen (at a high rate)
For interaction of enzyme and substrate to occur, a substrate must have a correct
A catalyst is a substance (as an enzyme) that enables a chemical reaction to proceed at a usually faster rate under different conditions (as at a lower temperature) than otherwise possible.
Catalase converting hydrogen peroxide to water and oxygen (at a high rate)
For interaction of enzyme and substrate to occur, a substrate must have a correct
27
New cards
Identify the parts of an enzymatic reaction in a chemical reaction that involves an enzyme
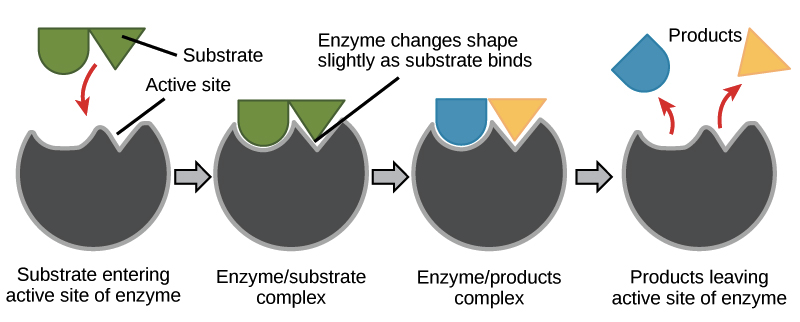
28
New cards
Define activation energy
the minimum quantity of energy which the reacting species must possess in order to undergo a specified reaction.
29
New cards
How are enzymes denoted?
denoted by the suffix “ase”
30
New cards
Explain why enzymes are characterized as highly specific.
each enzyme catalyzes only one type of reaction in only one compound or a group of structurally related compounds. The compound or compounds on which an enzyme acts are known as its substrates.
31
New cards
What is the difference between an endoenzyme and an exoenzyme?
exoenzymes are transported extracellularly \n \n endoenzymes are retained intracellularly and function there
32
New cards
Describe the structure of adenosine triphosphate.
consisting of a nitrogenous base (adenine), a ribose sugar, and three serially bonded phosphate groups.
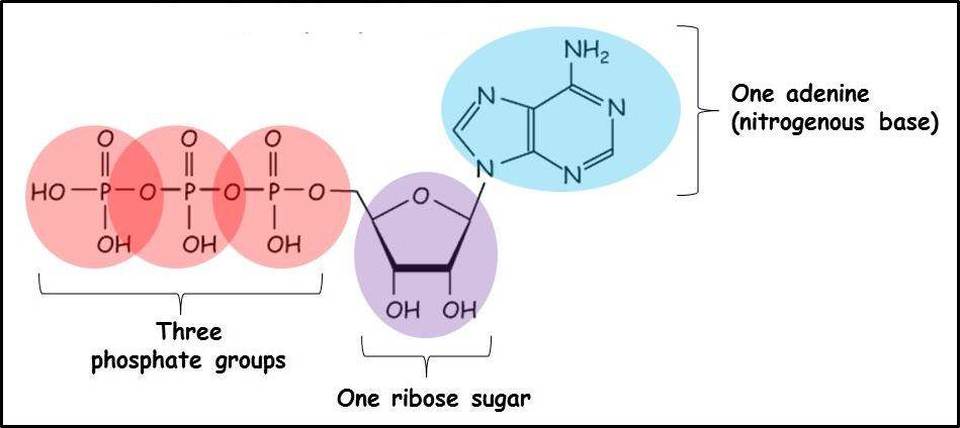
33
New cards
List 2 differences between ADP and ATP.
