Chapter 20- Carboxylic acids and Nitriles
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
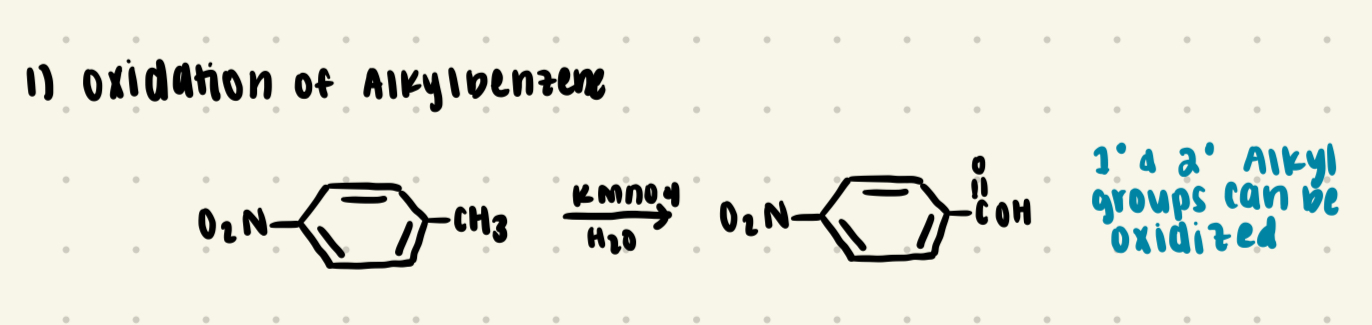
Oxidation of an Alkyl Benzne
Forms a Carboxylic Acid
Reagents: KMNO4/H2)
Primary and secondary alkyl groups can be oxidized.

Oxidation of Primary alcohol or aldehyde
Forms a carboxylic Acid
1) Uses KMNO4 or strong oxidizing agent
Carboxylation of Gringard reagents
Results in Carboxylic Acid
Reactands: Gringard + CO2
Carboxylation facilitated by CO2 and H3O+
Hydrolysis of Nitriles
Results in a carboxylic acid
1) Alkyl Halide + NaCN
2) NaOH/ H3O+
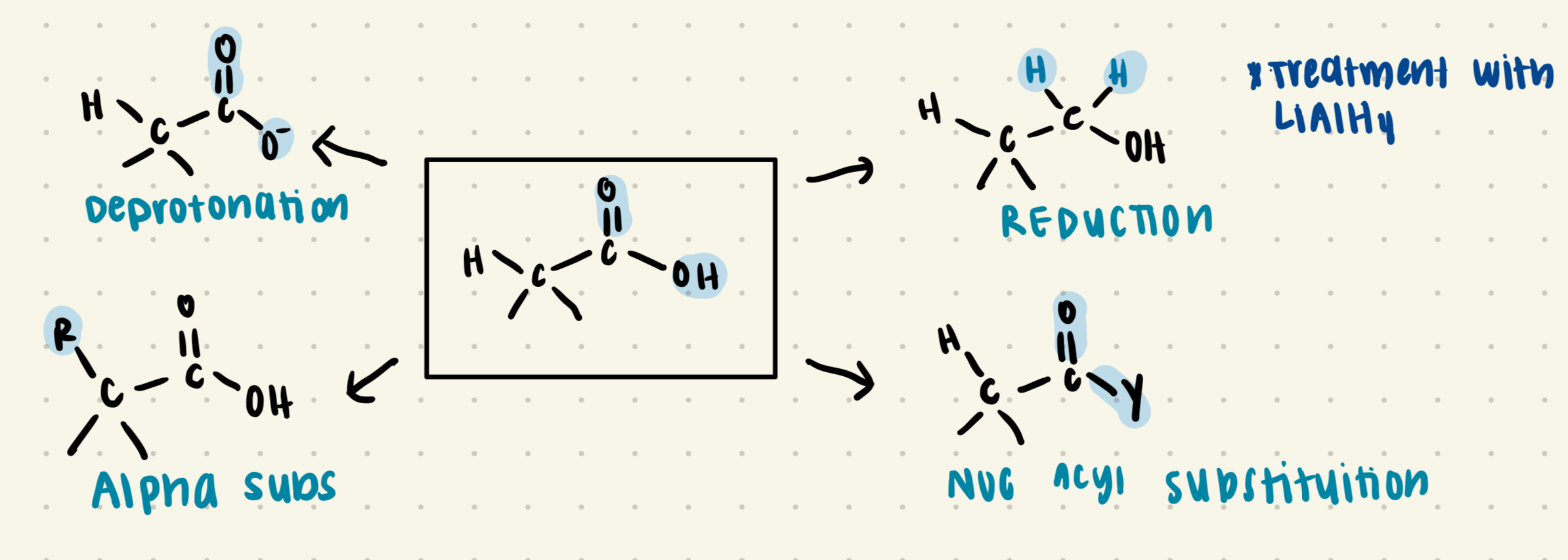
General Reactions of Carboxylic acids
Deprotonation
Alpha Substituition
Reduction
Nucleophilic Acyl Substituition
Henderson Hasselbach Reaction
pH=pKa+log([A-]/[HA−])
pKa is the acid dissociation constant,
[A−][A−] is the concentration of the conjugate base,
[HA][HA] is the concentration of the acid.
