Embryo: Cardiovascular, Heart & Arteries
1/7
Earn XP
Description and Tags
https://docs.google.com/document/d/1DlQwzrCZ5bK_PW_wsnl9x03Fzr3YlOXUlMMd_mTwnbY/edit?tab=t.0
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1. Nature of the Development of the Conducting System of the Heart
The conducting system does not originate from a separate lineage but rather differentiates from the working myocardium of the primitive heart tube under the influence of specific signaling pathways.
Origin: The cells of the conducting system are derived from the same cardiogenic mesoderm that forms the rest of the heart.
the cells of the conducting system are specialized cardiomyocytes that have lost their contractile function and gained the ability to generate and rapidly conduct electrical impulses.
Key Signaling Pathways: The development of the nodal system (SA and AV nodes) is primarily guided by the Shh (Sonic Hedgehog) signaling pathway.
In contrast, the development of the His-Purkinje system (the ventricular conduction network) is dependent on Endothelin signaling.
Developmental Process:
Primitive Pacemaker: Initially, the pacemaker activity is located in the caudal part of the primitive heart tube.
pacemaker activity located in the caudal part of the primitive heart tube.
Sinoatrial (SA) Node: As the heart loops and chambers form, the pacemaker function shifts cranially. The SA node forms in the right wall of the sinus venosus, which is incorporated into the right atrium. It becomes the dominant pacemaker.
primitive pacemaker is located in the caudal part of the primitive heart tube → primitive heart tube loops → chamber’s form → pacemaker function shifts cranially (toward the head) → the SA Node forms the right wall of the sinus venosis→ sinus venosis is incorporated into the right atrium. SA node becomes the dominant pacemaker.
the SA NODE becomes the DOMINANT pacemaker.
Atrioventricular (AV) Node and Bundle: The AV node and the atrioventricular bundle (Bundle of His) differentiate from a ring of specialized tissue located at the atrioventricular canal (what does this mean?) This tissue initially creates a slow conduction delay, allowing the atria to contract before the ventricles.