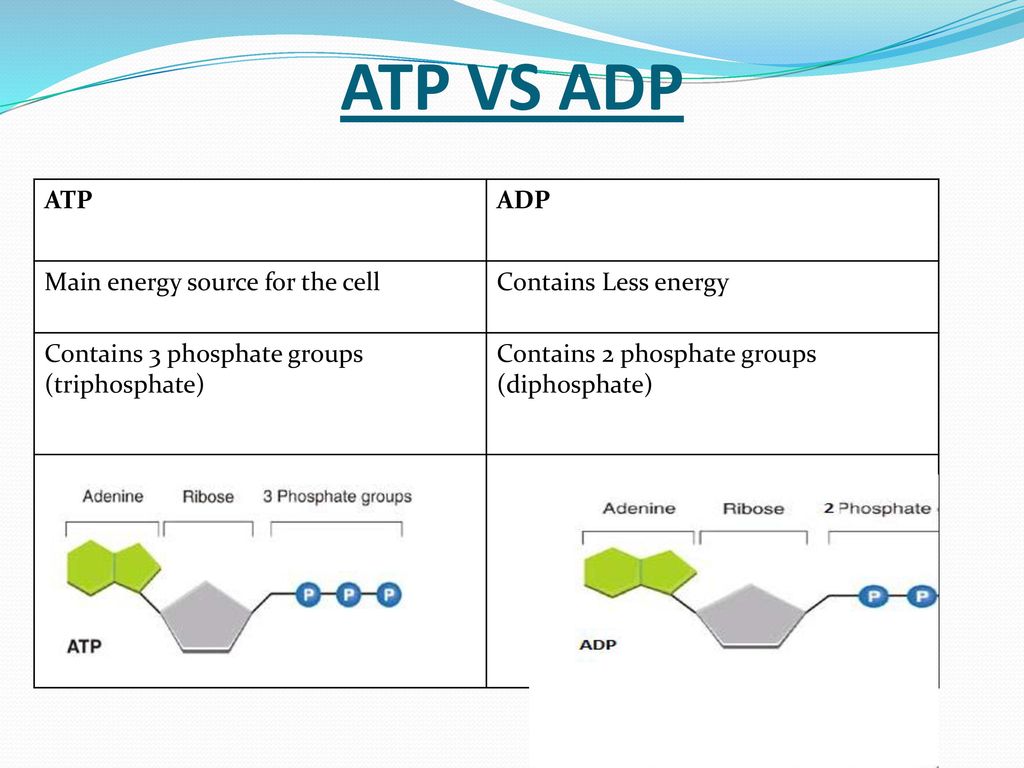
34
New cards
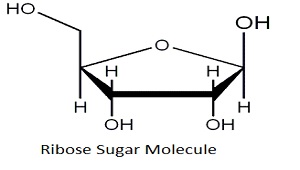
What is the chemical formula for ribose?
C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H.
35
New cards
What are the differences between substrate level phosphorylation and chemiosmosis?
30-32 molecules of ATP are generated by chemiosmosis during breakdown of one molecule of glucose. Whereas only about 8 molecules are formed through substrate level phosphorylation, that is transfer of a high energy phosphate group from a substrate to ADP.
36
New cards
What is the function of ATP synthase?
to synthesize ATP from ADP and inorganic phosphate (Pi) in the F1 sector.
37
New cards
How many ATP are generated from NADH and FADH2 during chemisosmotic phosphorylation?
only two ATP molecules
38
New cards
What is the difference between a phosphoryl shift and phosphoryl transfer?
Phosphoryl transfer is the name given to the chemical process of the transfer of the phosphoryl group (PO3) from a phosphate ester or anhydride to a nucleophile.
\
phosphoryl shift is a shift in the conversion of 3-phosphoglycerate into 2-phosphoglycerate.
\
phosphoryl shift is a shift in the conversion of 3-phosphoglycerate into 2-phosphoglycerate.
39
New cards
What is the difference between oxidation and reduction?
Oxidation is when the total number of electrons increases in a reaction
reduction is when the total number of electrons decreases in a reaction.
reduction is when the total number of electrons decreases in a reaction.
40
New cards
List 3 characteristics of glucose
* It's called simple sugar due to its monosaccharide.
* The chemical formula of glucose is C6H12O6.
* IUPAC's name for glucose is D-glucose.
* The chemical formula of glucose is C6H12O6.
* IUPAC's name for glucose is D-glucose.
41
New cards
Identify examples of each of the 7 chemical reactions of metabolism in Glycolysis and the Kreb Cycle.
42
New cards
List the products formed during Glycolysis and the Kreb Cycle.
In glycolysis, two ATP were invested, and four ATP and two NADH+H+ were produced.
