biochemistyr exam 3 sidequest Glyccolysis
1/15
Earn XP
Description and Tags
Glyccolysis
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
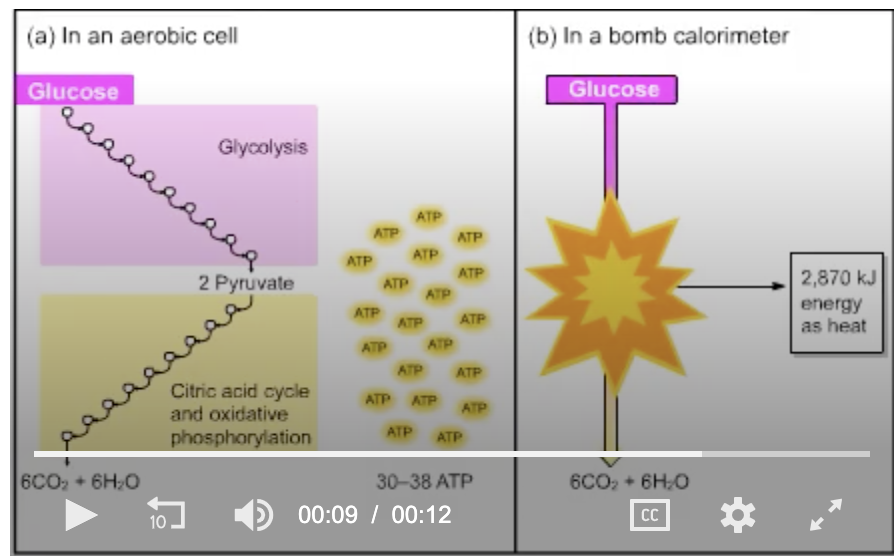
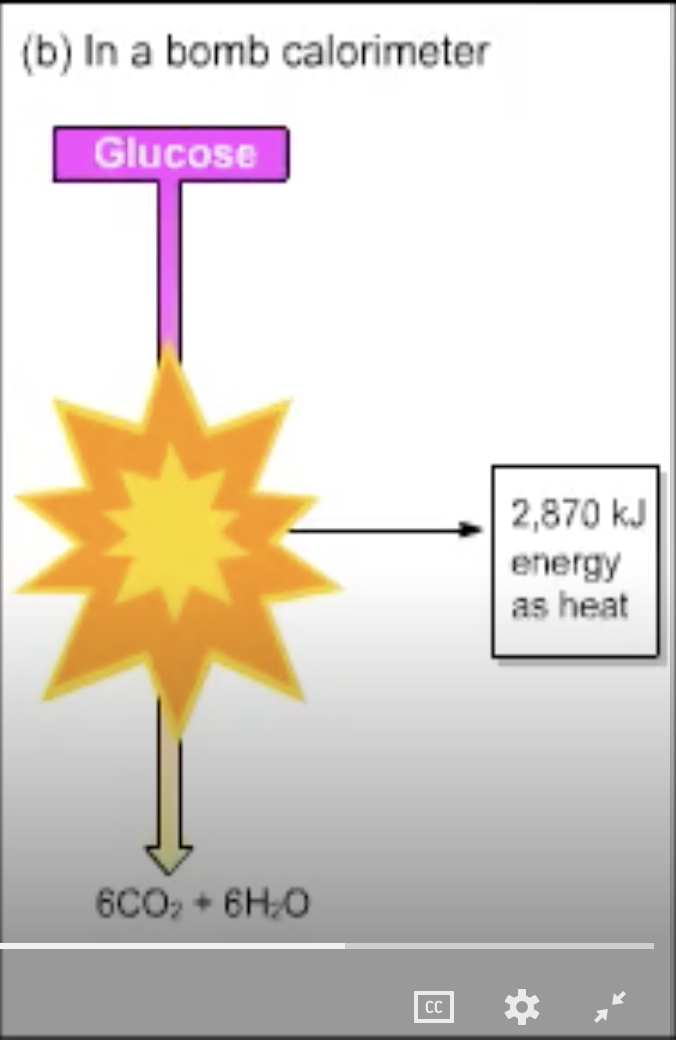
The starting material, glucose, and the end products, six molecules each of CO2 and H2O, are the same in parts (a) and (b). In (b), 2870 kJ are released as energy; where is that energy in (a)?