Purkinje Fibers: The left and right bundle branches and the Purkinje fiber network develop from the trabeculated myocardium of the ventricles and are connected to the AV bundle.
In summary, the conducting system develops through the specialization of specific regions of the myocardium into non-contractile, electrically active tissue, ensuring the coordinated sequence of atrial and ventricular contraction.
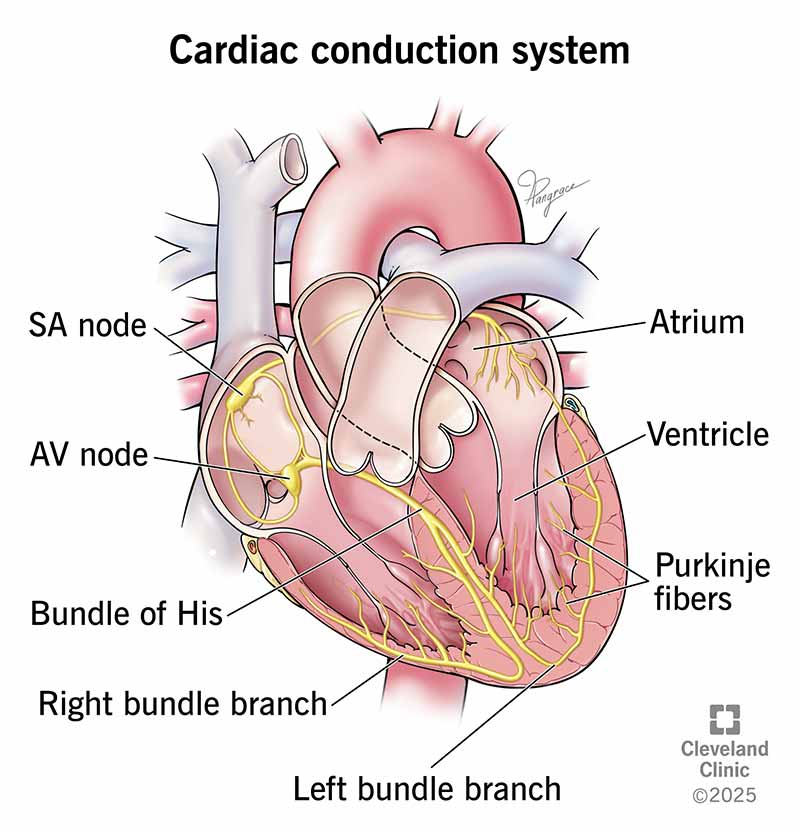
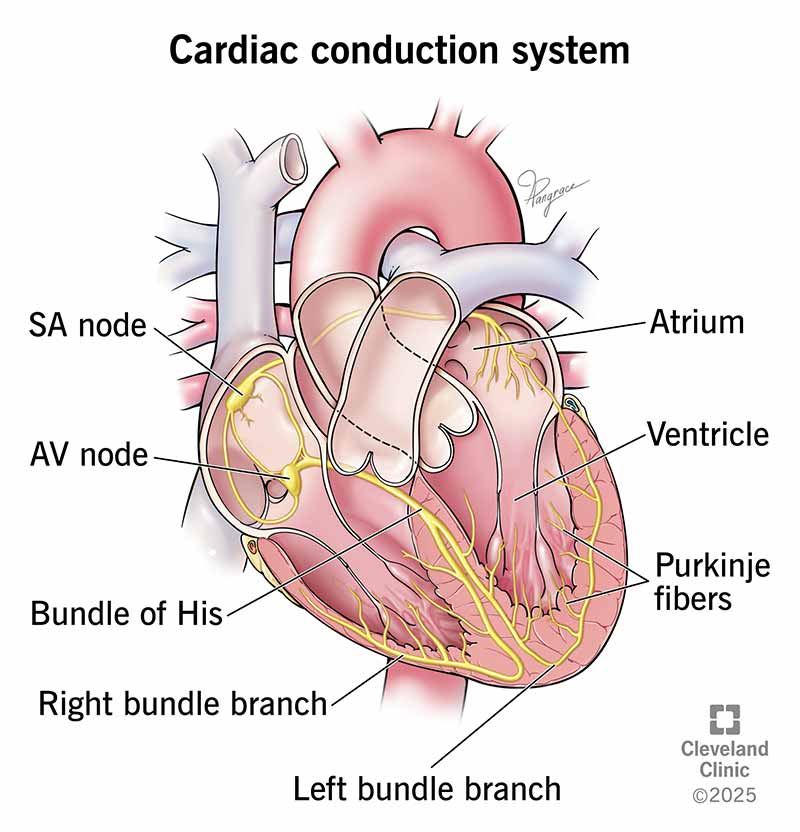
what is the conduction pathway?
The Pathway of Cardiac Conduction
SA node (the pacemaker) → atrial myocardium through gap junctions → Atrioventricular (AV) Node - The Gatekeeper → Bundle of His (AV Bundle) → Right and Left Bundle Branches → Purkinje Fibers → Ventricular Myocardium
1. Sinoatrial (SA) Node - The Pacemaker
Location: Found in the superior wall of the right atrium, near the entrance of the superior vena cava.
Function: This is the primary pacemaker of the heart. It spontaneously generates an electrical impulse at a rate of 60-100 times per minute under normal conditions. The impulse from the SA node spreads radially through both atria.
2. Atrial Myocardium
Conduction: The electrical wave from the SA node spreads directly through the gap junctions between atrial muscle cells. This causes the atria to contract, pumping blood through the open atrioventricular (AV) valves into the ventricles.
Anterior, Middle, and Posterior Internodal Tracts: While the impulse travels through general atrial muscle, there are also bands of preferential conduction that help speed the impulse toward the AV node.
3. Atrioventricular (AV) Node - The Gatekeeper
Location: Situated in the posteroinferior region of the interatrial septum, near the coronary sinus.
Function: The AV node is the only electrical connection between the atria and ventricles (which are otherwise separated by the fibrous cardiac skeleton). Its primary roles are:
To delay the impulse conduction by approximately 0.1 seconds. This delay is crucial as it allows the atria to fully empty their blood into the ventricles before ventricular contraction begins.
To act as a secondary pacemaker (40-60 beats/min) if the SA node fails.
4. Bundle of His (AV Bundle)
Location: The AV node continues as the Bundle of His, which penetrates through the fibrous skeleton.
