science finale semester 2
0.0(0)
Card Sorting
1/99
Earn XP
Description and Tags
Last updated 10:47 PM on 5/1/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
1
New cards
which is a property shared by most molecular compounds
low melting points
2
New cards
when an action bosses an electron, it becomes
a positive ion
3
New cards
magnesium bromide is an ionic compound with chemical formulas MgBr2. what does the two tell you
there are two bromide ions for ever one magnesium ion
4
New cards
in the chemical formula for an ionic compound, which item is written first?
the name of the positive ion
5
New cards
the chemical name for the compound with the formula Na2S is
sodium sulfide
6
New cards
what is a double bond
two pairs of electrons shared between two atoms
7
New cards
electrons involved in bonding between atoms are
valence electrons
8
New cards
the columns in the periodic table are called
groups
9
New cards
which molecule has a polar bond
HF
10
New cards
which of the following groups contain the most reactive elements
the alkali metals
11
New cards
covalent bonds usually form when a non metal combines with a
non-metal
12
New cards
solid metals are good conductors of eat and
electricity
13
New cards
a ___ is a neutral group of atoms joined by covalent bonds
molecule
14
New cards
because the electrons in a molecule of hydrogen fluoride new more strongly pulled towards the fluorine atom, the molecule is
polar
15
New cards
in the periodic table, the number of valence electrons in each element _________ from left to eight across each period
increases
16
New cards
the properties of solid metals can be explained by the __structure__ of and the bonding among the metal atoms
true
17
New cards
orderly crystal shapes, high melting points, and electrical conductivity when dissolved in water are properties of __Ionic__ compounds
true
18
New cards
when electrons new __transferred__ between two atoms, a covalent bond is formed
shared/false
19
New cards
a low melting point is one property of __molecular__ compounds
true
20
New cards
a __non polar__ bond is formed when two atoms shared electrons unequally.
polar/false
21
New cards
list three elements from the group containing the most reactive nonmetals
fluorine, chlorine, and bromine
22
New cards
in an electron dot diagram of aluminum , how many dots show be drawn around the symbol. explain
there should be three dots around aluminum because it is in group 3a/13. there should also be three because aluminum has three valence electrons.
23
New cards
how many atoms of a group 17 element should be needed to react with one atom of group 2 element. explain
