S1.3 Electronic configurations
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
How are frequency(f), energy and wavelength(λ) related
Wavelength is inversely related with frequency n energy
Long wavelength→Low frequency n energy
Short wavelength→High frequency n energy
Formula of speed of light
c=fλ
Emission spectrum levels
Always from higher energy level to lower as electron loses energy by emitting photon(From outer shells to inner)
Infrared: n=∞ → n=3
Visible light: n=∞ → n=2
Ultraviolet: n=∞ → n=3
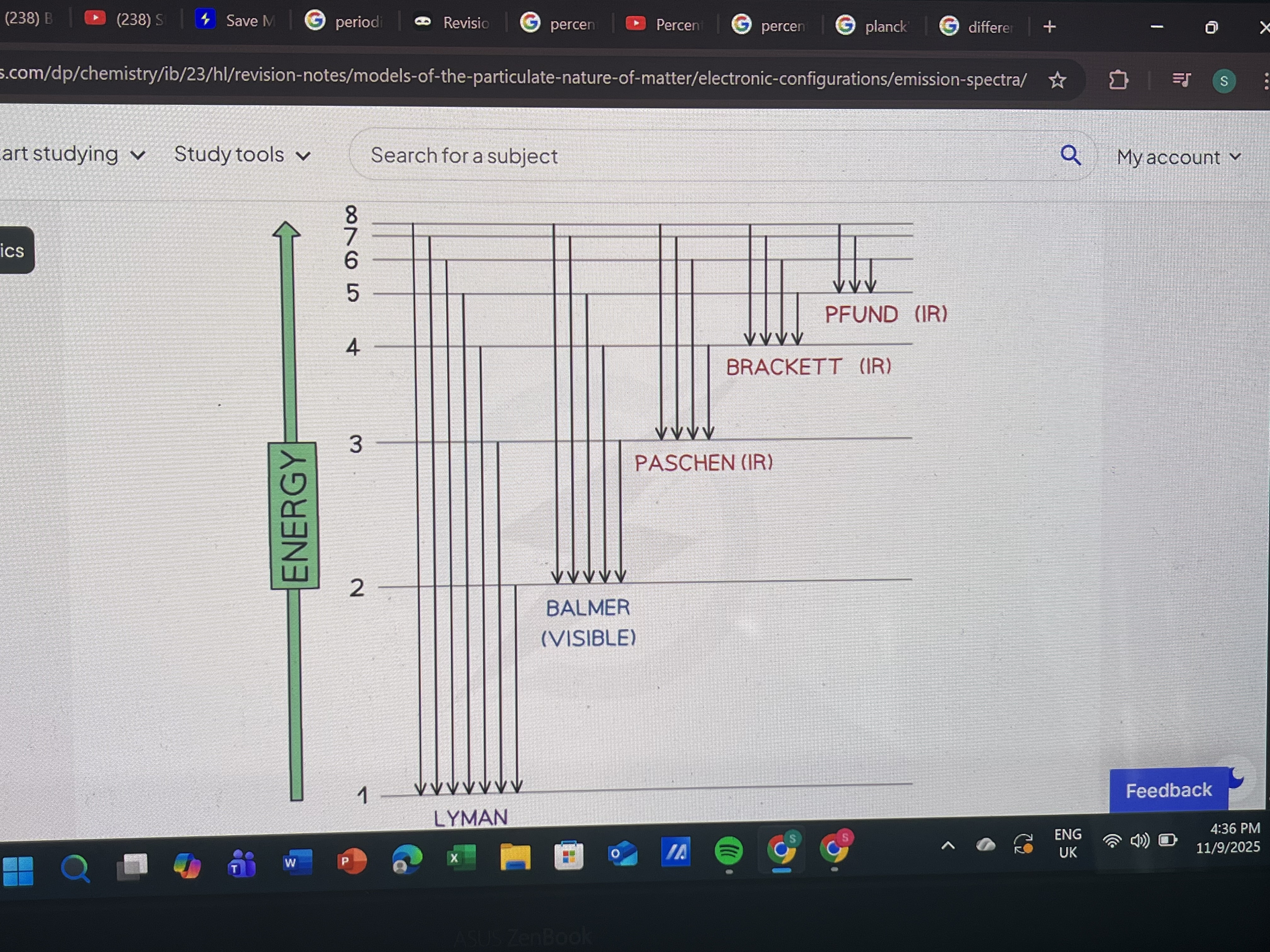
What happens when electrons move up energy levels?
They absorb light
What happens when electrons move down energy levels?
They emit light
How does mass spectrometer work?