In the Krebs cycle, two ATP, six NADH +H+, and two FADH2 were produced.
In the Krebs cycle, two ATP, six NADH +H+, and two FADH2 were produced.
43
New cards
List the products formed in the Electron Transport Chain.
ATP, NAD+, FAD, water, and protons.
44
New cards
Explain what happens to pyruvate during the transition steps in cellular respiration ?
The transition reaction converts the two molecules of the 3-carbon pyruvate from glycolysis (and other pathways) into two molecules of the 2-carbon molecule acetyl Coenzyme A (acetyl-CoA) and 2 molecules of carbon dioxide.
45
New cards
What is the difference between the role of an electron carrier and that of a coenzyme?
46
New cards
Describe the changes associated with pyruvate during the Transition Steps?
Before pyruvate can enter the next stage of cellular respiration it needs to be modified slightly. The transition reaction is a very short reaction which converts the two molecules of pyruvate to two molecules of acetyl CoA, carbon dioxide, and two high energy electron pairs convert NAD to NADH.
47
New cards
What is the difference between fermentation and anaerobic respiration?
Fermentation uses glycolysis only.
Anaerobic respiration uses all three parts of cellular respiration, including the parts in the mitochondria like the citric acid cycle and electron transport.
Anaerobic respiration uses all three parts of cellular respiration, including the parts in the mitochondria like the citric acid cycle and electron transport.
48
New cards
Define aerobic respiration.
(includes Glycolysis, Transition Steps, Krebs Cycle, Electron Transport Chain). The
final electron acceptor is oxygen
final electron acceptor is oxygen
49
New cards
Write the summary equation for photosynthesis.
6CO2 + 6H2O → C6H12O6 + 6O2.
50
New cards
What is the role of oxygen in photosynthesis and aerobic respiration?
In Photosynthesis, Oxygen is released as a byproduct.
In cellular respiration, oxygen is used to break down glucose, releasing chemical energy and heat in the process.
In cellular respiration, oxygen is used to break down glucose, releasing chemical energy and heat in the process.
51
New cards
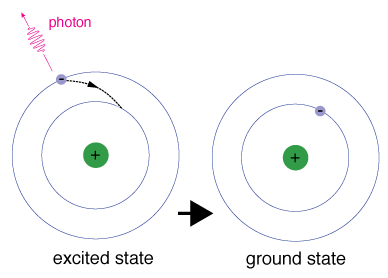
What is the difference between the ground and excited state?
The ground state configuration is the lowest energy, most stable arrangement. An excited state configuration is a higher energy arrangement
52
New cards
What is the difference between magnesium in the ground state and the excited state?
1s22s22p63s13p1 represents an atom of magnesium in an excited state. \n The ground state electronic configuration is 1s22s22p63s2. One electron is transferred from 3s to 3p level to form excited state. Mg has atomic number of 12. Thus it will have 12 electrons.
53
New cards
Define pigment?
substances that reflect the wavelength of light they cannot absorb (algae and blue-green algae, the reflected light we see color)
54
New cards
Identify the origin of electrons, the replacement electrons and the final electron acceptors in Photosystems II and 57. I of noncyclic photophosphorylation.
Light Dependent Reactions (Light Reactions) proceed only in the presence of light
(a) Noncyclic photophosphorylation – involves P680 (Photosystem II); generates molecular oxygen, NADPH, and ATP (680 wavelength is the highest to excite electrons)
(b) Cyclic photophosphorylation – involves only P700 (photosystem I); generates ATP
\
Light Independent Reactions (Dark Reactions) proceed in the absence or presence of light
(a) Calvin Cycle uses ATP and NADPH generated during the light reactions to synthesize glucose and other organic molecules.
(b) 2 electrons ejected from PS II upon reaction to sunlight, it goes to PS I, where it is ejected again until they get to the final destination NADP+. From H2O -> ½ O2 + 2H rxn.
(c) Sun hits both P680 & P700 (chlorophyll clusters). The original electrons from P680 comes from water. The original electrons for P700 comes from P680. Final destination for electrons leaving P680 is P700. Final destination for electrons leaving P700 is NADP (enzyme: Pyridine nucleotide reductase)
(d) For cyclic, only ATP is generated, no NADPH.