there should be 2 atoms form a groups 17 atom because when you transfer 1 valence electron from a group 2 atom, the group 17 atom would be stable. there group two atom would not be stable. there fore we must add 1 more group 17 atom to transferee there 1 valence electron from the group 2 atom. now all three atoms are stable.
24
New cards
water - ionic/covalent
covalent

25
New cards
HCN -ionic/covalent
covalent

26
New cards
Be + N - ionic/covalent
ionic
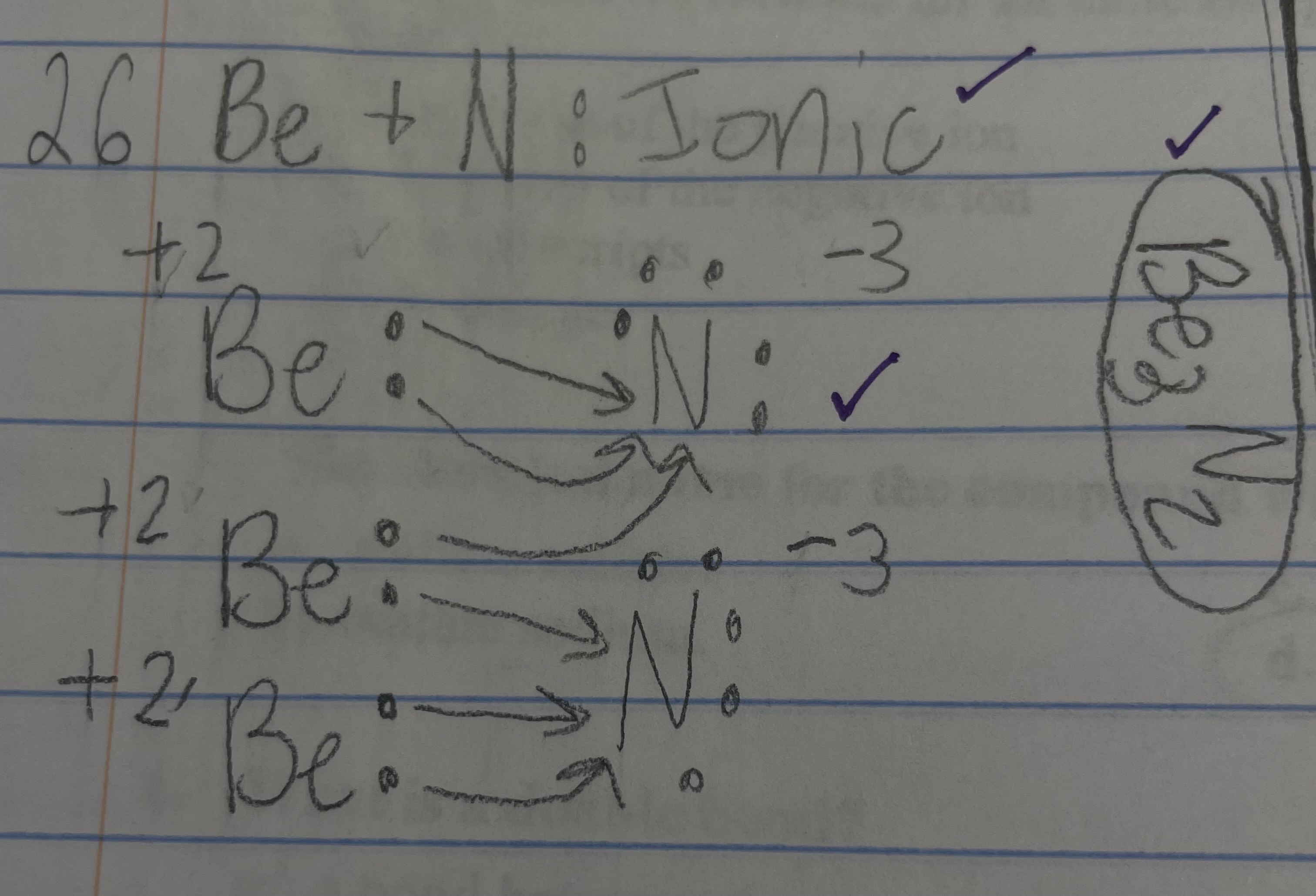
27
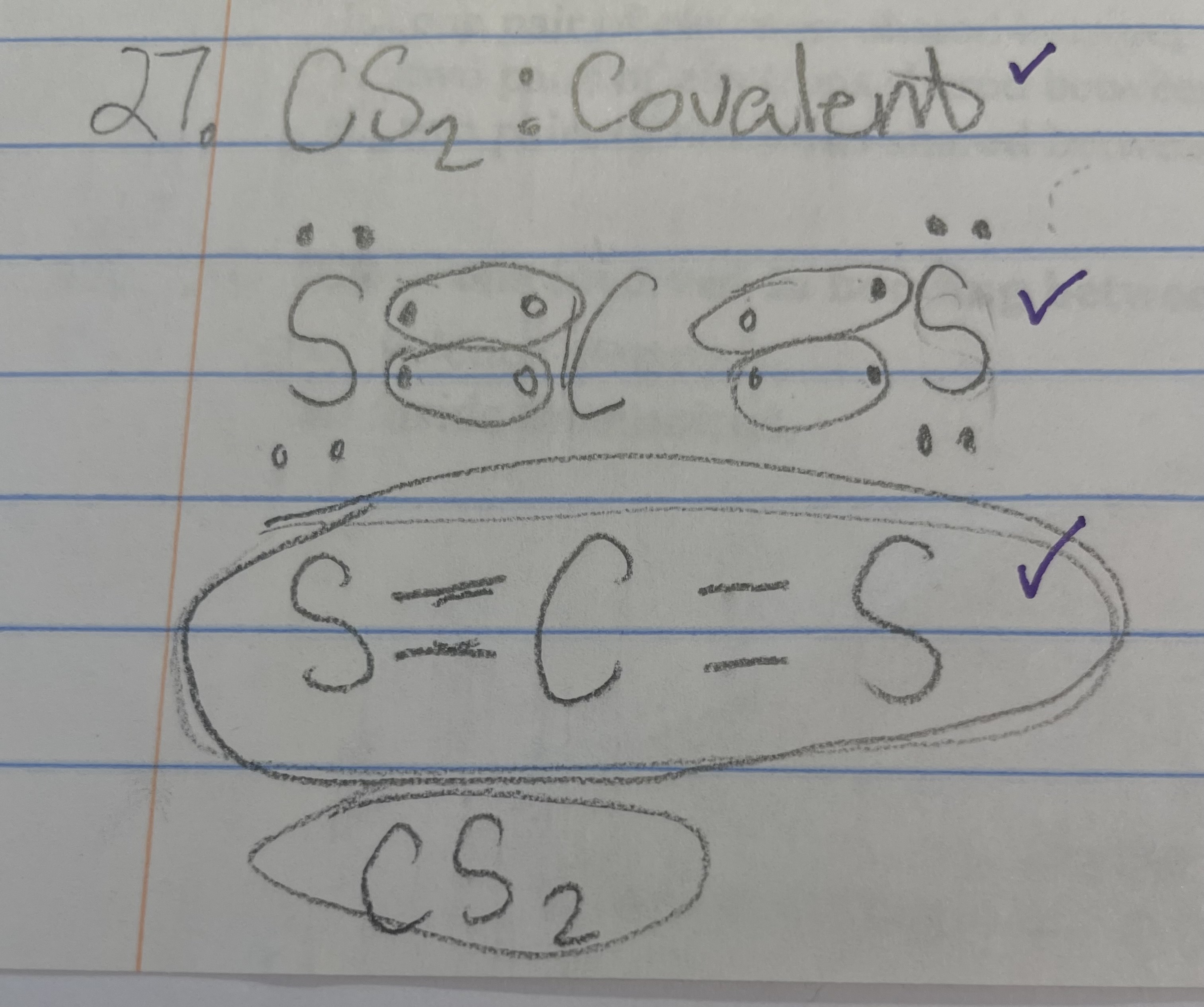
New cards
CS2 - ionic/covalent
covalent
28
New cards
NaCl - ionic/covalent
ionic
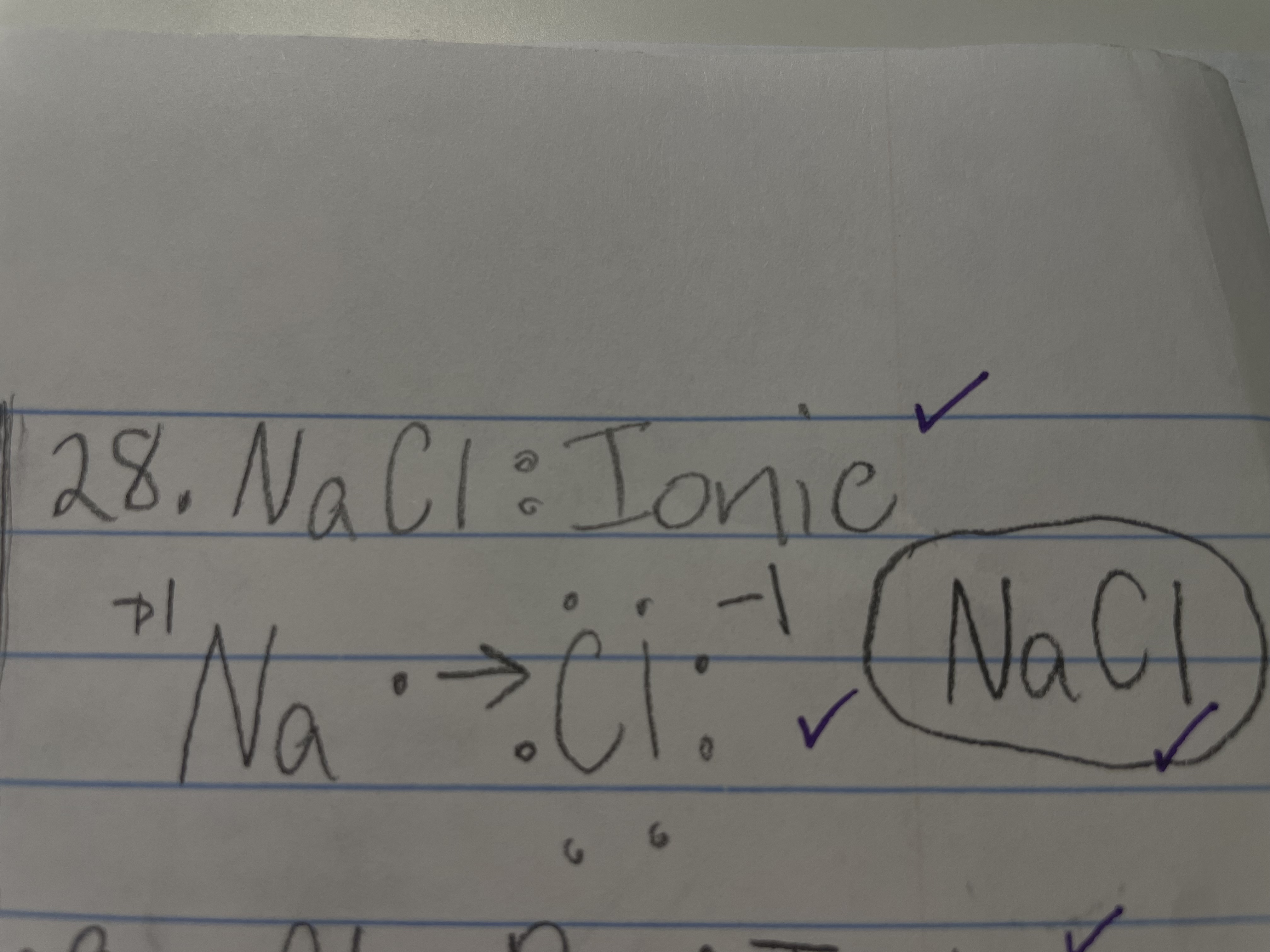
29
New cards
Al + Br
Ionic
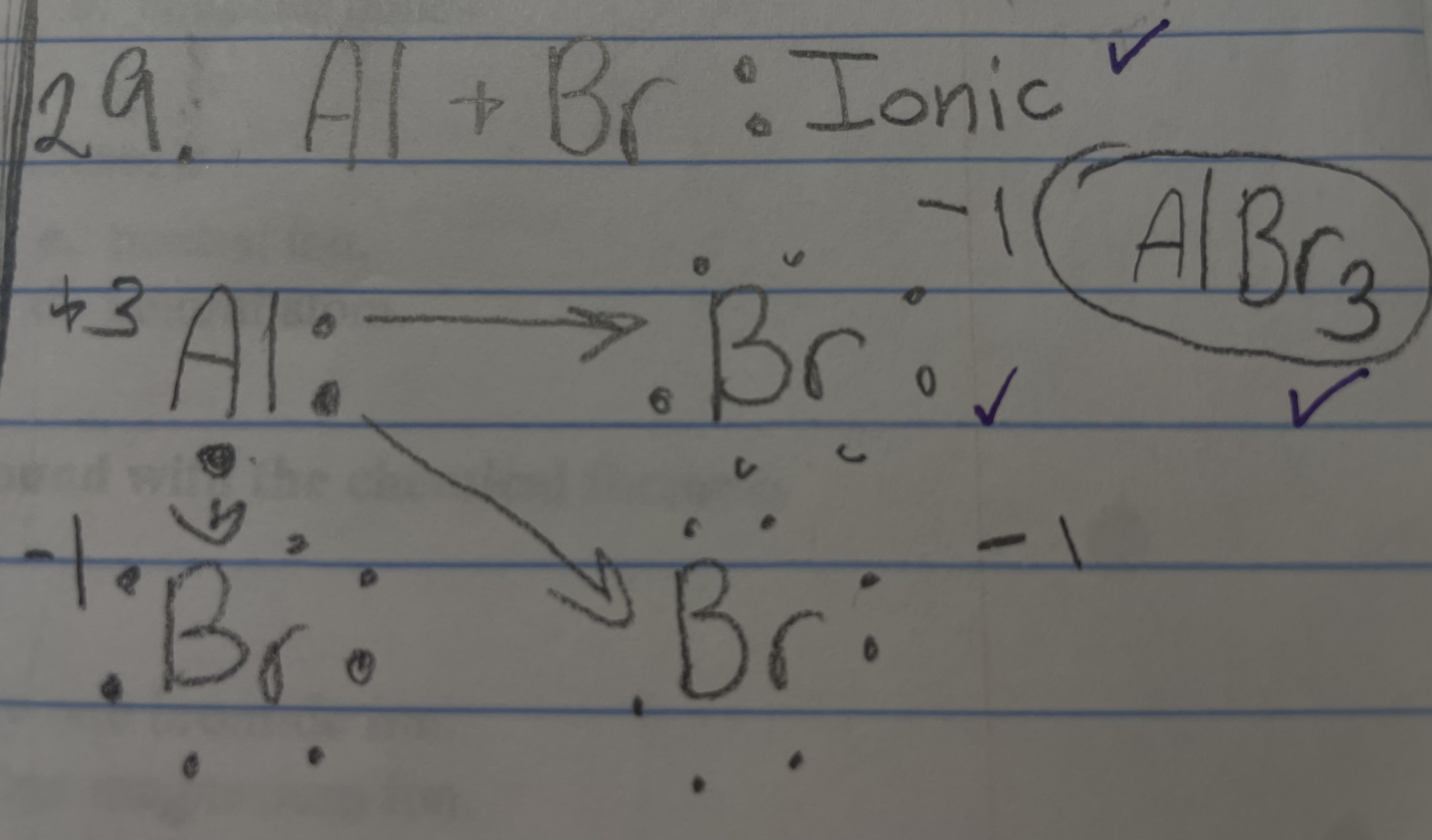
30
New cards
Ba + F - ionic/covalent
ionic
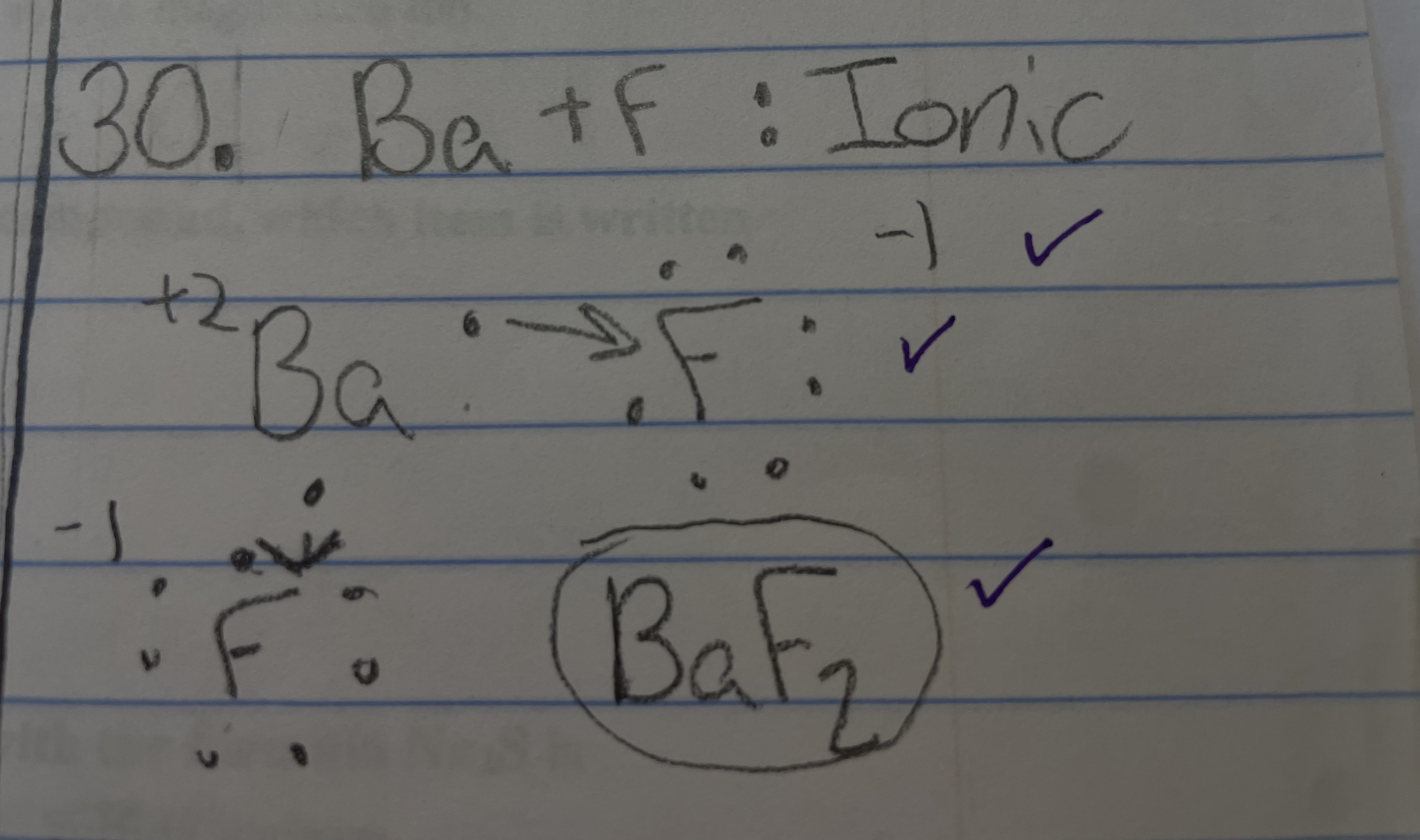
31
New cards
CCl4 - ionic/covalent
covalent
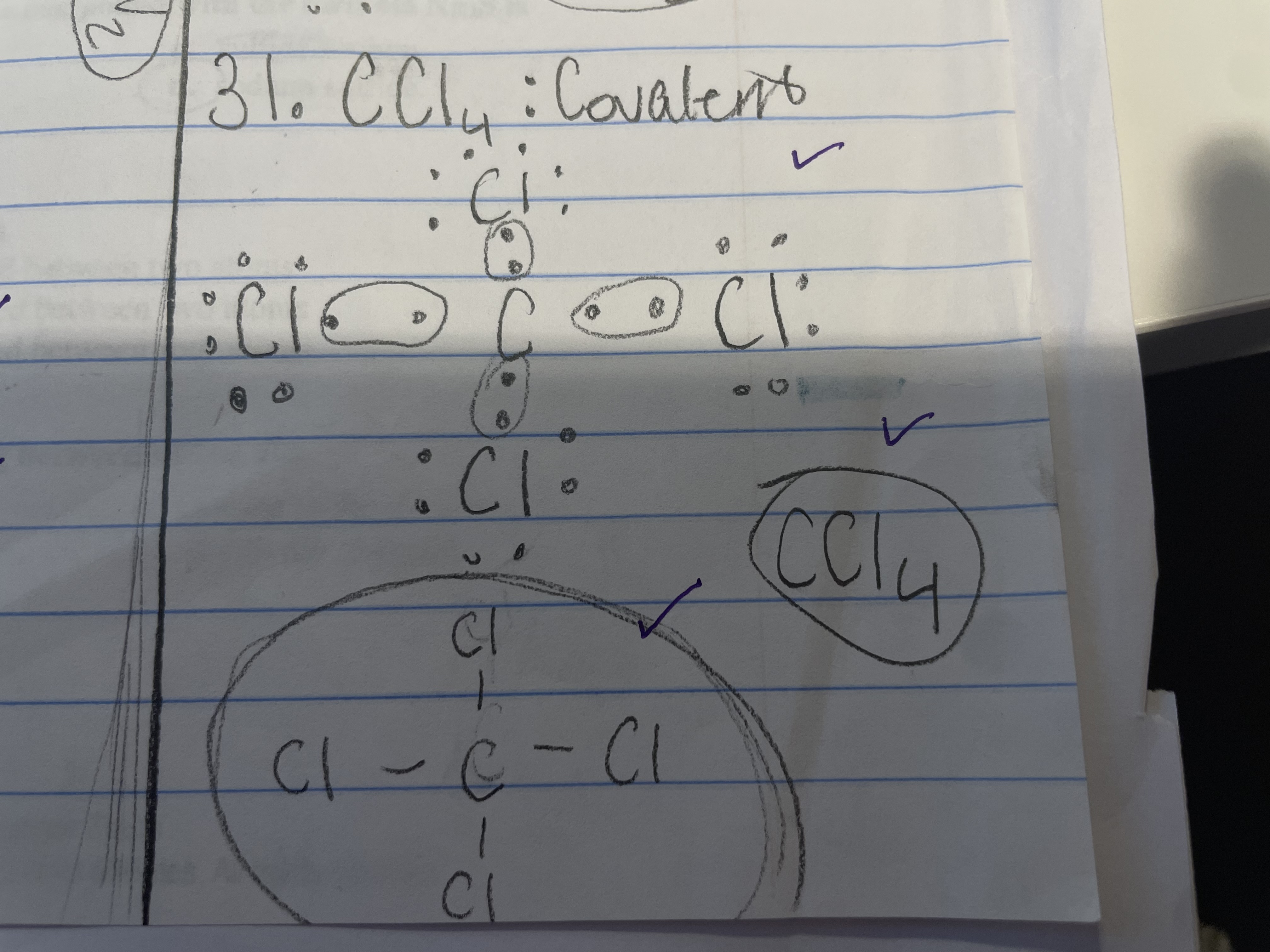
32
New cards
sodium nitrate
NaNO3
33
New cards
lithium oxide
Li2O
34
New cards
magnesium sulfate
MgS
35
New cards
potassium bicarbonate
KHCO3
36
New cards
beryllium carbonate
BeCO3
37
New cards
sodium chloride
NaCl
38
New cards
CaSO4
calcium Sulfate
39
New cards
Na2O
sodium oxide
40
New cards
MgCO3
magnesium carbonate
41
New cards
speed = distance divided by
time
42
New cards
when you know both the speed and direction of an objects motion, you know the
velocity of the object
43
New cards
the steepness of a line on a graph is called the
slope
44
New cards
the rate at which velocity changes is call
acceleration
45
New cards
which pf the following is the SI unit for acceleration
m/s^2
46
New cards
on a graph showing distance verses time, a horizontal line represents an object that is
no moving at all
47
New cards
the International Systiem of Units is used by scientist
all over the world
48
New cards
an object changing direction is an example of
acceleration
49
New cards
which of these is an example. of deceleration
a car approchant a red light
50
New cards
if you know a car traveled 300 kilometers in three hours, you can find its
average speed
51
New cards
suppose you are siting in a car at. red light when a car is moving toward the north begins to pass you. if you are passing the car as a reference point, the direction in which you paper to be moving towards the
south
52
New cards
the basic SI unit for length is the
meter
53
New cards
a golf ball ______ when either it speeds up or direction changes
accelerates
54
New cards
if a car is speeding up, its initial speed is ____ than its final speed
less
55
New cards
if two lines appear on the same motion graph, ht Eline with the steeper ____ indicates a greater speed.
