PHR 936 - Block 2: Pain kaitlyn
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
more than __% of Americans are living with some form of chronic pain
30
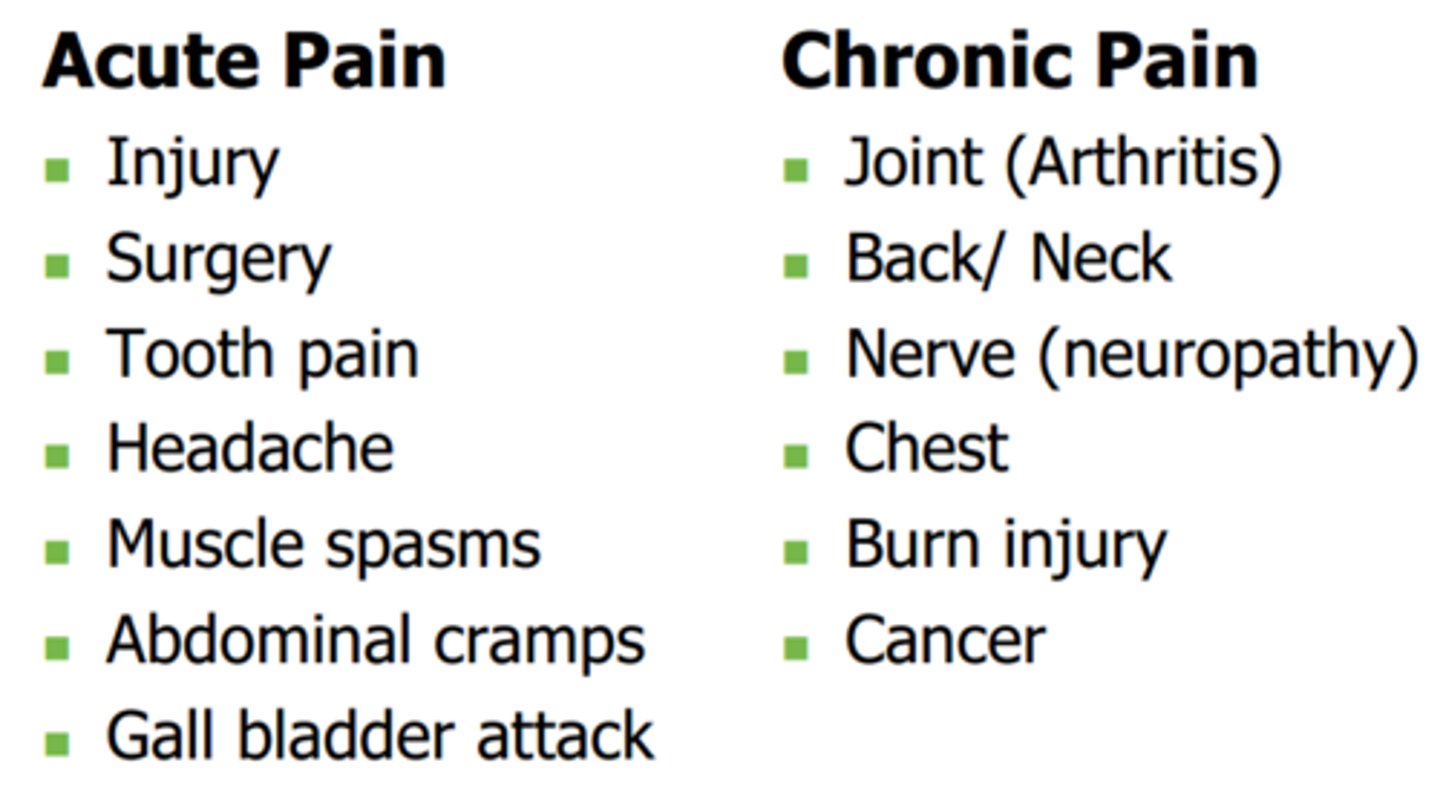
pain
an unpleasant sensory and emotional experience
- associated with actual/ potential tissue damage

nociception
the perception and sensation of pain
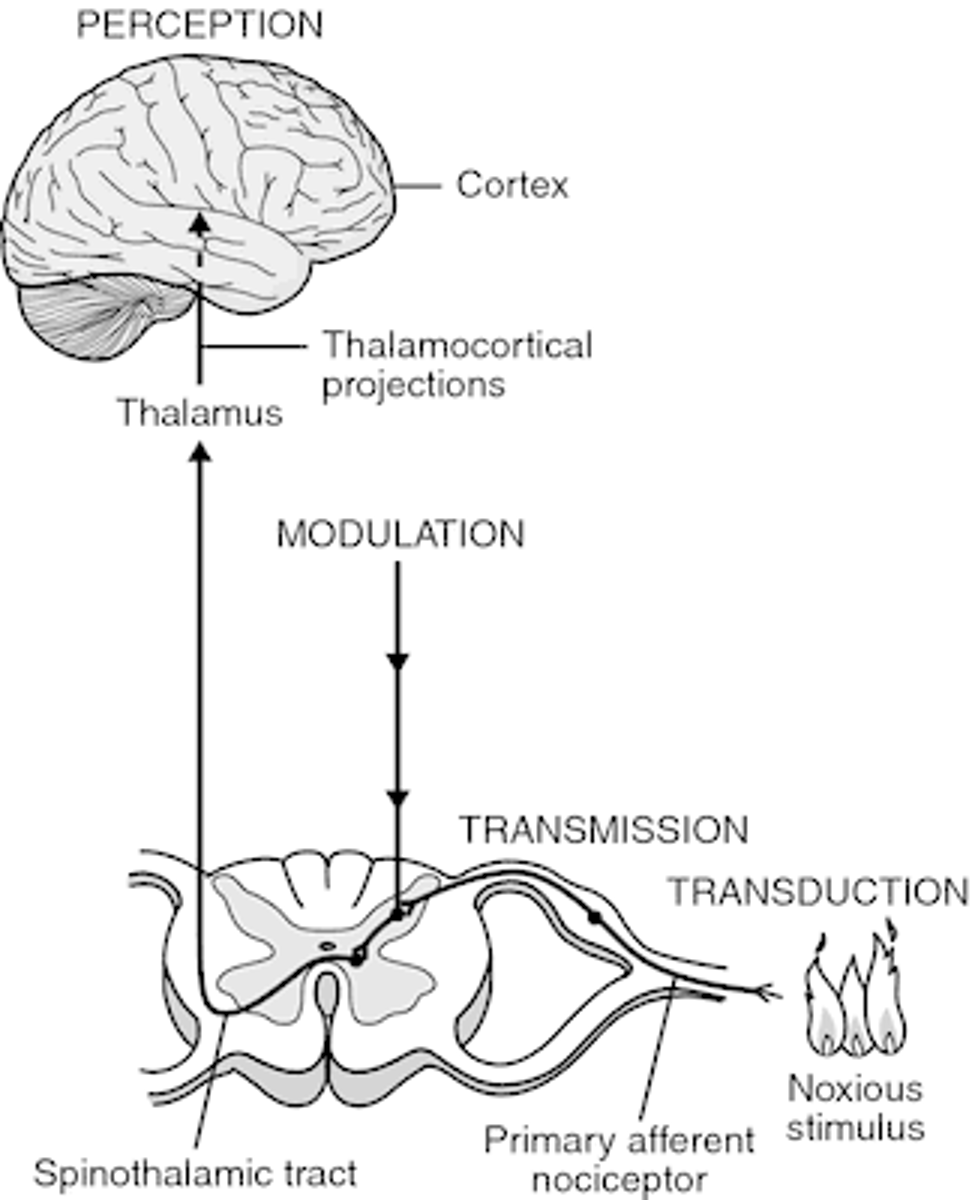
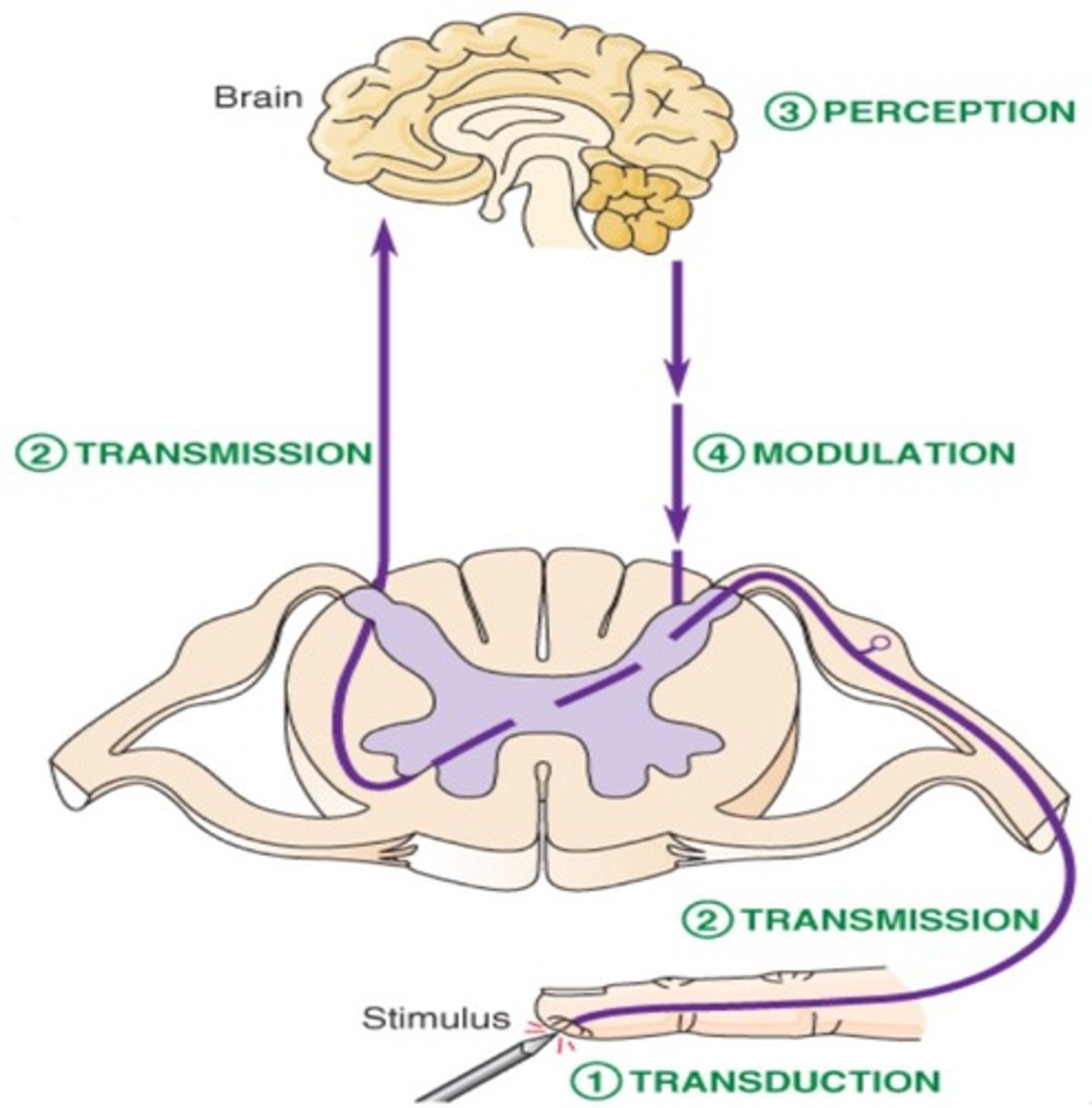
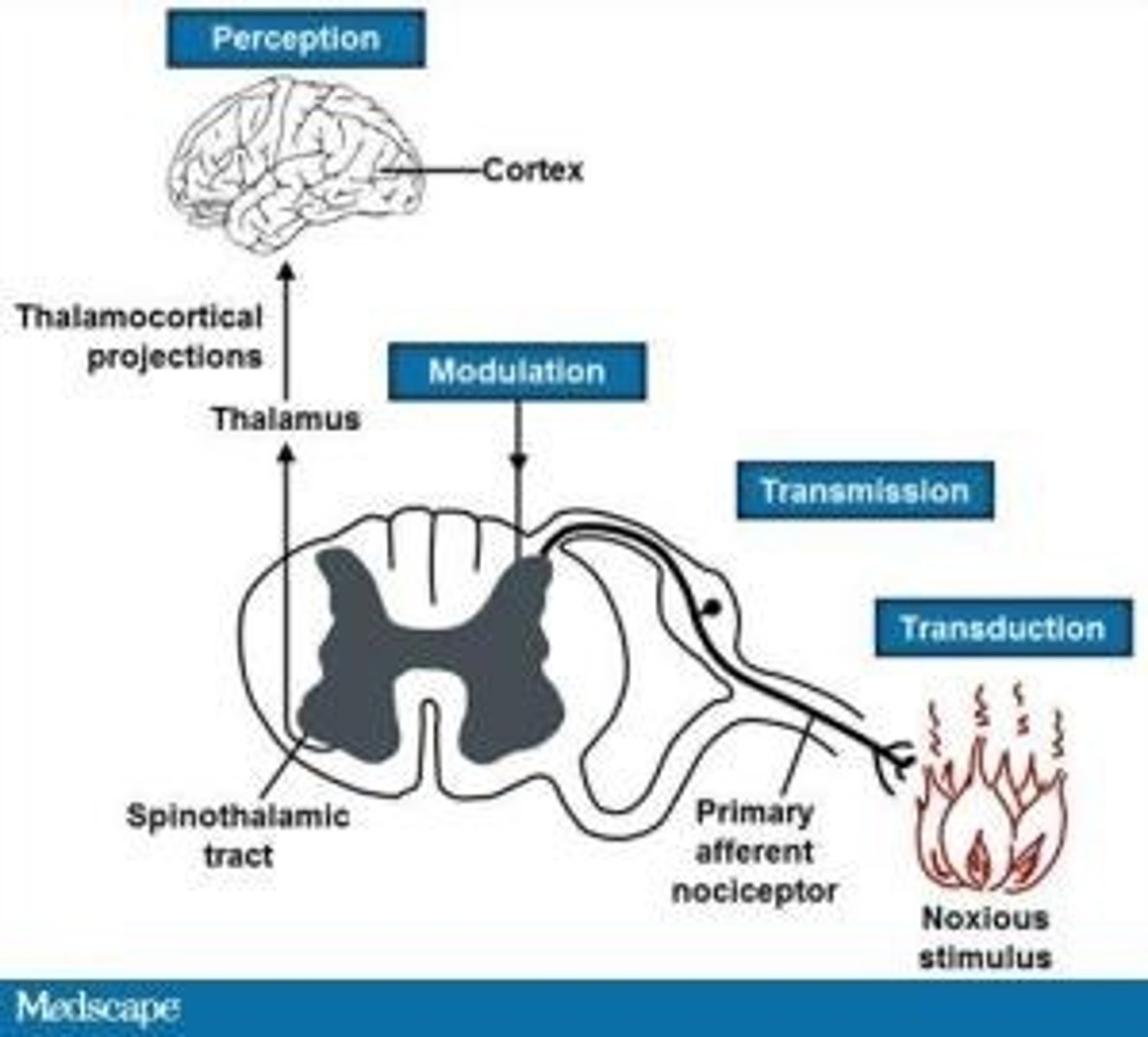
4 steps of nociception
1. transduction
- transformation of a physical stimulus into an electrical signal
2. transmission
- sending the electrical signal to the spinal cord and brain
- afferent
3. modulation
- amplifying or reducing pain signals based on various factors
- efferent
4. perception
- conscious awareness and interpretation of the pain signal by the brain (thalamocortical projections)
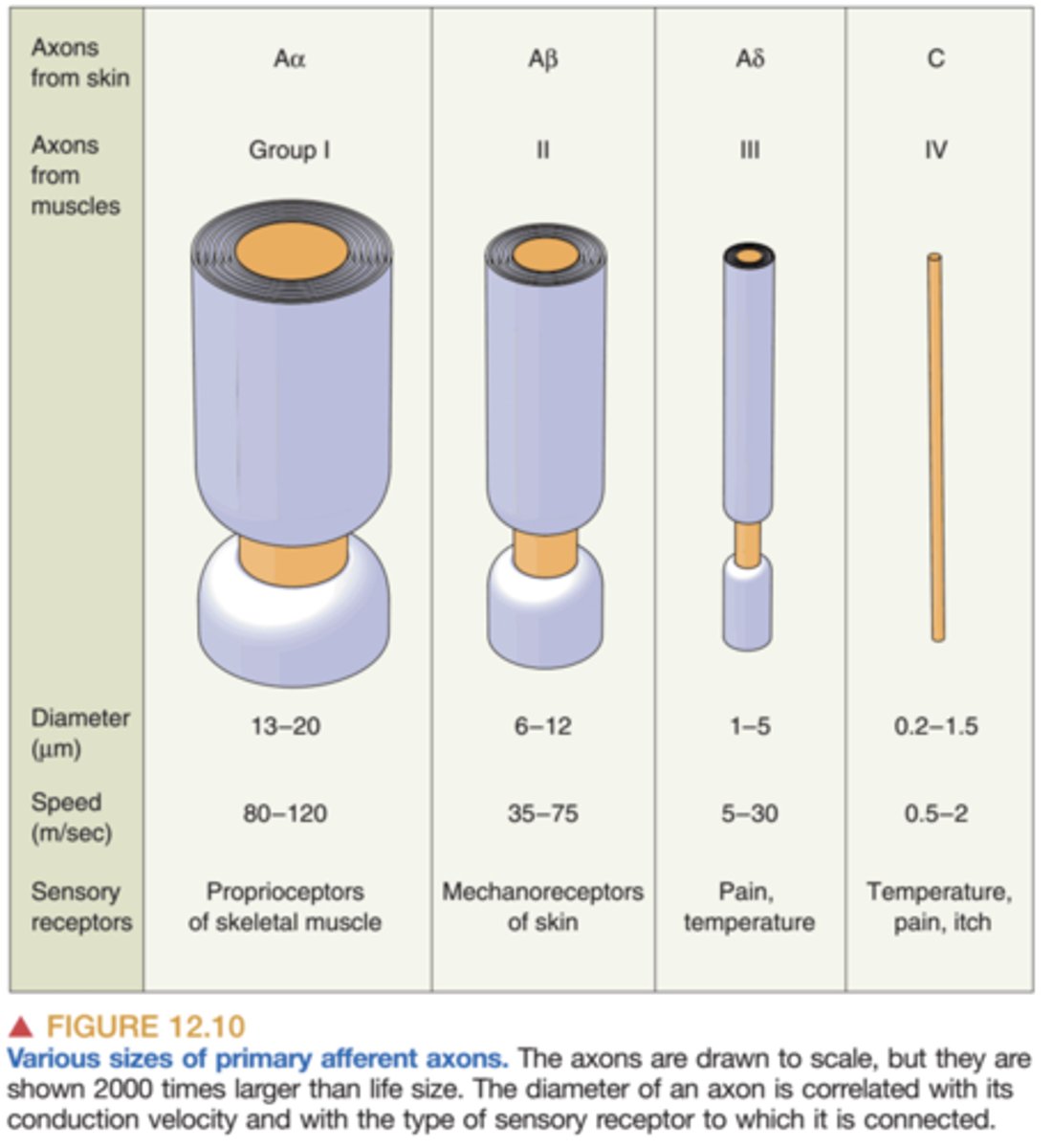
primary afferent axons
1st order
- the gateway by which sensory information is transmitted from the peripheral tissues to the spinal cord and brain