Carboxylic Acid + Gringard reagent
NO REACTION
Acid protons are too acidic, and can protonate gringard, nuetralizinf it before it can react with carboxylate group
Both COOH and CN are
Electrophiles and go through Nucleophilic addition rxns
Prep of simple nitirle
SN2 reaction of CN- with a primary or secondary Alkyl Halide
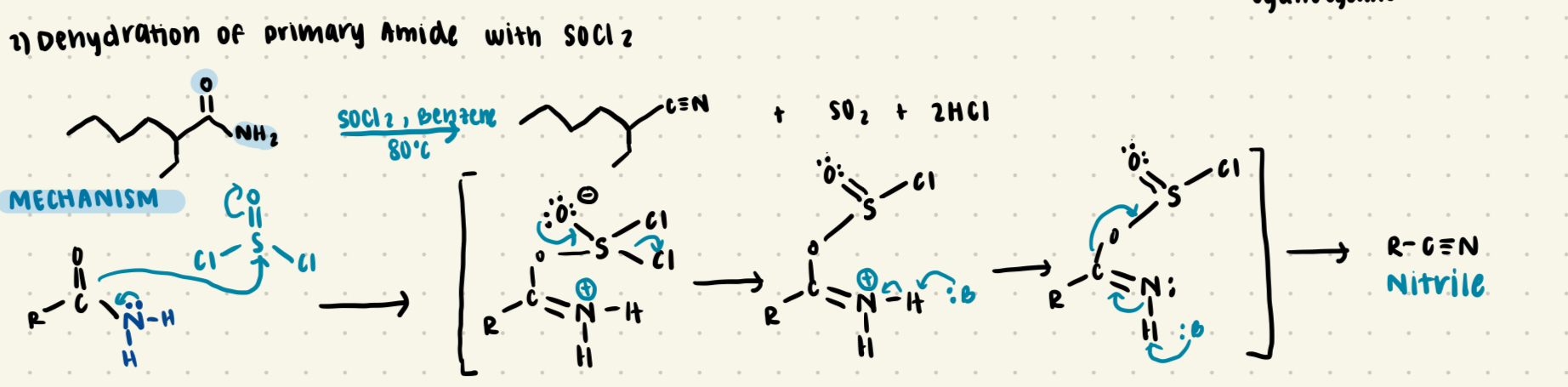
Preparation of complex nitriles
Dehydration of primary amide with SOCl2
results in R-C:::N
