AQA A-Level Chemistry - Thermodynamics
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Enthalpy Change of Reaction
The number of moles of reactant specified in the balanced equation reacting together
Enthalpy of Formation
The enthalpy change required to form one mole of a substance from its constituent elements under standard conditions in standard states
Enthalpy of Combustion
The enthalpy change required to completely burn one mole of a substance in the presence of oxygen, with all substances in their standard states
First Ionisation Enthalpy
The enthalpy change when one mole of gaseous atoms loses one electron per atom to produce gaseous +1 ions
Second Ionisation Enthalpy
The enthalpy change when one mole of gaseous +2 ions are formed from one mole of +1 ions
First Electron Affinity
The enthalpy change when one mole of gaseous atoms gains one electron per atom to produce gaseous -1 ions
Second Electron Affinity
The enthalpy change when one mole of gaseous -1 ions gains one electron per ion to form gaseous -2 ions
Enthalpy of Atomisation
The enthalpy change when one mole of gaseous atoms are produced from an element in its normal state
Hydration Enthalpy
The enthalpy change when one mole of gaseous ions become hydrated (dissolved in water)
Enthalpy of Solution
The enthalpy change when one mole of an ionic solid dissolves in an amount of water large enough so that the dissolved ions are far apart enough to not interact with one another
Bond Dissociation Enthalpy
The enthalpy change when one mole of covalent bonds are broken in the gaseous state
Lattice Enthalpy of Formation
The enthalpy change when one mole of a solid ionic compound is formed from its constituent ions in the gas phase
Lattice Enthalpy of Dissociation