(a) Noncyclic photophosphorylation – involves P680 (Photosystem II); generates molecular oxygen, NADPH, and ATP (680 wavelength is the highest to excite electrons)
(b) Cyclic photophosphorylation – involves only P700 (photosystem I); generates ATP
\
Light Independent Reactions (Dark Reactions) proceed in the absence or presence of light
(a) Calvin Cycle uses ATP and NADPH generated during the light reactions to synthesize glucose and other organic molecules.
(b) 2 electrons ejected from PS II upon reaction to sunlight, it goes to PS I, where it is ejected again until they get to the final destination NADP+. From H2O -> ½ O2 + 2H rxn.
(c) Sun hits both P680 & P700 (chlorophyll clusters). The original electrons from P680 comes from water. The original electrons for P700 comes from P680. Final destination for electrons leaving P680 is P700. Final destination for electrons leaving P700 is NADP (enzyme: Pyridine nucleotide reductase)
(d) For cyclic, only ATP is generated, no NADPH.
55
New cards
List the products of noncyclic photophosphorylation.

56
New cards
What happens during the Hill Reaction?
Water is split into hydrogen and oxygen; the electrons released from hydrogen are used as replacement electrons for Photosystem II (P680)
57
New cards
What is the difference between anoxygenic an oxygenic photosynthesis?
Oxygenic photosynthesis – oxygen releasing because the replacement electrons come from the hydrogen in water (H2O)
Anoxygenic photosynthesis – non-oxygen releasing, because the replacement electrons come from the hydrogen in compounds such as hydrogen sulfide (H2S)
Anoxygenic photosynthesis – non-oxygen releasing, because the replacement electrons come from the hydrogen in compounds such as hydrogen sulfide (H2S)
58
New cards
Define microbial growth
an increase in the number of cells in a population
59
New cards
What are the differences between budding and binary fission?
Fission – the splitting of a parent cell into two (2) genetically-identical daughter cells.
Budding – the outgrowth or projection of a parent cell called a bud differentiates into a single () genetically identical daughter cell (hydra or yeast-eukaryotic cell)
Budding – the outgrowth or projection of a parent cell called a bud differentiates into a single () genetically identical daughter cell (hydra or yeast-eukaryotic cell)
60
New cards
Define fragmentation.
the breakage or fragmentation of a filamentous multicellular individual. Each
piece continues to thrive as a separate individual (spirogyra)
piece continues to thrive as a separate individual (spirogyra)
61
New cards
Define temperature.
The average kinetic energy or degree of hotness of a system
62
New cards
Define psychrophile, mesophile, and thermophile
Psychrophiles – requires cold temperatures for growth (-4 C to 22 C)
2\. Mesophiles – requires moderate temperatures for growth (12 C to 45 C)
3\. Thermophiles – requires hot temperatures for growth (42 C to 72 C) – can’t reproduce in body
(a) Extreme thermophiles – require extremely hot temperatures for growth (62 C – 120 C)
\- Water freezes a 0 C; water boils at 100 C, normal body temperature is about 37 C.
2\. Mesophiles – requires moderate temperatures for growth (12 C to 45 C)
3\. Thermophiles – requires hot temperatures for growth (42 C to 72 C) – can’t reproduce in body
(a) Extreme thermophiles – require extremely hot temperatures for growth (62 C – 120 C)
\- Water freezes a 0 C; water boils at 100 C, normal body temperature is about 37 C.
63
New cards
What is the difference between the minimum and maximum temperature for growth?
Minimum temperature required for growth – lowest temperature (some organisms like bacteria and fungi can live at temperature below the minimum but they will not be able to reproduce or grow and will not die
\
Maximum temperature required for growth – highest temperature (will reproduce slowly, but above the maximum you will kill them)
\
Maximum temperature required for growth – highest temperature (will reproduce slowly, but above the maximum you will kill them)
64
New cards
Define optimum temperature for growth.
best temperature
65
New cards
Define buffer
substances that resist changes in pH
66
New cards
How are hydrogen and hydroxyl ions symbolized?