- thicker = faster (more myelin sheath)
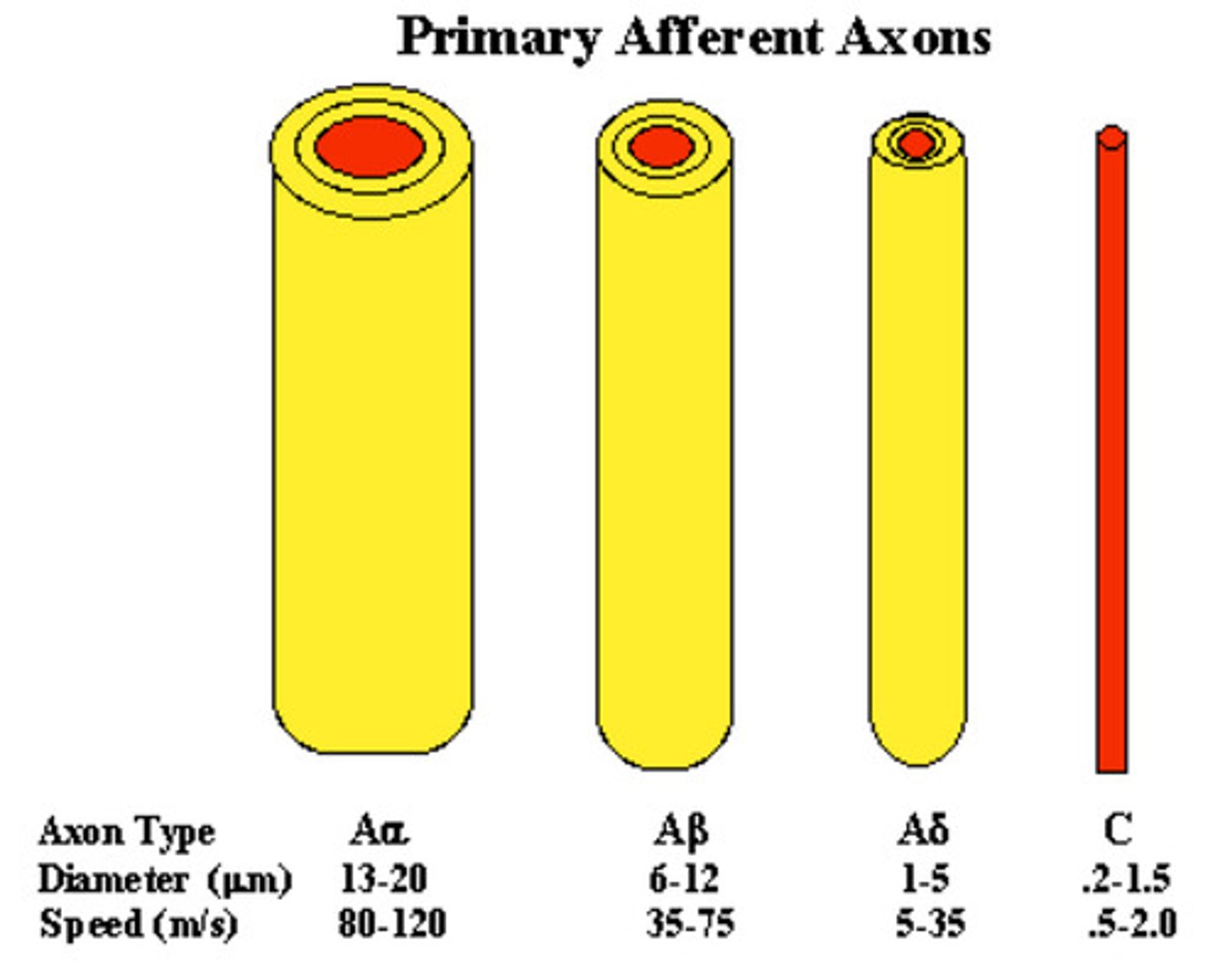
a fibers and c fibers
a fibers: myelinated and detect quick pain
- stabbing, pricking pain
- acute
c fibers: found throughout the body and are un-myelinated
- dull, aching pain
- lasts longer
nociceptors
primary afferent nerve fibers that detect environmental stimuli
1. high or low threshold mechanoreceptors
2. thermal receptors
3. chemical receptors
also have high levels of voltage-gated sodium and potassium channels
VGSC and VGPC
voltage-gated sodium channels (Navs) and voltage-gated potassium channels
- Nav 1.7, 1.8, & 1.9 are mainly expressed in C-fibers
Navs
voltage-gated sodium channels
- focus on the three in the PNS: 1.7, 1.8, and 1.9
Nav 1.7: olfaction, nociception
Nav 1.8: nociception
Nav 1.9: threshold maintenance
- when dysfunctional -> pain
transmission
ascending pathway, afferent
- relay of action potential
pathway:
1. dorsal root ganglion (DRG)
2. to 2nd order neuron (thru spinothalmamic tract)