slope
56
New cards
if a toy car traveling at 10 cm/s asses a toy car moving at 10 cm/s in the opposite direction, both cares have the some __velocity__
speed/ false
57
New cards
motion is measured relative to a reference point
true
58
New cards
__Average speed__ is the rate at which am object is moving at a given instant
institanious speed/false
59
New cards
an SI unit of __velocity__ is the meter per second per second
acceleration/false
60
New cards
a strait diagonal line on a __distance-verses-time__ graph indicates constant speed
true
61
New cards
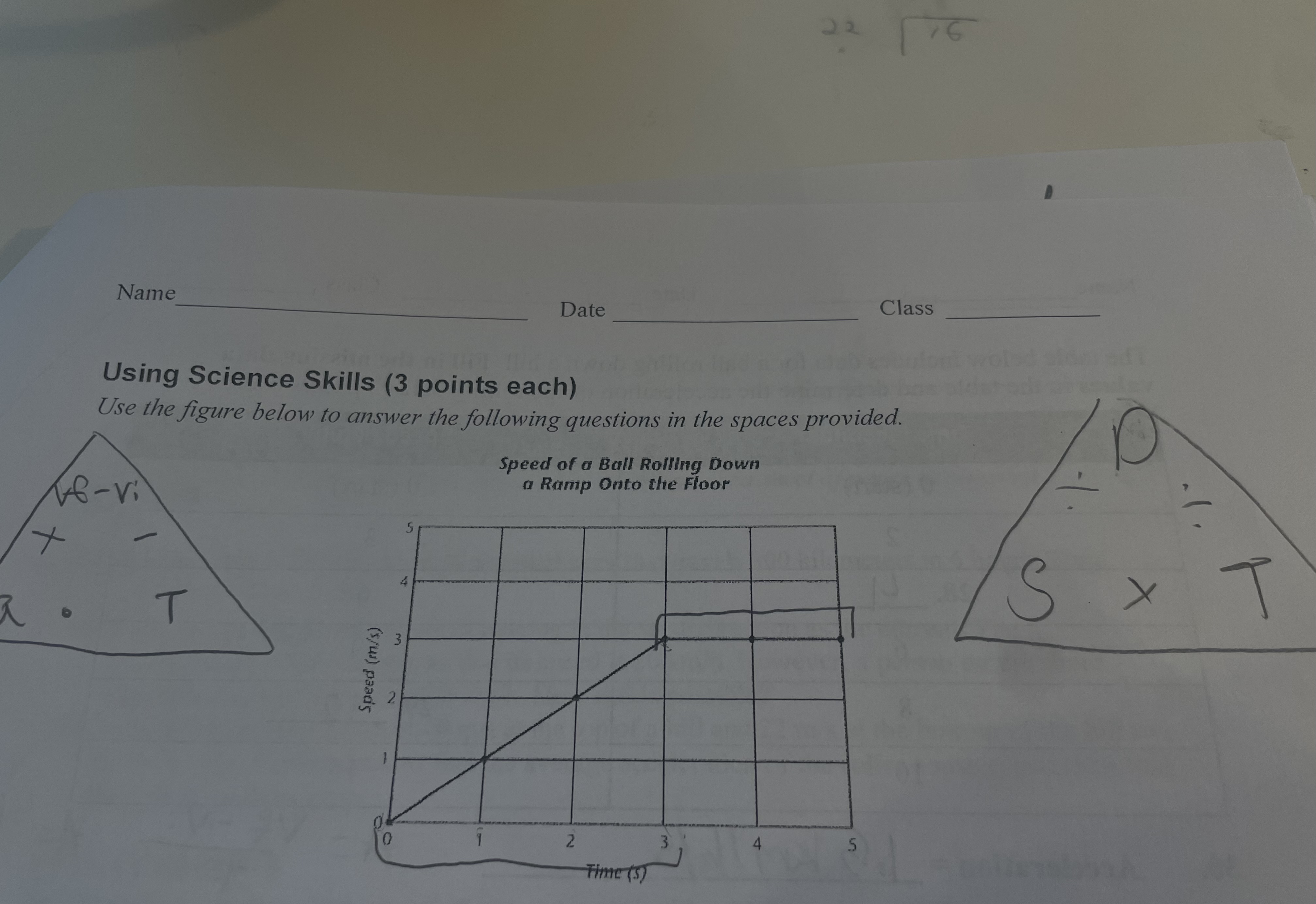
does the graph indicate that the ball decelerated? explain