Hydrogen ion=H+
Hydroxyl ion=OH−
Hydroxyl ion=OH−
67
New cards
Define pH
measurement of the hydrogen ion concentration of a solution
68
New cards
What is the difference between an acidic and basic solution?
Acidic solutions have a high concentration of hydrogen or hydronium ions.
In contrast, basic solutions have a lower concentration of hydronium ions and a higher concentration of hydroxide ions.
In contrast, basic solutions have a lower concentration of hydronium ions and a higher concentration of hydroxide ions.
69
New cards
Describe a neutral solution.
Neutral solutions are those in which the concentrations of hydrogen and hydroxide ions are the same
70
New cards
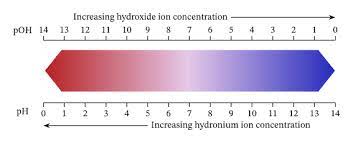
What is the relationship between the pH scale and the hydrogen ion concentration of a solution?
The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 1). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH. The pH scale ranges from 0 to 14.
71
New cards
Give some examples of acids and bases.
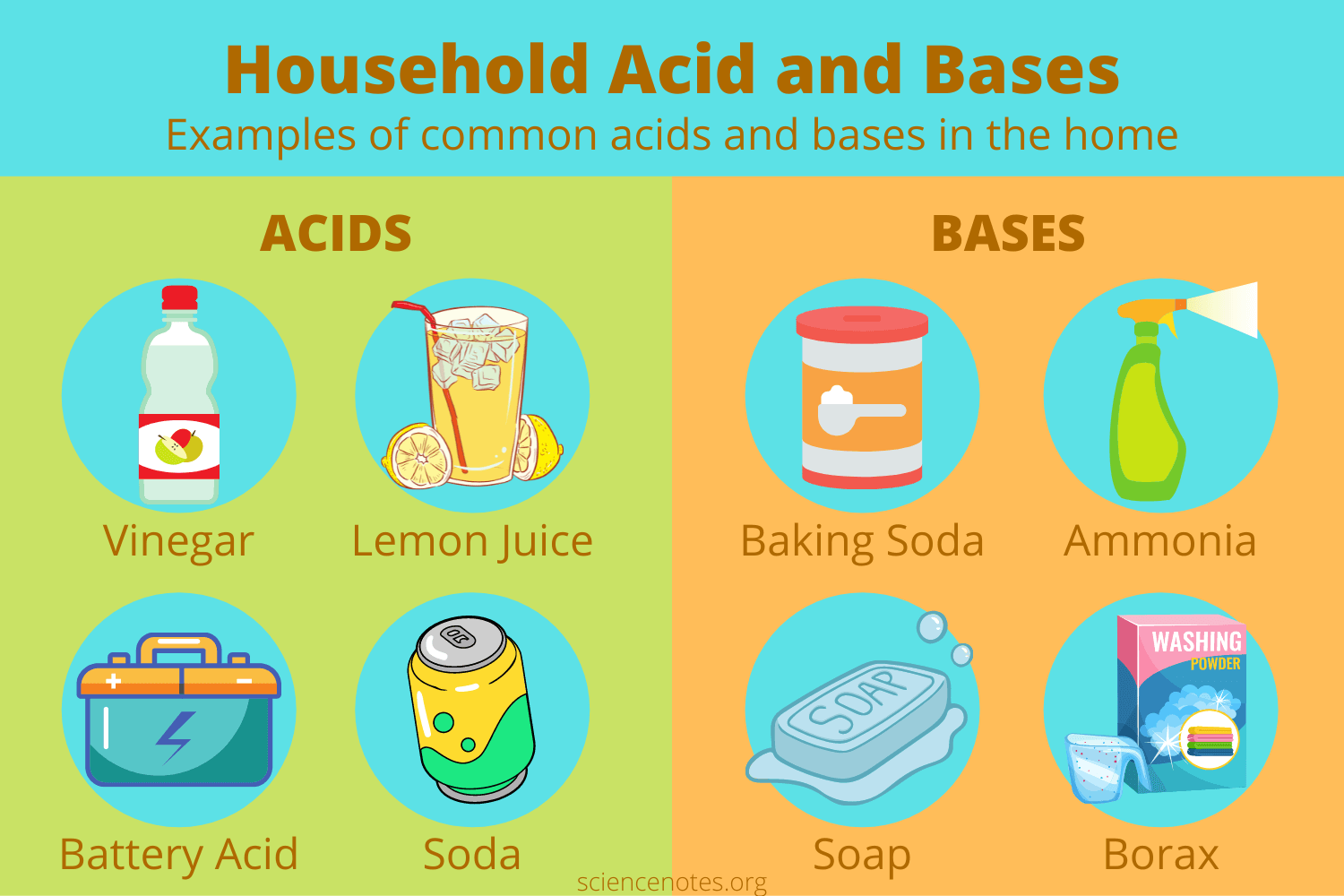
72
New cards
Define *per cent*?