3. to thalamus (relay station) to synapse with 3rd order neuron
1st to 2nd order neuron transmission
1. glutamate activates NMDA & AMPA receptors to allow influx of Ca and Na -> action potential propagation
2. substance P bind to NK-1 receptors -> activates PKC -> removes Mg from NMDA receptor -> Ca influx
3. CGRP changes receptor expression/ function -> central sensitization
modulation
descending pathway
- centrally originating neuron acts to inhibit release of substance P from 1st order neuron
- serotonergic/ noradrenergic neuron
- interneuron stimulation leads to enkephalin release
> inhibits pre and post synaptic activity in the ascending pathway
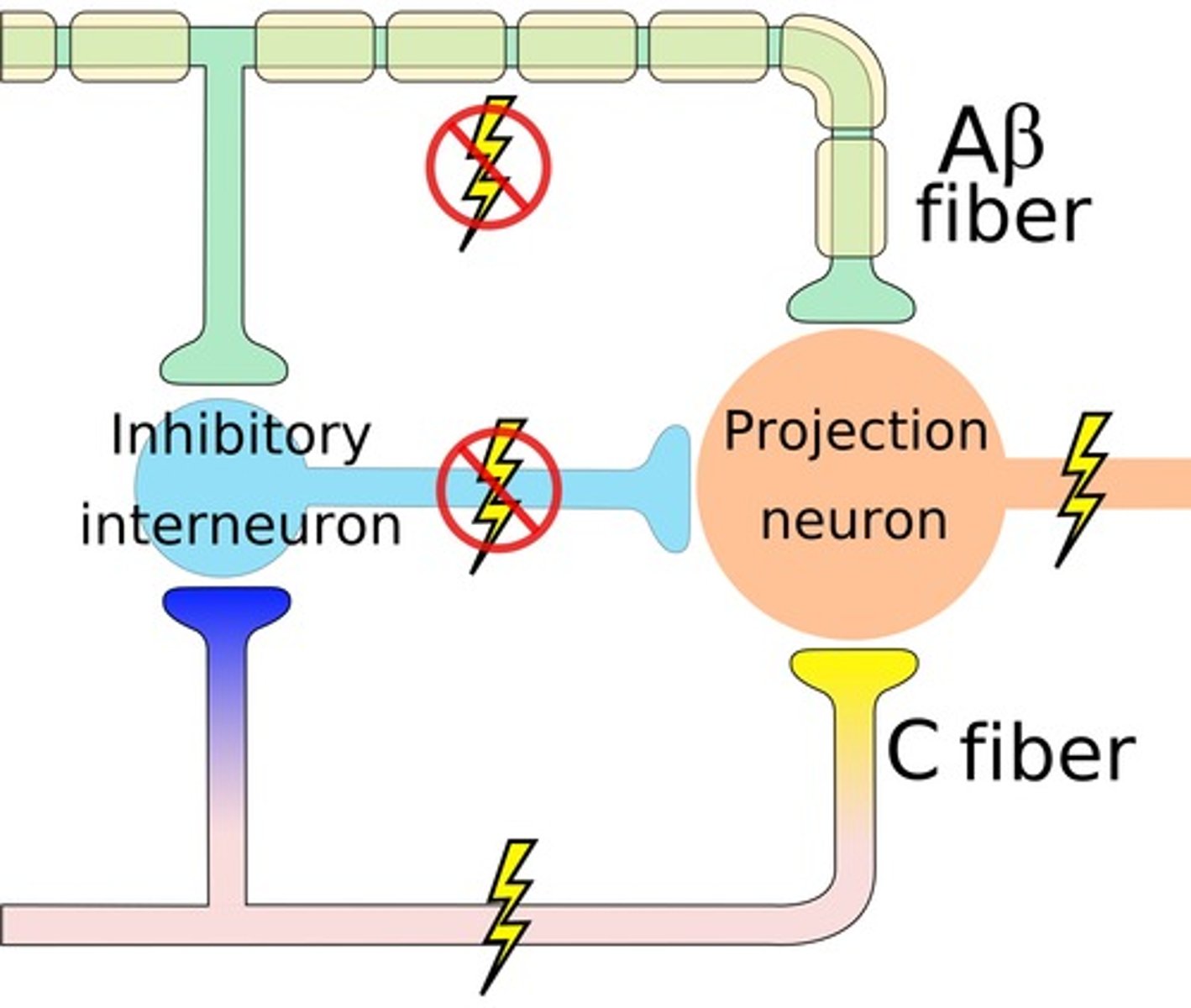
gate control theory
the theory that the spinal cord contains a neurological "gate" that blocks pain signals or allows them to pass on to the brain
- the "gate" is opened by the activity of pain signals traveling up small nerve fibers and is closed by activity in larger fibers or by information coming from the brain