Function: It is the pathway for the electrical impulse to travel from the atria to the ventricles. It then divides into the right and left bundle branches.
5. Right and Left Bundle Branches
Location: These branches run along the right and left sides of the interventricular septum.
Function: They conduct the impulse toward the apex (bottom) of the heart. The Left Bundle Branch often divides further into anterior and posterior fascicles.
6. Purkinje Fibers
Location: The bundle branches fan out into a vast network of Purkinje fibers that penetrate the ventricular myocardium.
Function: These fibers are specialized for extremely rapid conduction (up to 4 m/s). They distribute the wave of excitation from the apex upward through the walls of both ventricles.
7. Ventricular Myocardium
Conduction: The impulse spreads from the Purkinje fibers through gap junctions in the ventricular muscle cells. This causes the ventricles to contract from the apex toward the base, effectively ejecting blood into the pulmonary artery and aorta.
Summary of the Sequence
Step | Structure | Action |
|---|---|---|
1 | Sinoatrial (SA) Node | Impulse initiation. Pacemaker cells fire. |
2 | Atria | Impulse spreads, causing atrial contraction. |
3 | Atrioventricular (AV) Node | Impulse is delayed to allow ventricles to fill. |
4 | Bundle of His | Impulse travels to the interventricular septum. |
5 | Right & Left Bundle Branches | Impulse travels down the septum toward the apex. |
6 | Purkinje Fiber Network | Impulse rapidly spreads throughout ventricular walls. |
7 | Ventricles | Ventricular contraction from apex upward. |
This highly coordinated pathway ensures efficient pumping: the atria contract first to top off the ventricular volume, and a fraction of a second later, the powerful ventric
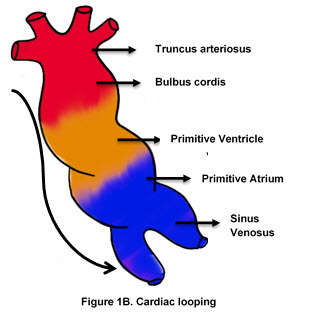
2. Development of the Cardiac Valves, Heart Chambers, and Septae
The heart starts as a simple, linear tube and undergoes complex looping and septation to form the four-chambered heart.
heart starts as a simple, linear tube
complex looping
separation to form the four chambered heart.
A. Heart Chambers
The primitive heart tube has five regions (attached picture):
Truncus Arteriosus
Bulbus Cordis
Primitive Ventricle
Primitive Atrium
Sinus Venosus.
As the heart tube elongates and bends (cardiac looping), it forms a U-shaped structure, bringing the future chambers into their correct anatomical positions. The bulbus cordis is incorporated into the ventricles to form the conus arteriosus (outflow tract) of the right ventricle and the aortic vestibule of the left ventricle.
B. Septation (Partitioning):
This process occurs between the 4th and 7th weeks and involves the growth of tissue masses that fuse to divide the heart.
Atrial Septation:
Septum Primum: A sickle-shaped crest grows from the roof of the common atrium towards the endocardial cushions. The opening between its lower edge and the cushions is the ostium primum.
Ostium Secundum: Before septum primum closes the ostium primum, programmed cell death creates perforations in its upper portion, forming the ostium secundum.
Septum Secundum: A thicker, muscular septum grows to the right of septum primum. It leaves an oval-shaped opening called the foramen ovale. The foramen ovale allows right-to-left shunting of blood in the fetus. The thin, flexible septum primum acts as a one-way valve.
Ventricular Septation:
Muscular Interventricular Septum: A muscular ridge grows upward from the floor of the primitive ventricle.
Endocardial Cushions: Tissue proliferations in the AV canal (superior and inferior endocardial cushions) fuse to form the AV septum, dividing the common AV canal into right and left orifices.
Membranous Septum: The final closure of the interventricular foramen (between the muscular septum and the fused endocardial cushions) is achieved by tissue from the right and left bulbar ridges and the AV cushions. This forms the thin membranous part of the interventricular septum.
Division of the Outflow Tract (Truncus Arteriosus):
Bulbar Ridges: A pair of opposing ridges (neural crest cell-derived) spiral within the truncus arteriosus and the conus cordis.