stored as H2O
given off as heat
stored as ATP
none of the above
given off as heat
stored as ATP
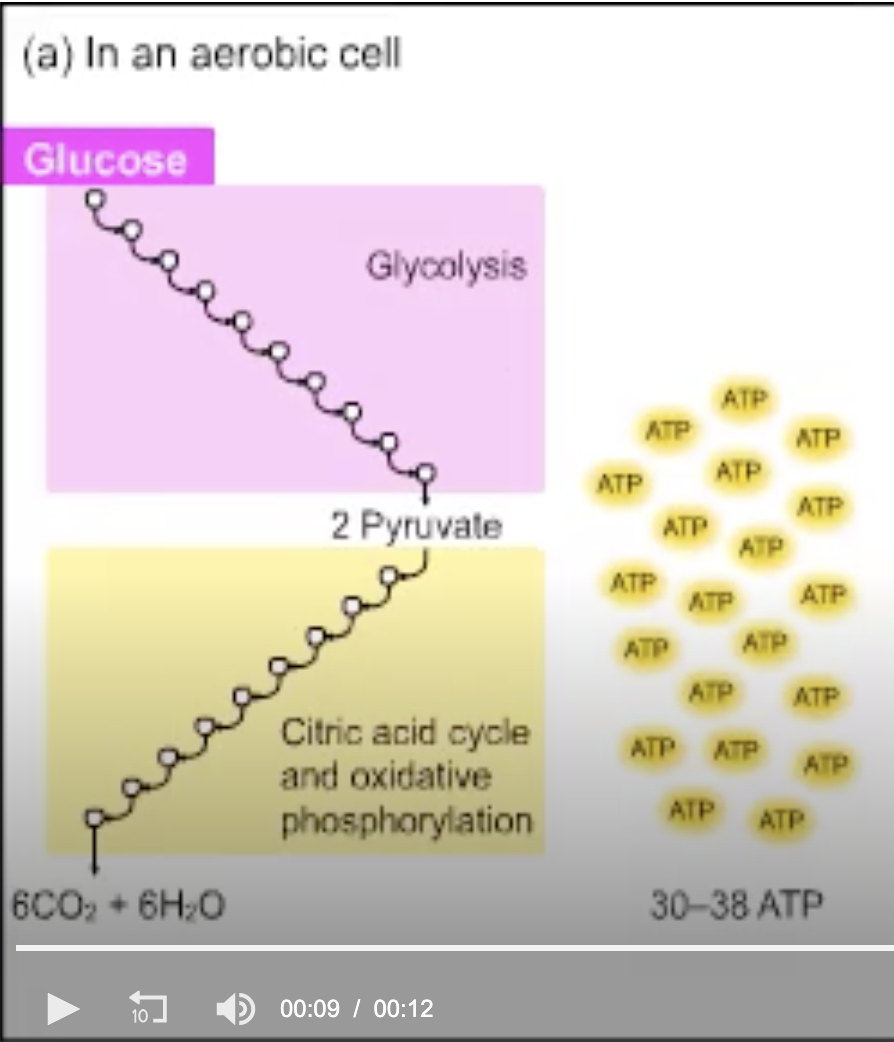
Why does (a) show a variable number of ATPs produced?
Different tissues have different ATP yields.
Assume that the maximum number of ATPs is produced (38). At pH 7, and in the presence of excess Mg2+, how much of the energy in one mole of glucose is stored as ATP?
Each mole of ATP stores 30.5 kJ of energy. 30.5 kJ/mol x 38 mol ATP = 1159 kJ
Assuming the maximum number of ATPs are produced at pH 7 and in the presence of Mg2+, 1159 kJ of energy are stored as ATP.
What is the percent efficiency?
40% 1159/2870 ×100
How many ATPs per glucose are initially invested in priming the system?
2
How many net ATPs are directly generated in going from glucose to two pyruvates?
two - Four ATPs are directly generated, but two were initially invested, leaving a net product of two ATPs.
How many oxidation-reduction steps are involved in going from glucose to two pyruvates?
one - The only oxidation-reduction step takes place at the glyceraldehyde-3-phosphate to 1,3-biphosphoglycerate step (glyceraldehyde-3-phosphate dehydrogenase).
The overall purpose of this reaction is to isomerize glucose-6-phosphate into fructose-6-phosphate. This entails opening the six-membered pyranose ring and creating a five-membered furanose ring. The last two steps involve a general base extraction of hydrogen, followed by a general acid addition of hydrogen. At the stage just before these two steps take place, what is the structure?