1. activation of 1st order neuron (C fiber) -> 2nd neuron activation & inhibits inhibitory neuron -> nociception
2. activation of 1st order neuron (AB fiber) -> inhibits 2nd order neuron & de-inhibits inhibitory neuron -> no nociception
perception
2nd order neuron synapses with 3rd order neuron in the thalamus
-> relays information to cortex for perception of location and nature of the pain
NSAIDs +/- APAP are __ as other pain medications (including opioids) for acute pain
at least as effective
NSAID MOA
COX1 and COX2 inhibitors, inhibit metabotropic receptors which are activated in inflammation
- COX1: constitutive; PGE2 & PGI2
- COX2: inducible; PGI2
- COX3: regulation of pain response and fever
acetaminophen MOA
unclear
- could involve processes related to: COX inhibition, endocannabinoid system, TRP channels, nitric oxide, Ca/ K channels
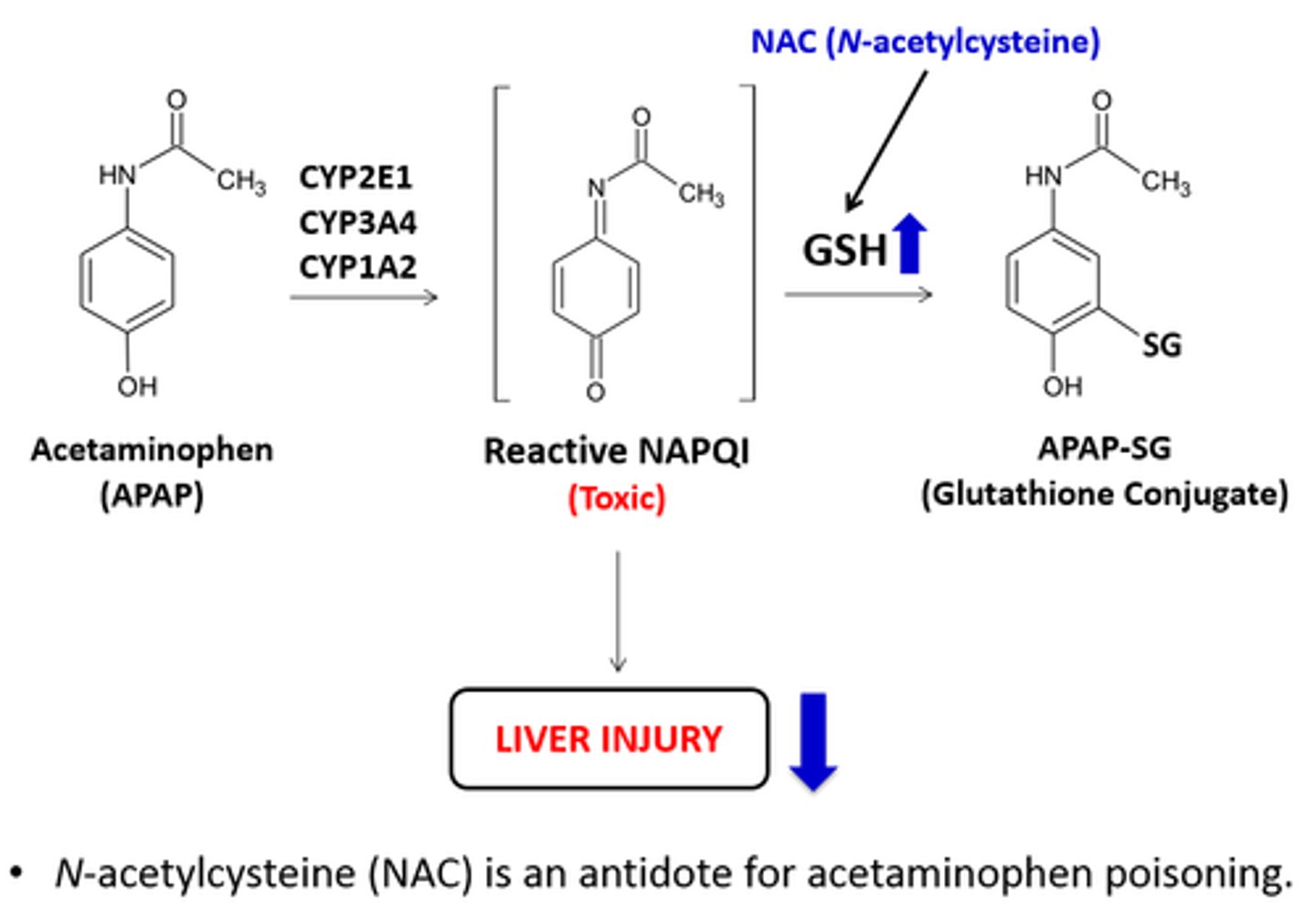
what is the cause of hepatoxicity with APAP?
there is not enough glutathione (GSH) to convert NAPQI into mercapturic acid for excretion
how is APAP dosing different from NSAIDs?