the graph does not indicate that the ball is decorating because the line is rising not falling
62
New cards
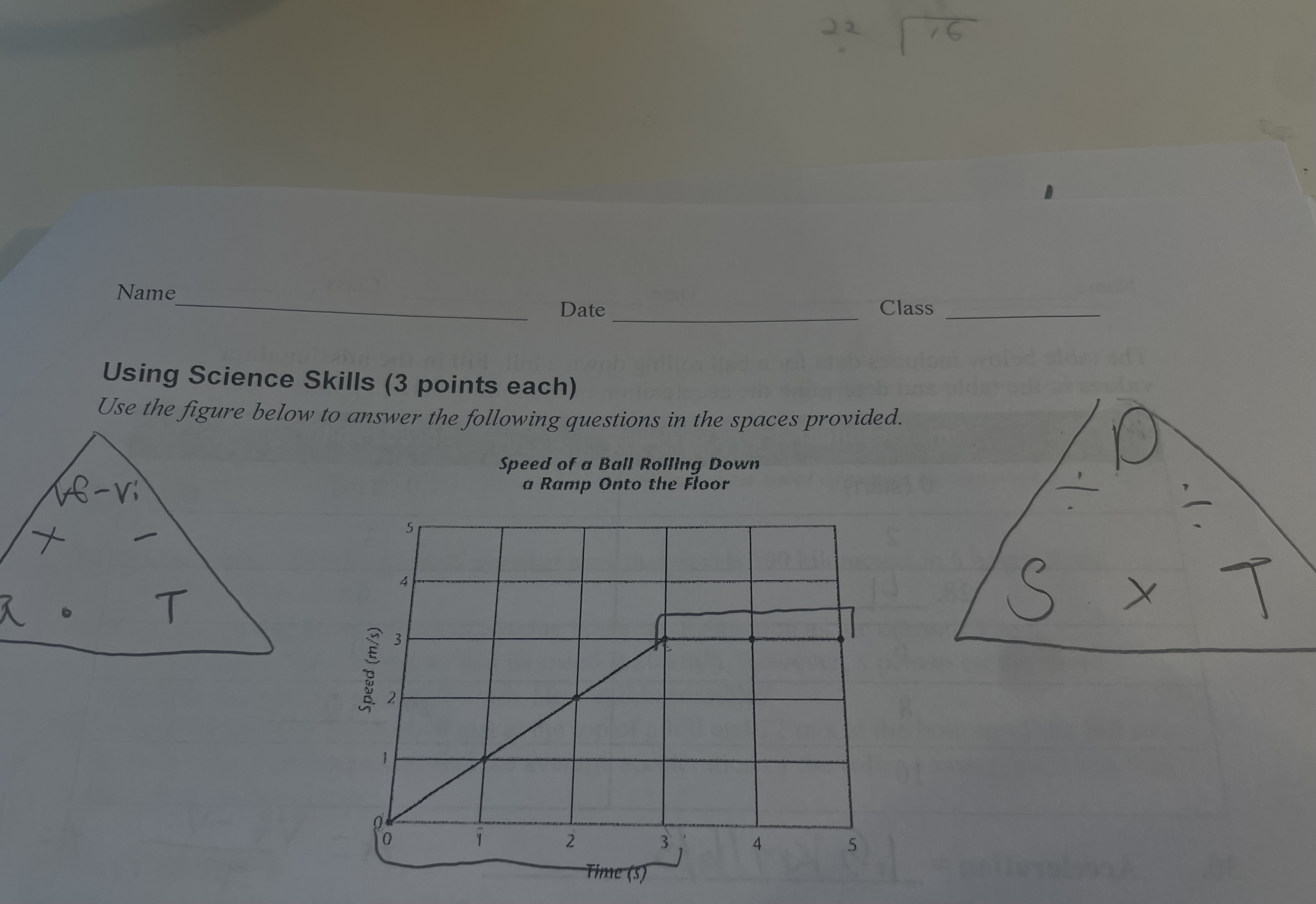
what happened to the speed of the ball during the final two seconds
the speed of the ball in the last two seconds stay at a constant speed of 3 m/s.
63
New cards
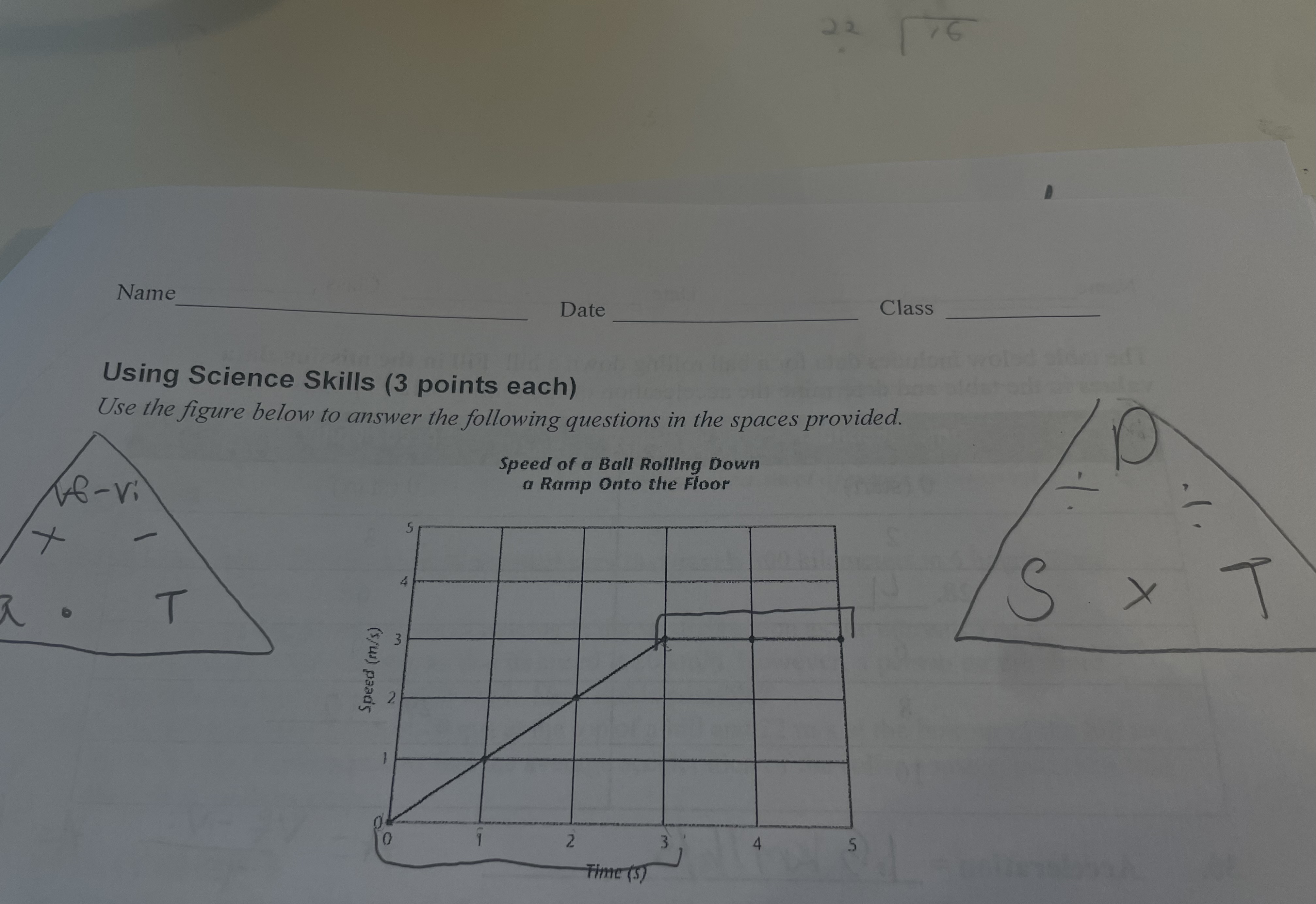
what is the acceleration of the ball between 0 and 3 seconds
the acceleration is 1m/s2 between 0 and 3 seconds
64
New cards
a skater increases her velocity from 2.0 m/s to 10.0 m/s in 3.0 seconds. what is the skaters acceleration
pic

65
New cards
a truck traces down the highway at a speed of 110 km/hr. how lang does the trip last if the ruck covered 2200 km