The linear form of fructose-6-phosphate
The linear form of glucose-6-phosphate
A mixed anhydride
An ether
The linear form of fructose-6-phosphate - Exactly parallel to the opening of the ring form of glucose-6-phosphate to give the linear form, the linear form of fructose-6-phosphate is formed and then closed to the ring form.
The overall purpose of this reaction is to isomerize glucose-6-phosphate into fructose-6-phosphate. This entails opening the six-membered pyranose ring and creating a five-membered furanose ring. Normally, a C-H bond, such as the bond in the C2 position, is very stable. Why is the H at that position so easily removed by the enzyme?
Its proximity to the OH group on C2
Its proximity to the OH groups on both C2 and C3
Its proximity to a carbonyl group after ring cleavage
Its position as an axial proton
Its proximity to a carbonyl group after ring cleavage - Once the ring is initially opened, the C-H bond is next to a carbonyl group, which destabilizes the C-H bond.
The overall purpose of this reaction is to isomerize glucose-6-phosphate into fructose-6-phosphate. This entails opening the six-membered pyranose ring and creating a five-membered furanose ring.
During the animation, there is first a general base extracting a hydrogen and then a general acid donating a hydrogen. At this stage, what is the resulting structure?
The linear form of fructose-6-phosphate
The linear form of glucose-6-phosphate
A mixed anhydride
An ether
The linear form of glucose-6-phosphate
The conversion of fructose-1,6-bisphosphate to DHAP and G3P is where the six-carbon carbohydrates are broken into two three-carbon carbohydrates. The aldol cleavage mechanism shown here can be found many times in biochemical pathways. What is the name given to the class of structure that results from the reaction of the α-amino group of the enzyme and the carbonyl group of FBP?
Aldol condensation
Ketamine
Schiff base
Gem diol
Schiff base - This is a generalized reaction that occurs in many places in biochemical reactions.
The conversion of fructose-1,6-bisphosphate to DHAP and G3P is where the six-carbon carbohydrates are broken into two three-carbon carbohydrates. The aldol cleavage mechanism shown here can be found many times in biochemical pathways. A generalized aldol cleavage always results in what two products?
ADHAP and G3P
DHAP and an aldehyde
G3P and a ketone
An aldehyde and a ketone
An aldehyde and a ketone - The reaction catalyzed by fructose bisphosphate aldolase is a specific example of a generalized reaction.
This enzyme is almost a "perfect" enzyme in that it catalyzes at a rate so rapid that it is essentially instantaneous. This allows DHAP to be converted to G3P (and vice versa) very rapidly. It also allows biochemists to consider all of the DHAP to be converted to G3P for purposes of calculating energies and stoichiometries for the glycolytic pathway. Question Content Area
What is the role of glutamic acid in the creation of the enediol intermediate?
It does not have a role
It donates a hydrogen to the carbon bound to the OH group
It accepts a hydrogen from the carbon bound to the OH group
It accepts a hydrogen from the carbon bound to the OH group
This enzyme is almost a "perfect" enzyme in that it catalyzes at a rate so rapid that it is essentially instantaneous. This allows DHAP to be converted to G3P (and vice versa) very rapidly. It also allows biochemists to consider all of the DHAP to be converted to G3P for purposes of calculating energies and stoichiometries for the glycolytic pathway.What is the role of glutamic acid in the reaction with the enediol intermediate?
It does not have a role
It donates a hydrogen to the C2 carbon
It accepts a hydrogen from the C2 carbon
It donates a hydrogen to the C2 carbon
This is the first reaction in the glycolytic pathway that involves redox reactions. G3P is oxidized from an aldehyde to an acid and NAD+ is reduced. This is also the point at which inorganic phosphate is introduced (as well as a site susceptible to arsenic poisoning because of the similarity of AsO43- to HPO33-). From what intermediate was the hydride transferred to NAD+?
A thioester
A thiohemiacetal
An aldehyde
An acid
A thiohemiacetal
This is the first reaction in the glycolytic pathway that involves redox reactions. G3P is oxidized from an aldehyde to an acid and NAD+ is reduced. This is also the point at which inorganic phosphate is introduced (as well as a site susceptible to arsenic poisoning because of the similarity of AsO43- to HPO33-). The high energy acyl phosphate is formed by the attack of inorganic phosphate on which of the following?
A thioester
A thiohemiacetal
An aldehyde
An acid
A thioester