give enough APAP: 1000 mg Q6H (scheduled)
give the minimum of NSAIDs: 400 mg Q6H to avoid GI bleed or CV events
- higher dose is not better
selective COX2 inhibitor
celecoxib
- lower bleed risk
- similar CV risk
choosing an NSAID
all have similar efficacy
- consider celecoxib for bleed concern
- consider ADR risks, cost, and dosing schedule
- begin with scheduled dose, transition to PRN
- can try a different one if needed
ibuprofen (Motrin, Advil), Celecoxib (Celebrex), diclofenac (Voltaren), meloxicam (Mobic), naproxen (Aleve)
NSAID + PPI
may be considered for high risk patients: 64+, hx of PUD/ upper GI bleed, anticoag/platelet therapy
not generally recommended: PPIs should be taken on an empty stomach, NSAIDs should be taken with food
opioid vs opiate
opioid: natural, semi-synthetic, and fully synthetic opioids
- fentanyl, methadone, meperidine, oxycodone
opiate: natural derived opioid
- heroin, morphine, codeine

narcotic
a drug or other substance that affects mood or behavior and is consumed for non-medical purposes, especially one sold illegally
opioid pharmacology
opioid binds to Gi GPCR μ-receptor → inhibits adenylyl cyclase:
presynaptic: closes voltage-gated Ca²⁺ channels → ↓ release of neurotransmitters (substance P, glutamate) → ↓ pain signal transmission
postsynaptic: opens K⁺ efflux channels → hyperpolarization → ↓ neuronal excitability
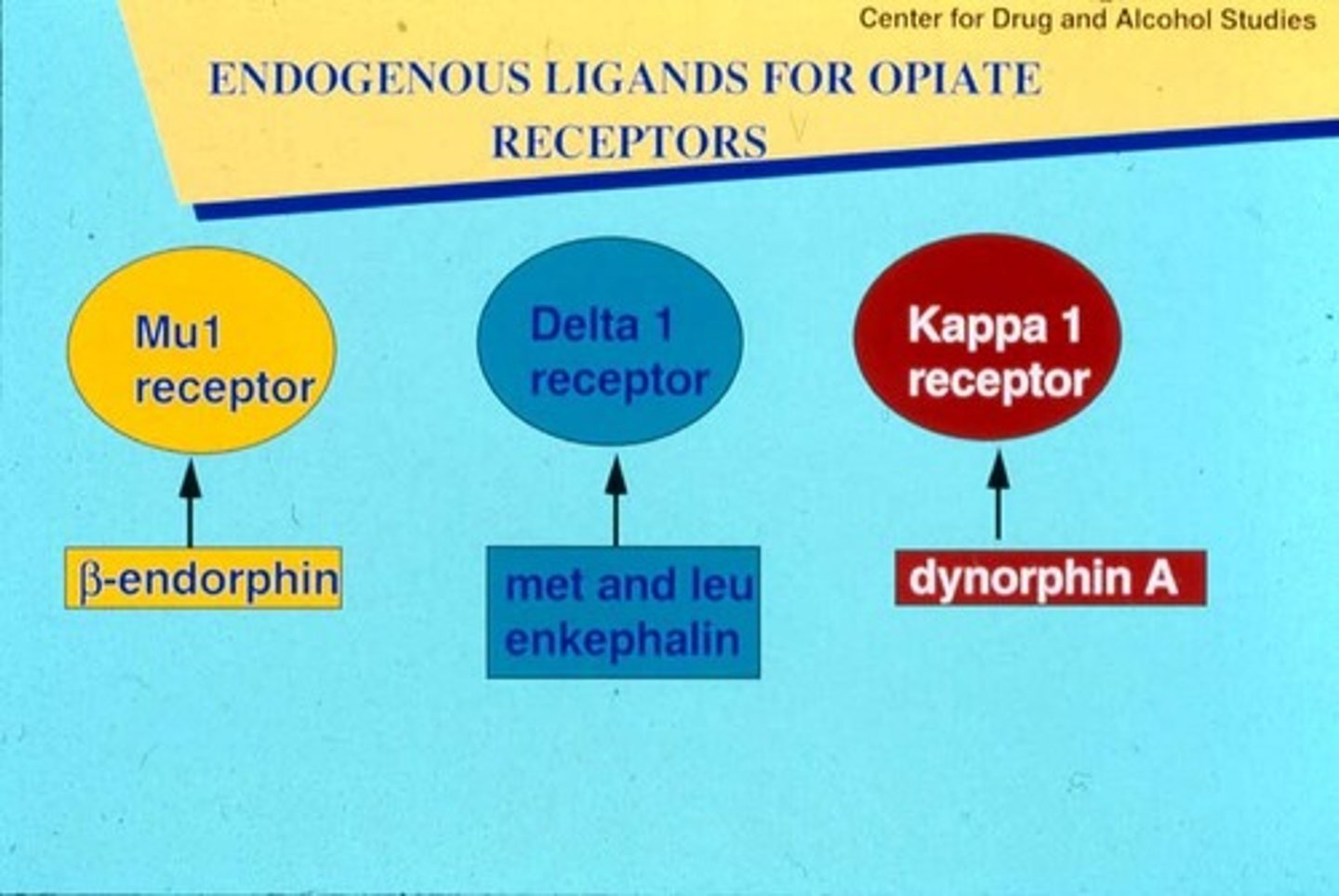
naturally occurring opioids: endorphins, dynorphins, enkephalins

opioid receptors
mu, kappa, delta
most common: mu receptor (MOR)
- functions in analgesia, respiratory function, GI motility, mood, thermoregulation, hormone secretion, and immune function
5 categories of opioids
1. phenanthrenes (most common)
- morphine
- codeine
- hydrocodone
- oxycodone
- oxymorphone
- heroin
- naloxone
- naltrexone
2. benzomorphans
- diphenoxylate
- loperamide
- pentazocine
3. phenyl-piperadines
- fentanyl
- nils
- meperidine
- IMFs
4. diphenyl-heptanes
- methadone
- propxypehne
5. phenyl-propylamines
- tramadol
- tapentadol
morphine
phenanthrene
- naturally occurring opioid found in opium
- standard for comparison (MME)
- IV is the most common, but has MANY formulations
- metabolized via glucuronidation
codeine
phenanthrene
- an inactive prodrug that is converted to morphine via CYP2D6
- BLACK BOX: contraindication in children
> kids are rapid CYP2Dg metabolizers = OD
opioid allergies
RARE!
ADE ≠ allergy
common ADEs: nausea, drowsiness, constipation
pseudoallergies: also not allergies
- 1st dose drives histamine release -> flushing, uticaria, pruritus
- uncommon with synthetic opioids
real allergy: mediated by IgE -> anaphylaxis
fentanyl
phenylpiperadine
- 50-100x more potent than IV morphine
- fully synthetic (= no histamine release)
- highly lipid soluble (obese pts = prolonged exposure)
- may see chest wall rigidity with rapid IV infusions (NOT respiratory depression)
transdermal fentanyl
Duragesic
phenylpiperadine
- for moderate-severe chronic pain requiring 60+ MME/day for at least 1 week
- takes 12 hours for onset and offset (not for acute pain)
- apply Q72H
- do NOT cut or heat
- always use the package insert for conversions!!!!!