pic
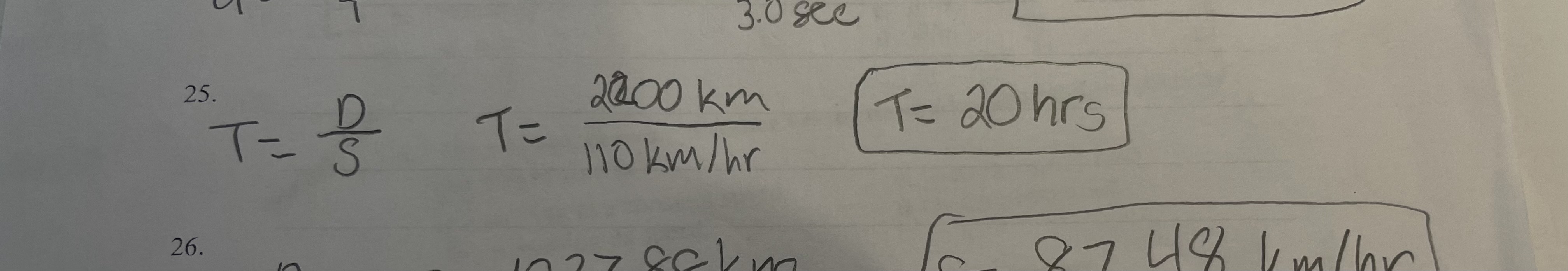
66
New cards
a car traveled 1027.88 km from Harrisburg, pA to Chicago, il in 11.75 hours. what was the average speed of the car
pic
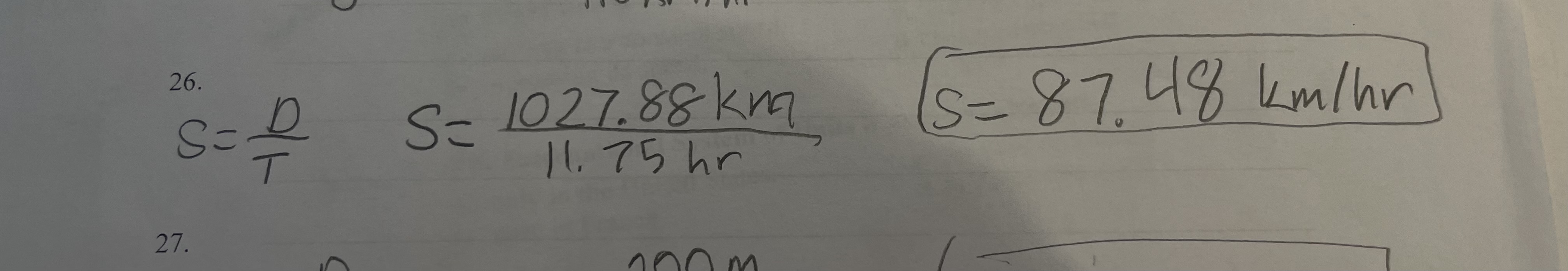
67
New cards
at the circus, the human cannon ball is fired from its cannon at a speed of 350 m/s. how long does it take him to fly 200 m
pic

68
New cards

what is the acceleration
1\.5 km/h/s
69
New cards
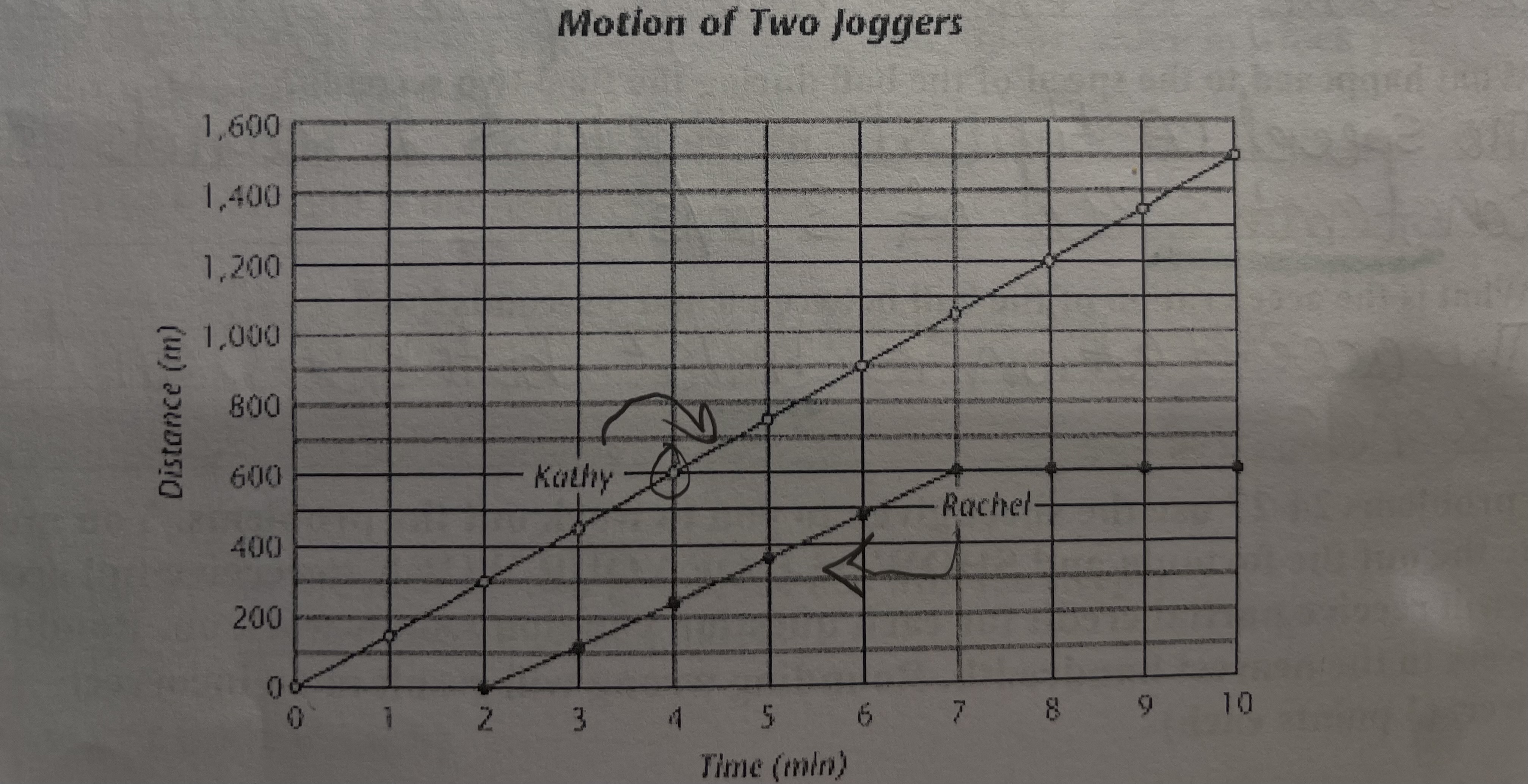
what two variables are plotted on the graph
the two variables are time in minutes and distance in meters
70
New cards
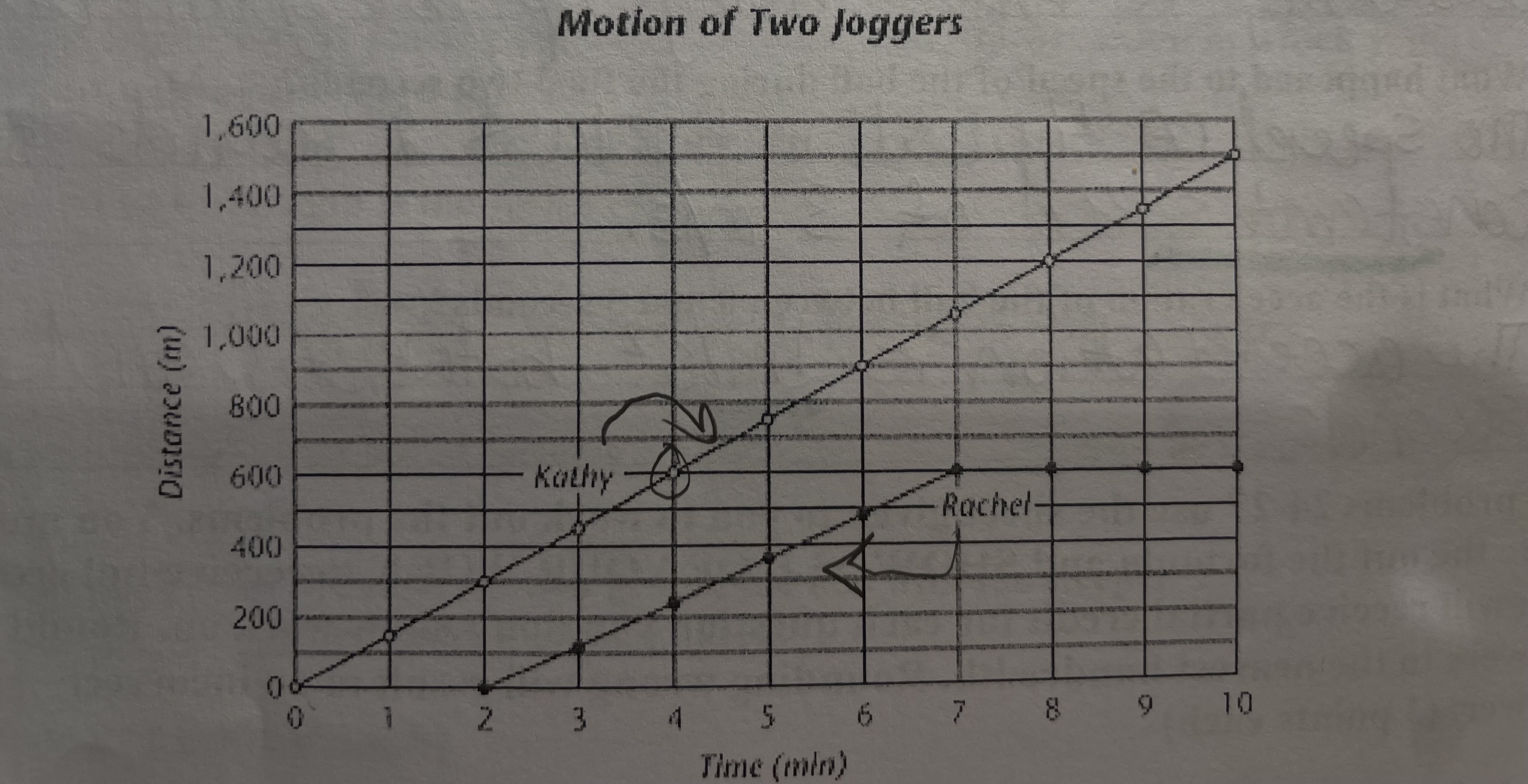
how far did Kathy jog in the first four minutes
in the first 4 minutes, Kathy jogged 600 meters
71
New cards
describe the two joggers speed, is it constant, changing, instantaneous, how do you know
Kathy has a constant speed because she has a constant speed of 150 m/min. Rachle has a changing speeds ultimately leading to her stopping from minutes 7-10.
72
New cards
73
New cards
74
New cards
75
New cards
76
New cards
77
New cards
78
New cards
79
New cards
80
New cards
81
New cards
82
New cards
83
New cards
84
New cards
85
New cards
86
New cards
87
New cards
88
New cards
89
New cards
90
New cards
91
New cards
92
New cards
93
New cards
94
New cards
95
New cards
96
New cards
97
New cards
98
New cards
99
New cards
100
New cards