means part per 100 parts
73
New cards
How do you prepare a *per cent* solution?
How do you prepare a 8% NaCl solution?
8 parts NaCl / 8 parts NaCl + 92 parts H2O or Add 8 parts NaCl to 92 parts H2O
8 parts NaCl / 8 parts NaCl + 92 parts H2O or Add 8 parts NaCl to 92 parts H2O
74
New cards
What is the difference between a dilute and a concentrated solution?
dilute solution” refers to a solution in which a small amount of solute is dissolved in a large amount of solvent. The term “concentrated solution” refers to a solution in which the amount of solute dissolved is more compared to the amount of solvent.
75
New cards
Define osmosis
Water moves from a high concentrated to a low concentration and continues until the two solution are equal in concentration
76
New cards
What is the difference between an acidophile and a halophile?
acidophiles-microorganisms that live in high concentration of acids
halophile-microorganisms that live in high salt concentrations. Includes Archaea, Bacteria, Fungi, and Algae species.
halophile-microorganisms that live in high salt concentrations. Includes Archaea, Bacteria, Fungi, and Algae species.
77
New cards
What are the differences between a hypotonic and hypertonic solution?
Hypertonic – high concentration; water moves out of the cell causing the cell to shrink/the decrease in size
\
low concentration; water moves into the cell causing the cell to increase in size – it may swell and even burst
\
low concentration; water moves into the cell causing the cell to increase in size – it may swell and even burst
78
New cards
Define isotonic.
Isotonic – same concentration; water moves in and out of the cell at the same rate – cell remains the same size
79
New cards
What are the carbon and energy sources for a photoheterotroph, chemoheterotroph, photoautotroph, and a chemoautotroph?
Photoautotroph – use light as the energy source and carbon dioxide as the carbon source (algae,
bacteria, plants)
4\. Photoheterotroph – use light as the energy source and carbon-containing compounds other than
carbon dioxide as the carbon source (bacteria, archaea)
5\. Chemoautotroph – use inorganic substances as the energy source and carbo dioxide as the
carbon source (archaea, bacteria)
6\. Chemoheterotroph – use carbon-containing compounds other than carbon dioxide as the carbon
source and energy sources (bacteria, archaea, protozoa, fungi, animals)
bacteria, plants)
4\. Photoheterotroph – use light as the energy source and carbon-containing compounds other than
carbon dioxide as the carbon source (bacteria, archaea)
5\. Chemoautotroph – use inorganic substances as the energy source and carbo dioxide as the
carbon source (archaea, bacteria)
6\. Chemoheterotroph – use carbon-containing compounds other than carbon dioxide as the carbon
source and energy sources (bacteria, archaea, protozoa, fungi, animals)
80
New cards
Why are carbonates used in a culture medium?
put in medium sodium carbonate or sodium bicarbonate (CO2 would go in
atmosphere if put in medium, but CO3 will release CO2 when added to water
atmosphere if put in medium, but CO3 will release CO2 when added to water
81
New cards
Give some examples of carbonates?
calcite or calcium carbonate, CaCO3
82
New cards
What is the difference between an obligate anaerobe and an obligate aerobe?