The enthalpy change when one mole of a solid ionic compound is broken up into its constituent ions in the gas phase
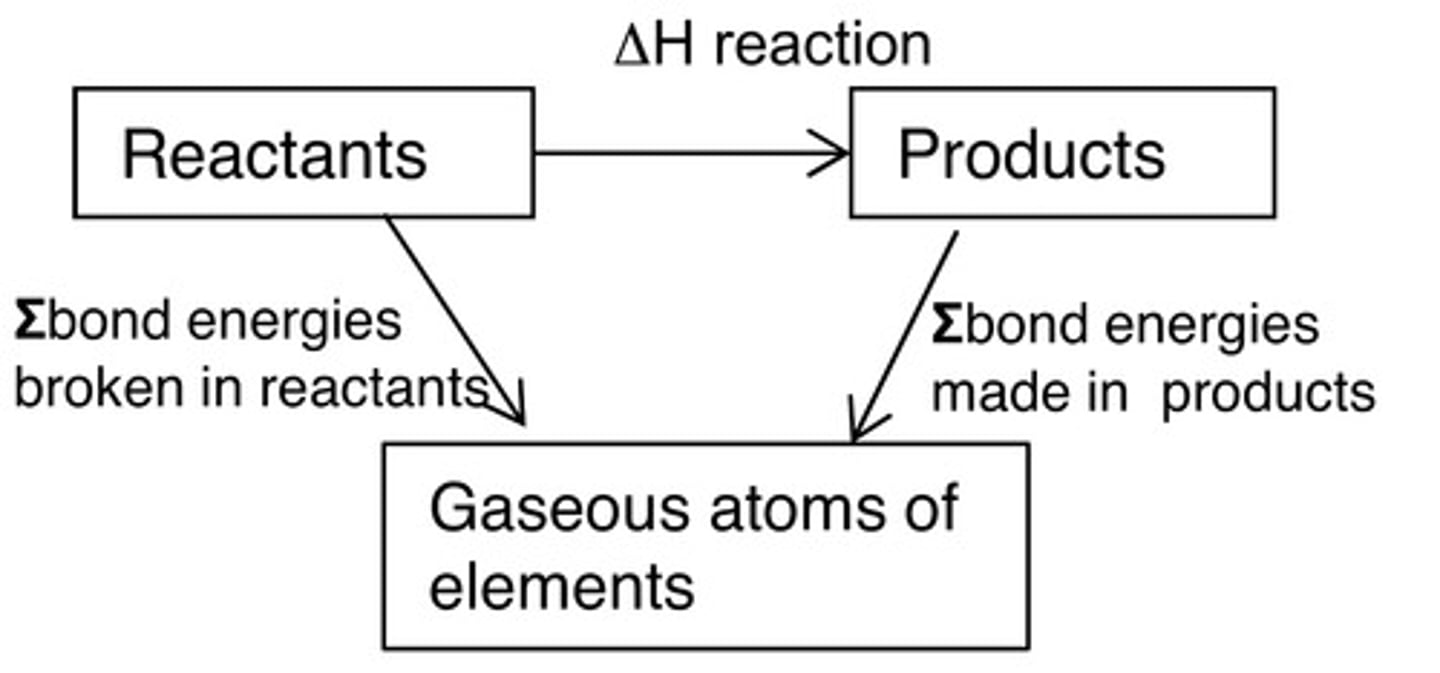
Hess's Law
The sum of energy changes in the clockwise direction is always equal to the energy changes in the anticlockwise direction of a Hess's Cycle
Hess's Cycle
A physical representation of the different pathways by which our desired product can be formed, including the enthalpy of formation and enthalpy of combustion
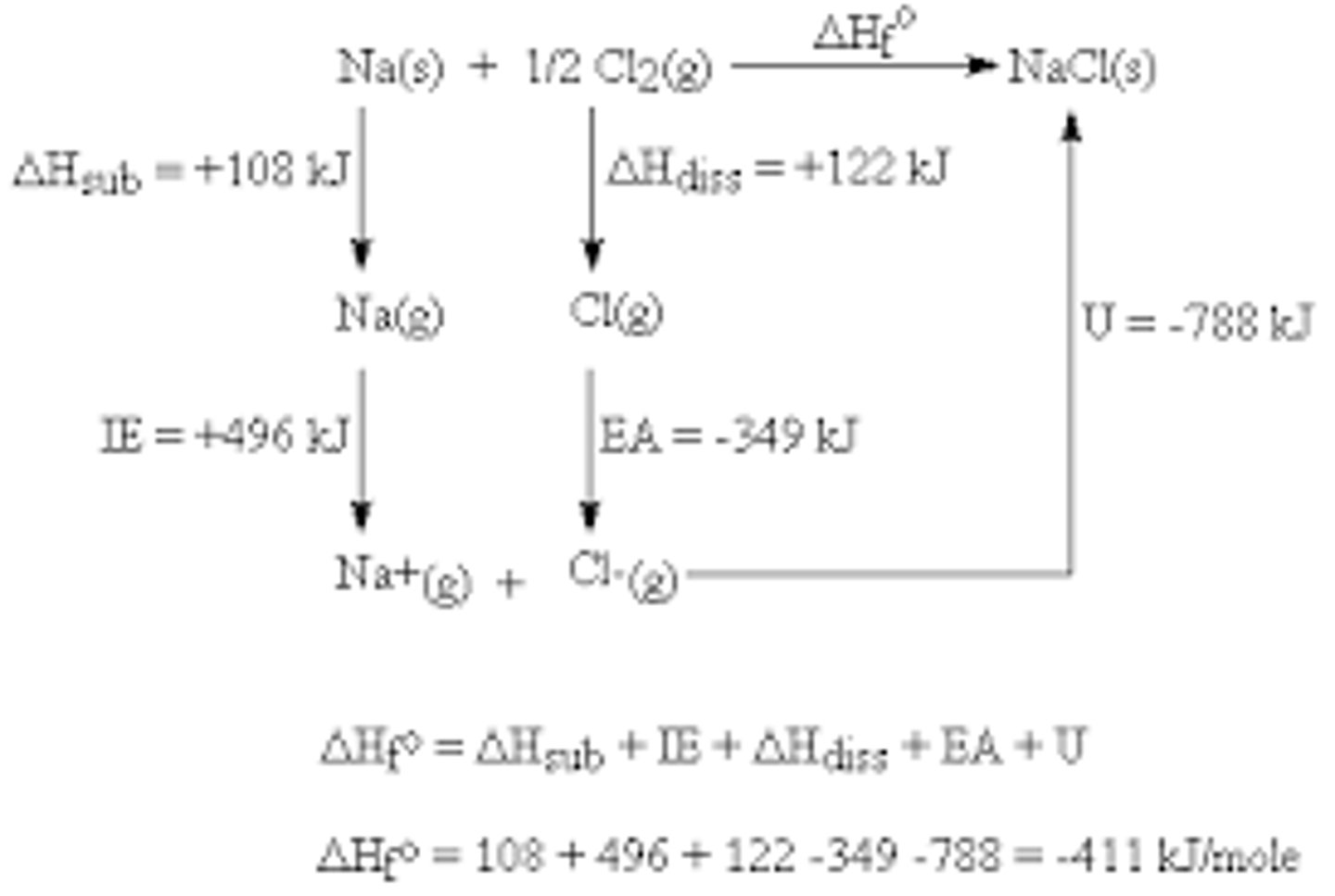
Born-Haber Cycle
A physical representation of a complete reaction and the numerous stages involved to calculate a enthalpy value
Enthalpy of Formation BHC
The sum of all other enthalpy diagrams in a Born-Haber cycle. This value is always negative
Positive
Are the following values positive of negative?
- Enthalpy of atomisation
- Ionisation enthalpies
- Enthalpy of vapourisation
- Second electron affinity
- Lattice enthalpy of dissociation
Negative
Are the following values positive of negative?
- First electron affinity
- Lattice enthalpy of formation
- Enthalpy change of reaction
M (s) --> M (g)
enthalpy of atomisation of a metal
1/2 X2 --> X (g)
enthalpy of atomisation of a diatomic
Cl2 --> 2 Cl (g)
Bond dissociation enthalpy of chlorine
Na+ (g) + Cl- (g) --> NaCl (s)
Formation lattice enthalpy of sodium chloride
NaCl(s) --> Na+ (g) + Cl- (g)
Dissociation lattice enthalpy of sodium chloride
CH3CH2OH (l) + 3O2 (g) --> 2CO2 (g) + 3H2O (l)
enthalpy of combustion of ethanol
X (g) --> X+ (g) + e-
1st ionisation enthalpy
X+ (g) --> X2+ (g) + e-
2nd ionisation enthalpy
X (g) + e- --> X- (g)
1st electron affinity
X- (g) + e- --> X2- (g)
2nd electron affinity
H2O (l) --> H2O (g)
enthalpy of vaporisation of H2O
H2O (s) --> H2O (l)
enthalpy of fusion of H2O