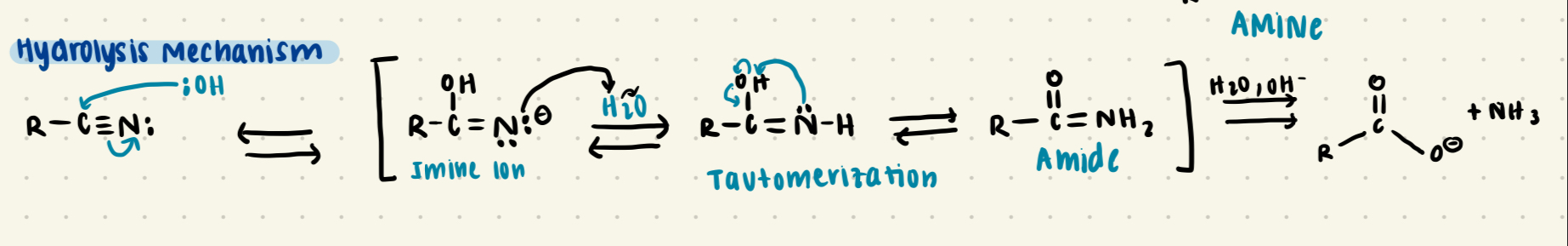
Nitrile + H2O
Forms an amide through Imine intermediate, which tautomerizes to form the amide form
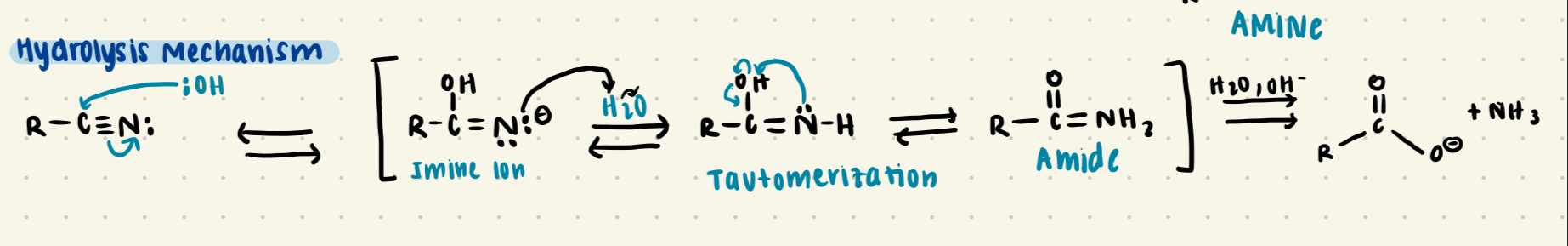
Amide + H2O
Forms a carboxylic Acid
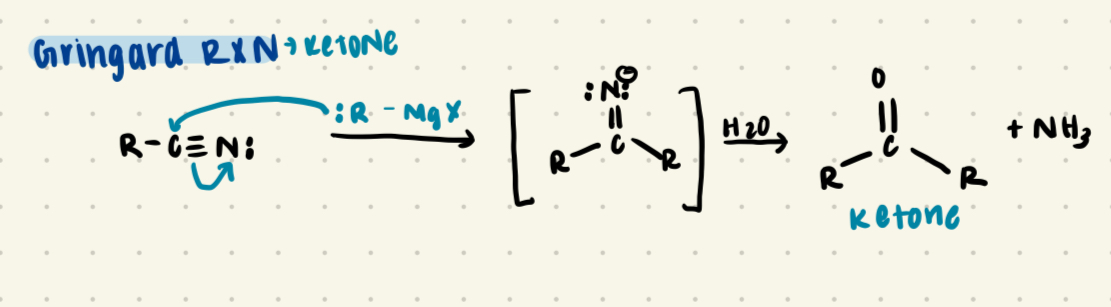
Nitrile + gringard reagent in water
Forms a KETONE + NH3
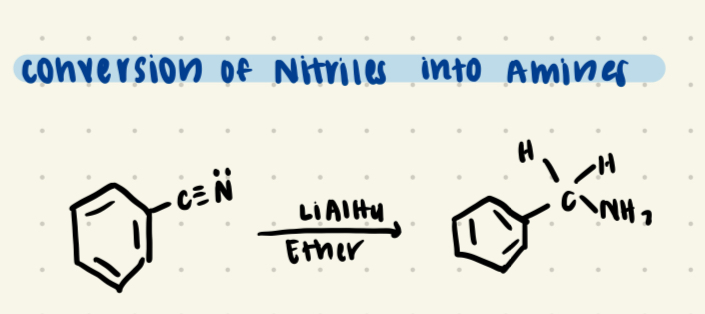
Nitirle plus strong oxidizing agent (LIalH4)/Ether
Forms an Amine (NH2)
Carboxylic acid + LiALH4
Reduction reaction- results in an Alchol