Chemical sample placed into device and It’s heated up till it’s vaporized
Electron gun emits of beam of high energy electrons through sample
This causes atoms in sample to lose some electrons and become positively charged
Positively charged ions pass through electric field(accelerates movement of cations)
They pass through magnet that deflects them and changes their direction
Atoms with lowest mass and highest charge are deflected more
Atoms pass through detector to register location of atoms and determine mass and charge
Mathematical relationship between number of electrons and principal energy level
Energy level: n(n=3)
Number of electrons: 2n²(2×9=18)
Increasing energy order of subshells
s<p<d<f
What is the shape of s orbital
Spherical
Size increases with increasing shell number
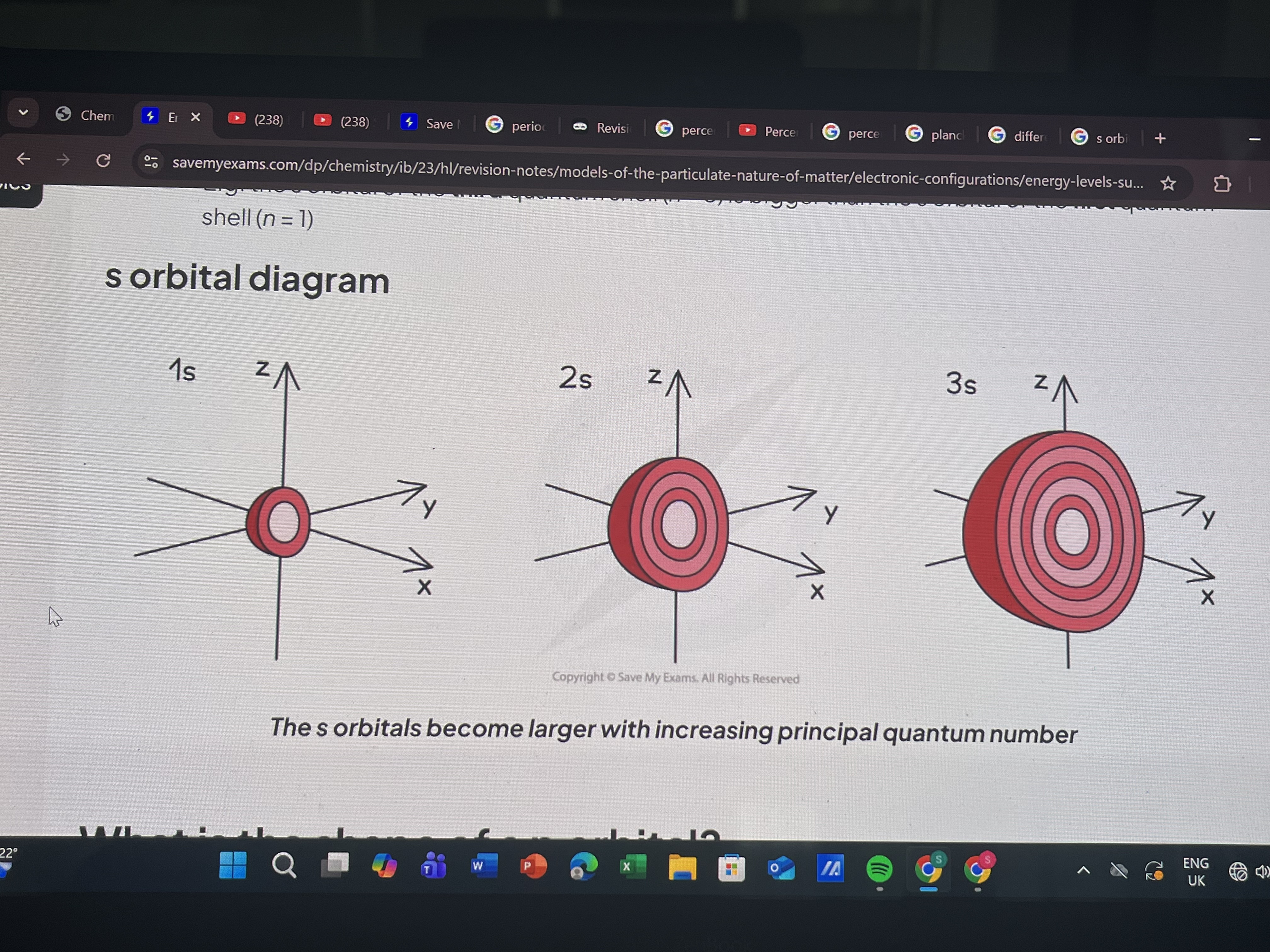
What is the shape of p orbital
Dumbbell-shape
Each shell(except n=1) has 3 p orbitals(px, py, pz)
They lie on the x, y, z axes respectively
What are limitations of the Bohr’s model?
Assumes electrons have fixed positions
Assumes all energy levels are spherical
Only accurately explains hydrogen spectrum(fails for atoms w <1 electron)
What is the limit of convergence?
The point where lines in an emission spectrum merge into continuous spectrum
What does limit of convergence represent?
Energy required to remove an electron from an atom (ionization energy)
Conversion of nm to m
x 10^-9
Energy formula
E=hf
h: planck’s constant
f: frequency
True or false: E=hf calculates energy of one mole of electrons
False
It calculates energy of one photon
Always multiply by avogadro’s constant to find ionisation energy(per mole)
Note to remember
Convert wavelength from nm to m(x 10^-9) when calculating frequency
Why is it easier to removed a paired electron from an orbital than a single electron
Spin pair repulsion: the 2 electrons repel each other, making it easier for one of them to leave
What do large jumps and small jumps in IE show
Large→change in shell
Small→changes within subshell