tramadol
Ultram
phenylpropylamine
- dual action mu agonist + SNRI
- 1:1 racemic mixture
> R,R(+) tramadol = 5HT
> S,S(-) tramadol = NE
> M1/M5 metabolite = mu agonist
- CYP2D6 polymorphisms
methadone
Dolophine
diphenylheptane
- mu receptor agonist + NMDA agonist
- 50:50 racemic mixture of 2 enantiomers
> R(-) methadone = mu and delta receptor agonist
> S(+) methadone = voltage-gated K channel blocker -> QTc prolongation
- both enantiomers act as NMDA antagonist and SNRIs
- for chronic pain, OUD
- difficult to dose

methadone PK
bi-exponential distribution curve
- a phase: transfer from central compartment to tissue; analgesia
- B phase: drug elimination; suppression of withdrawal
- long half life
- IV to PO conversion: 1:2
- extensive hepatic metabolism -> renal excretion of inactive metabolites
most effective strategy for pain
multimodal analgesia
XR opioids
designed for 1-2x daily administration
- OxyContin
- MSContin
- Duragesic
- increased risk of non-fatal overdose, even when pt characteristics are accounted for
abuse-deterrent opioid formulations
designed to prevent a given route of abuse (injecting, snorting)
ex: Suboxone (buprenorphine/naloxone)
different mechanisms:
- physical barrier (prevent crushing/ extraction)
- chemical barrier (alter drug release/ solubility)
- agonist/antagonist combo (Suboxone)
- aversive agent (undesired effect with excessive dose)
- prodrug/ novel drug delivery system (action dependent on GI tract)
opioid ADE
- constipation
- respiratory depression - consider antagonist co-prescription, avoid co-sedating meds
- opioid-induced hyperalgesia - increasing pain with escalating doses