Obligate aerobes – strict requirement for oxygen
Obligate anaerobe – cannot live in the presence of oxygen (Clostridium botulinum – botulin) – Gram-positive bacteria in this group, will O2 will kill vegetative cell but not the endospore (can be found in swamps, deep in soil, in caves, causes diseases in people)
\
Obligate anaerobe – cannot live in the presence of oxygen (Clostridium botulinum – botulin) – Gram-positive bacteria in this group, will O2 will kill vegetative cell but not the endospore (can be found in swamps, deep in soil, in caves, causes diseases in people)
\
83
New cards
Define facultative anaerobe.
Facultative anaerobe – can live in the presence or absence of oxygen; if oxygen is present it uses it
84
New cards
What is the difference between an aerotolerant anaerobe and a microaerophilic organism?
Aerotolerant anaerobe – can live in the presence of oxygen but do not use it
Microaerophilic – requires very small amounts of oxygen (in large O2 it will kill it)
Microaerophilic – requires very small amounts of oxygen (in large O2 it will kill it)
85
New cards
Identify the elements in proteins and nucleic acids.
Nucleic acids contain the same elements as proteins: carbon, hydrogen, oxygen, nitrogen; plus phosphorous (C, H, O, N, and P)
86
New cards
What are organic growth factors? List some examples of organic growth factors?
Growth factors are organic compounds.
Such as amino acids , purines , pyrimidines , and vitamins
Such as amino acids , purines , pyrimidines , and vitamins
87
New cards
Define fastidious.
has very complicated nutritional requirements
88
New cards
What is agar?
A solidifying agent, consisting of a polysaccharide derived from certain marine alga that cannot be digested by most organisms
89
New cards
Why is agar used in a culture medium?
When heated to 100 C, then cool it down, forms something that isharder than Jell-O, it is a semi-solid. Reason for growing bacteria in semi-solid, is so that bacteria will just grow on the surface, and we can scoop off from the surface without any of the nutrients from the broth… pure bacteria or fungus
90
New cards
What is the difference between a broth and a semi-solid culture medium?
(a) Liquid – broth
(b) Semi-solid (broth + agar)
(b) Semi-solid (broth + agar)
91
New cards
What is the difference between a culture and a culture medium?
\-Culture medium: nutrients required for growing microorganisms in the laboratory \n -Culture: microorganisms growing in a culture medium
92
New cards
What is the difference between chemically-defined and a complex medium?
\-Chemically-defined: you know the exact chemical composition of every ingredient in the culture medium \n -Complex: you do not know the exact chemical formula of every ingredient in the culture medium. It varies from batch to batch.
93
New cards
Why are peptones, tryptones, and other *–one* compounds added to a culture medium?
They contain C, H, O, N, and S
94
New cards
What is the difference between a selective and differential media?
* selective media are used to isolate a particular strain of microorganisms whereas
* differential media are used to identify and differentiate a closely-related group of microorganisms.
* differential media are used to identify and differentiate a closely-related group of microorganisms.
95
New cards
Why is velvet used to replica-plate?
elveteen is utilized in the replica plating of yeast cells. The velveteen's texture allows the cells to be copied to a new plate exactly without morphing the appearance or location of a colony.
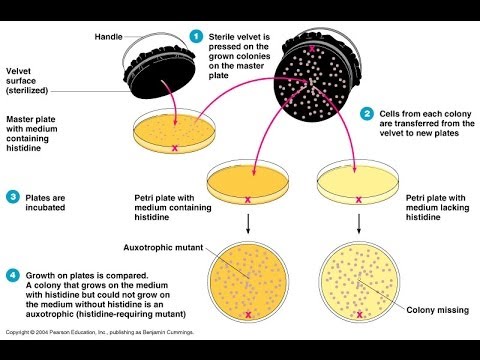
96
New cards
What is the difference between a pure and mixed culture?
\-Pure Culture: contains only one type of microorganism growing in a culture medium \n -Mixed culture: contains more than one type of microorganism growing in a culture medium (soil, food, water, feces, saliva, air)
97
New cards
Define streak plating.
rub a thin line of the specimen over the surface of a spread (agar) plate.
98
New cards
Define colony
descendants of a single cell that landed in a region (clones)
99
New cards
Define generation time.
time it takes a cell to divide.
100
New cards
Identify the symbols in b = B \* 2n.
\-b: final concentration of cells \n -B: initial concentration of cells \n -n: number of doublings/generations