Aorticopulmonary Septum: These ridges grow toward each other and fuse, forming a spiral aorticopulmonary septum. This divides the truncus into the aorta and pulmonary trunk and ensures the right ventricle connects to the pulmonary artery and the left ventricle to the aorta.
C. Valve Development:
Semilunar Valves (Aortic & Pulmonary): Develop from three swellings of subendocardial tissue at the orifices of the aorta and pulmonary trunk. These swellings hollow out and reshape to form the three thin-walled, cup-shaped leaflets of each valve.
Atrioventricular Valves (Tricuspid & Mitral): After the AV canals are divided, the valves form from localized proliferations of tissue around the orifice. The connective tissue of the valves hollows out on the ventricular side, leaving thin leaflets connected to the papillary muscles via the chordae tendineae.
3. Partitioning of the Great Vessels
This process is directly linked to the septation of the outflow tract, as described above.
The truncus arteriosus is the common outflow vessel of the primitive heart.
Neural crest cells migrate into the truncus arteriosus and conus cordis and form the bulbar and truncal ridges.
These ridges fuse to form the spiral aorticopulmonary septum. The spiral nature of this septum is why the pulmonary artery twists around the aorta.
This septum divides the single great vessel into two: the aorta (from the left ventricle) and the pulmonary trunk (from the right ventricle).
4. Formation of Extra- and Intra-Embryonic Blood Vessels
Blood vessel formation (angiogenesis) occurs via two primary mechanisms: vasculogenesis (the de novo formation of blood vessels from angioblasts) and angiogenesis (the formation of new vessels from pre-existing ones).
Intra-Embryonic Vessels:
Vasculogenesis: Angioblastic cells (from mesoderm) form isolated cell clusters called blood islands within the mesoderm. The peripheral cells of these islands flatten to form endothelial cells, while the central cells become hematopoietic stem cells (blood cells).
Plexus Formation: These endothelial-lined spaces coalesce to form a primitive, diffuse vascular plexus throughout the embryo.
Remodeling (Angiogenesis): This primitive network is then remodeled via angiogenesis—sprouting, splitting, and pruning—into the definitive arteries and veins (aortae, cardinal veins, etc.). This process is guided by hemodynamic forces and genetic signals.
Extra-Embryonic Vessels:
The same process occurs simultaneously outside the embryo, primarily in the yolk sac and connecting stalk (future umbilical cord).
Blood islands form in the yolk sac mesoderm. They coalesce to form a network of vessels, including the vitelline veins and arteries that connect the yolk sac to the intra-embryonic circulation.
In the connecting stalk, vessels form to become the umbilical arteries and veins, connecting the placenta to the embryo.
5. Development of the Aortic Arches
The aortic arches are a series of six paired arteries (though the 5th is rudimentary or absent) that run through the pharyngeal arches and connect the ventral aortic sac (from the truncus arteriosus) to the paired dorsal aortae.
Pharyngeal Arch: Each pharyngeal arch has its own aortic arch artery.
Fate of Each Arch:
1st Arch: Largely disappears. A small portion may contribute to the maxillary artery.
2nd Arch: Also largely disappears. Contributes to the stapedial artery.
3rd Arch: Forms the common carotid arteries and the proximal part of the internal carotid arteries. The external carotid arteries sprout from the ventral aortic sac.
4th Arch:
Left side: Forms part of the arch of the aorta (between the left common carotid and left subclavian arteries).
Right side: Forms the proximal part of the right subclavian artery.
5th Arch: Is transient and non-functional in humans.
6th Arch (Pulmonary Arch):
The proximal part of both right and left arches gives rise to the proximal pulmonary arteries.
The distal part of the right 6th arch degenerates.
The distal part of the left 6th arch persists as the ductus arteriosus, a crucial fetal shunt that allows blood to bypass the non-functional lungs. After birth, it closes to become the ligamentum arteriosum.
The dorsal aortae fuse caudally to form the single descending aorta. The subclavian arteries form from the 7th cervical intersegmental arteries, which connect to the dorsal aortae.
NINJA NERD VIDEO ON THE DEVELOPMENT OF THE HEART
develop a heart tube.
surrounding the heart tube, we try to develop a pericardial cavity.