- neurotoxicity - due to buildup of active metabolites
- hypogonadism
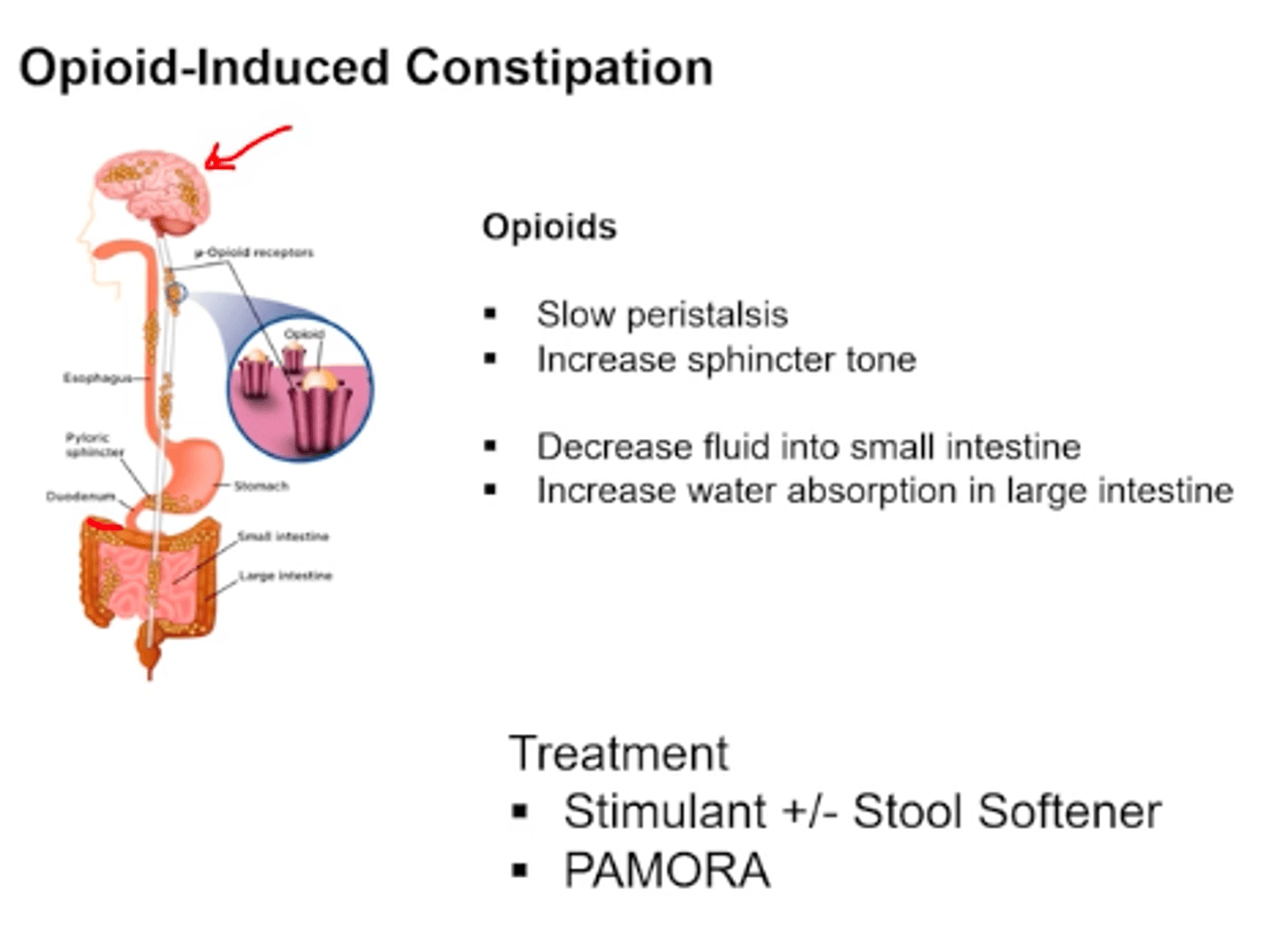
opioid-induced bowel dysfunction (OIBD)
generally self-limiting upper GI symptoms and opioid induced constipation (OIC)
- N/V
- abdominal pain
- dry mouth
managing opioid-induced constipation (OIC)
- assess bowel function BEFORE initiating therapy
- 1st line: non-pharm therapy + OTC stimulant/osmotic laxatives
- 2nd line: peripherally acting mu opioid receptor antagonists (PAMORA)
- generally avoid stool softeners: problem is not that the stool is hard, it is that the bowels are not contracting
PAMORAs
2nd line pharm therapy for OIC
- peripherally acting mu opioid antagonist with restricted access across the BBB
- all are substrates of CYP3A4: avoid with strong inducers/ inhibitors (kids)
> methylnaltrexone (Relistor)
> naloxegol (Movantik)
> naldemedine (Symproic)

opioid-induced neurotoxicity
associated with opioids with active metabolites typically
- morphine
- codeine
- oxycodone
- hydromorphone
-meperidine
signs: allodynia, delirium, hallucinations, hyperalgesia, hypersomnolence, myoclonus, tremor, seizures
risk factors: high dose, rapid dose increase, dehydration, renal failure, systemic infection, elderly
management:
- symptomatic treatment (benzos for myoclonus)
- dose reduction
- opioid rotation to non-toxic agen
- rehydration

opioid conversions
morphine is used as the baseline for conversion of doses
MME = morphine milligram equivalent
OME = oral morphine equivalent
MEDD = morphine equivalent daily dose
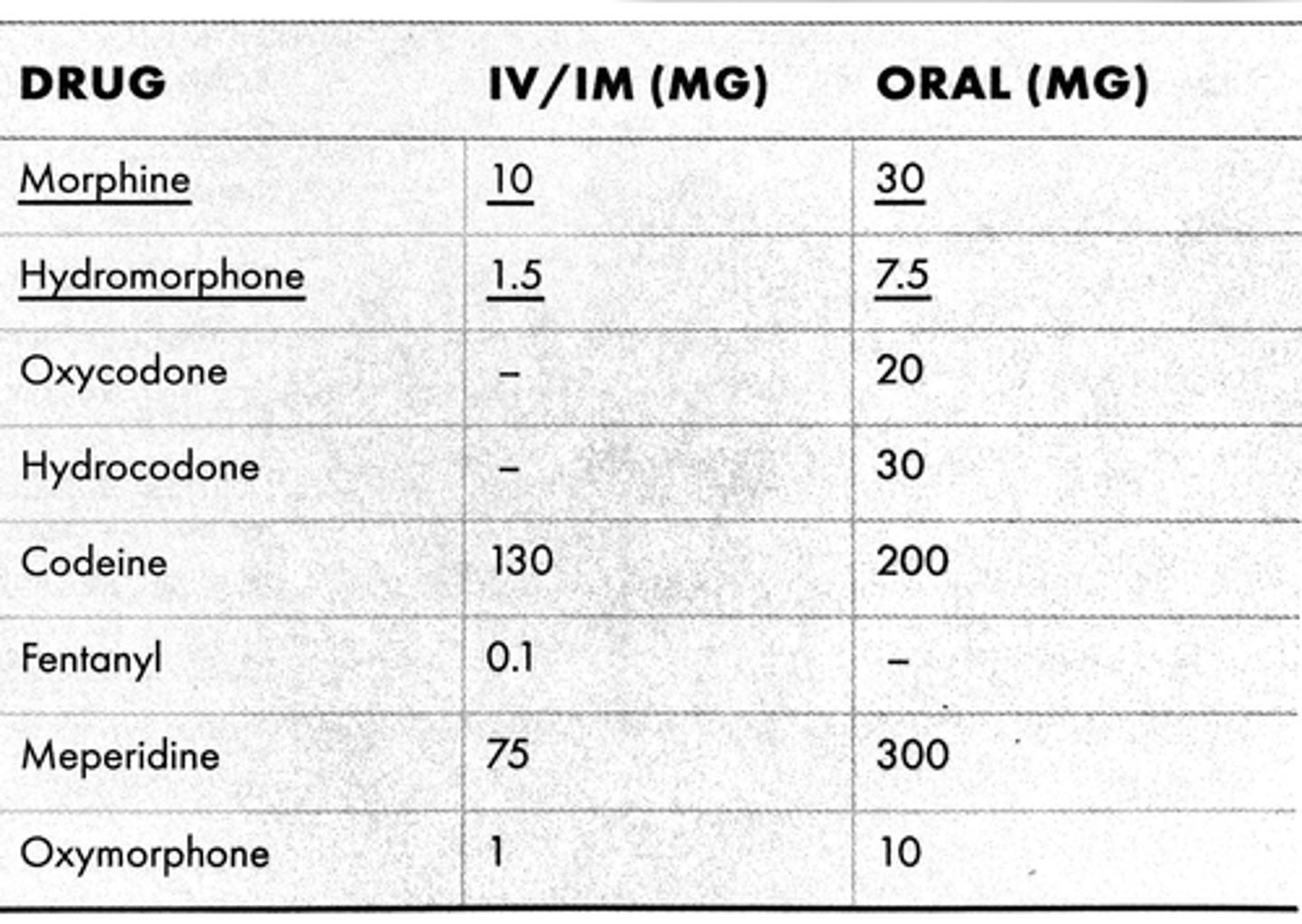
attempt to initiate opioid doses at <__ MME per day
50
avoid or justify initiating 90+ MME per day
use caution when initiating 50-89 MME per day
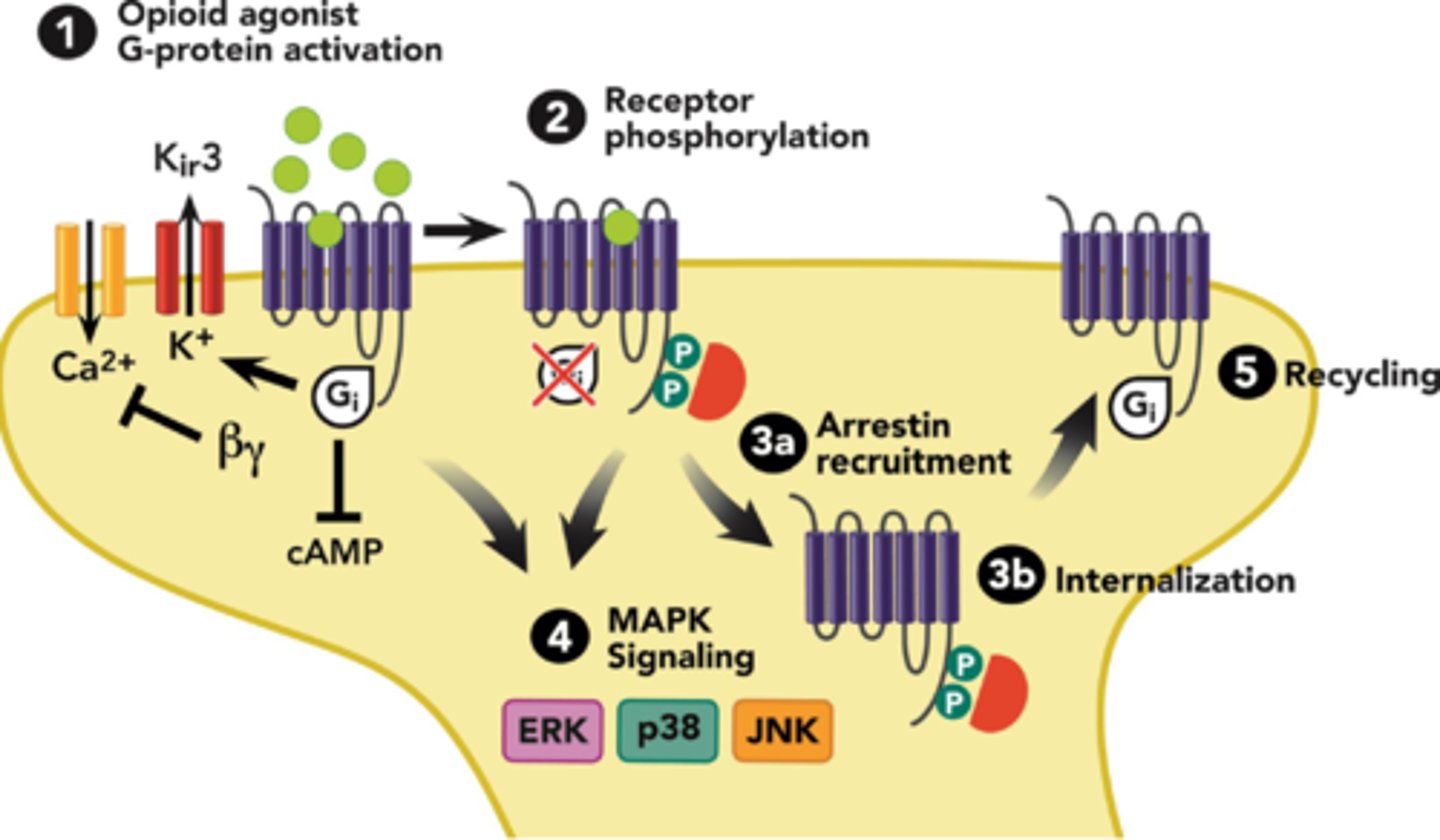
opioid tolerance
1. opioid binds to receptor
2. G-proteins dissociate and B-arrestin binds to newly phosphorylated receptors
3. receptor complex internalizes into endosomes
4. dephosphorylated and degraded OR recycled
- tolerance is DRUG specific
- reduce dose by 25-30% when switching opioids
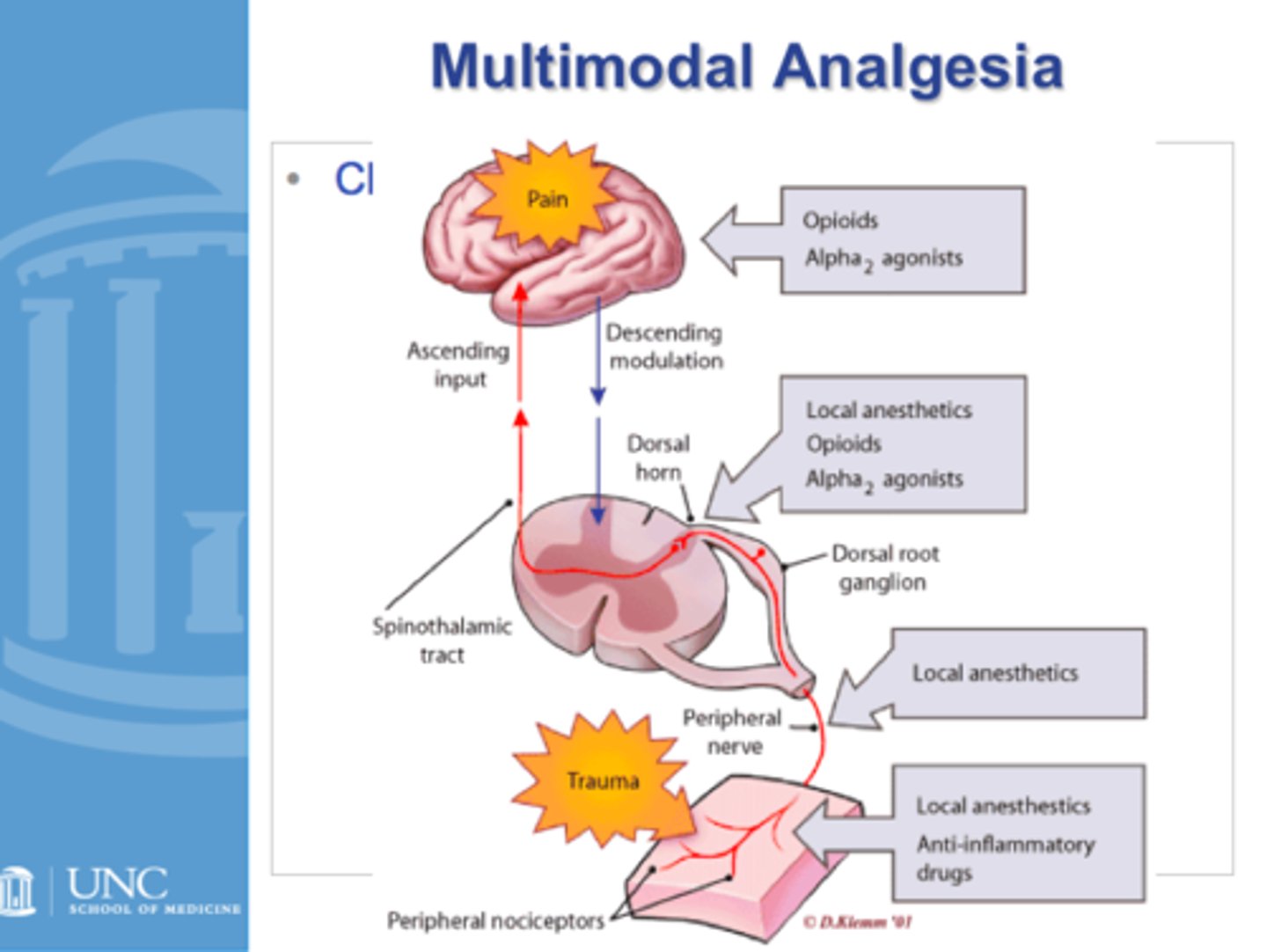
multimodal analgesia
the combination of various groups of analgesics with different mechanisms to provide superior pain control
may include:
- local anesthetics
- a2 agonists
- NMDA antagonists
- antiepileptics
- antidepressants
- antispasmodics
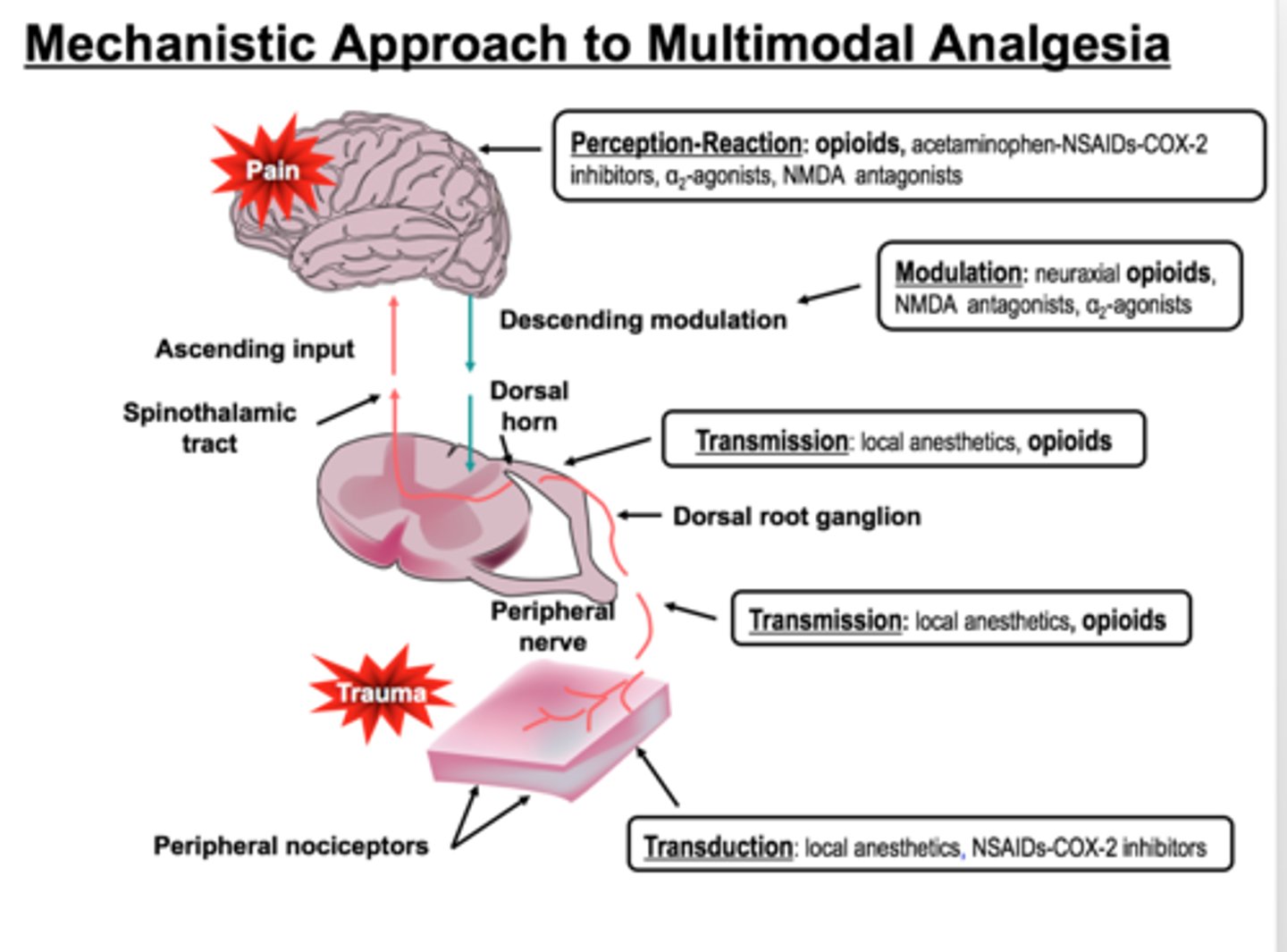
local anesthetics
non-selective Nav blockers
- caines: cocaine, prilocaine, lidocaine
- quick onset (1-5 minutes), quick offset (0.5-4 hours)
- can be combined with vasoconstrictors (epi) to prolong activity
- systemic administration can leads to tinnitus, metallic taste, seizures, coma, and death
topical lidocaine patch
Lidoderm
local anesthetic
- limited evidence of efficacy
- 12 hours on, 12 hours off
- apply to intact skin
- okay to cut prior to application
- avoid contact with face and eyes
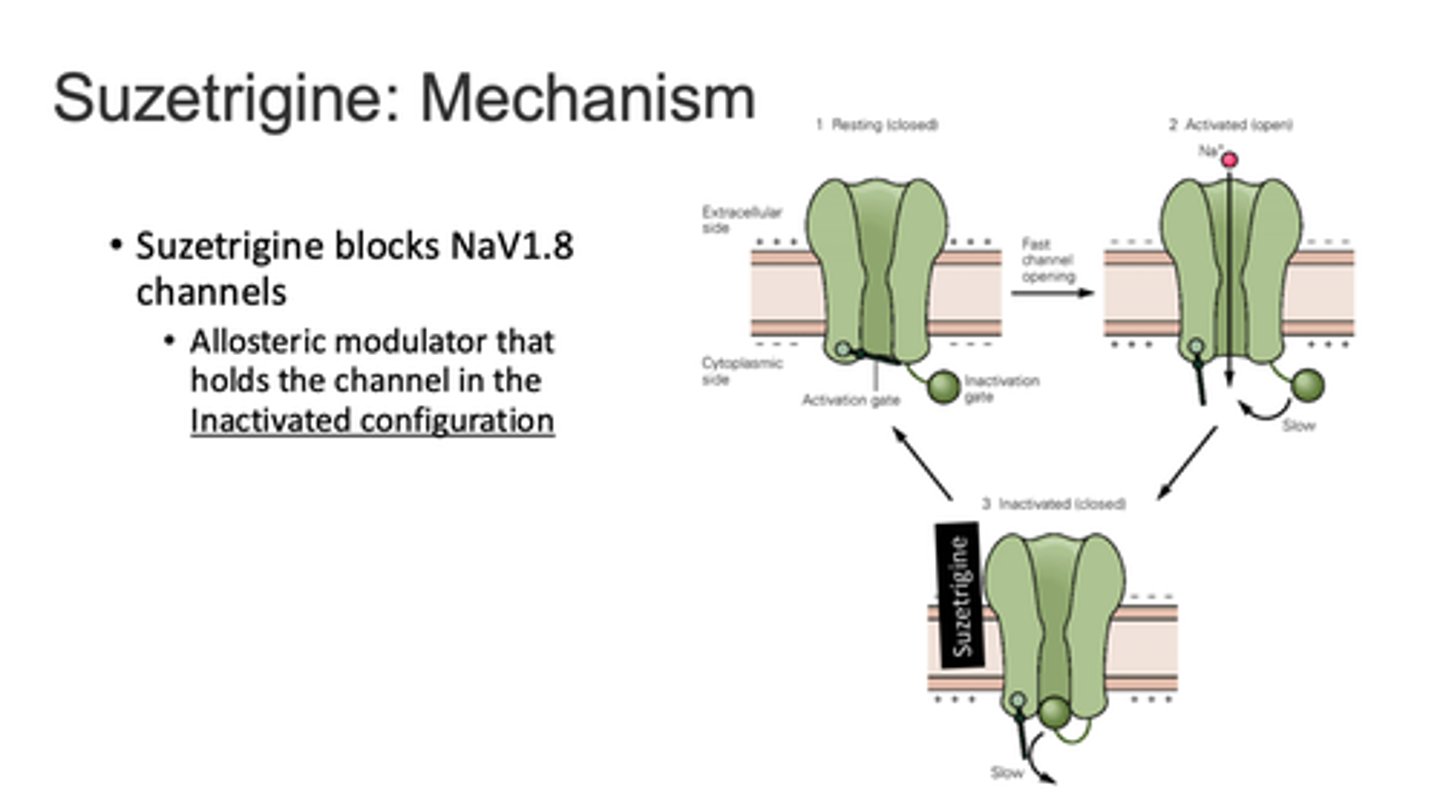
suzetrigine
Journavx (CVI)
Nav 1.8 inhibitor (oral)
new pain drug targeting transmission of inflammatory pain signal AND addresses SUD/OUD
- similar analgesic efficacy to hydrocodone
- similar ADRs: more headache, less N/V
- helpful in addiction long-term
- no CNS penetration, no abuse potential
- avoid in moderate-severe hepatic failure
- major CYP3A4 substrate
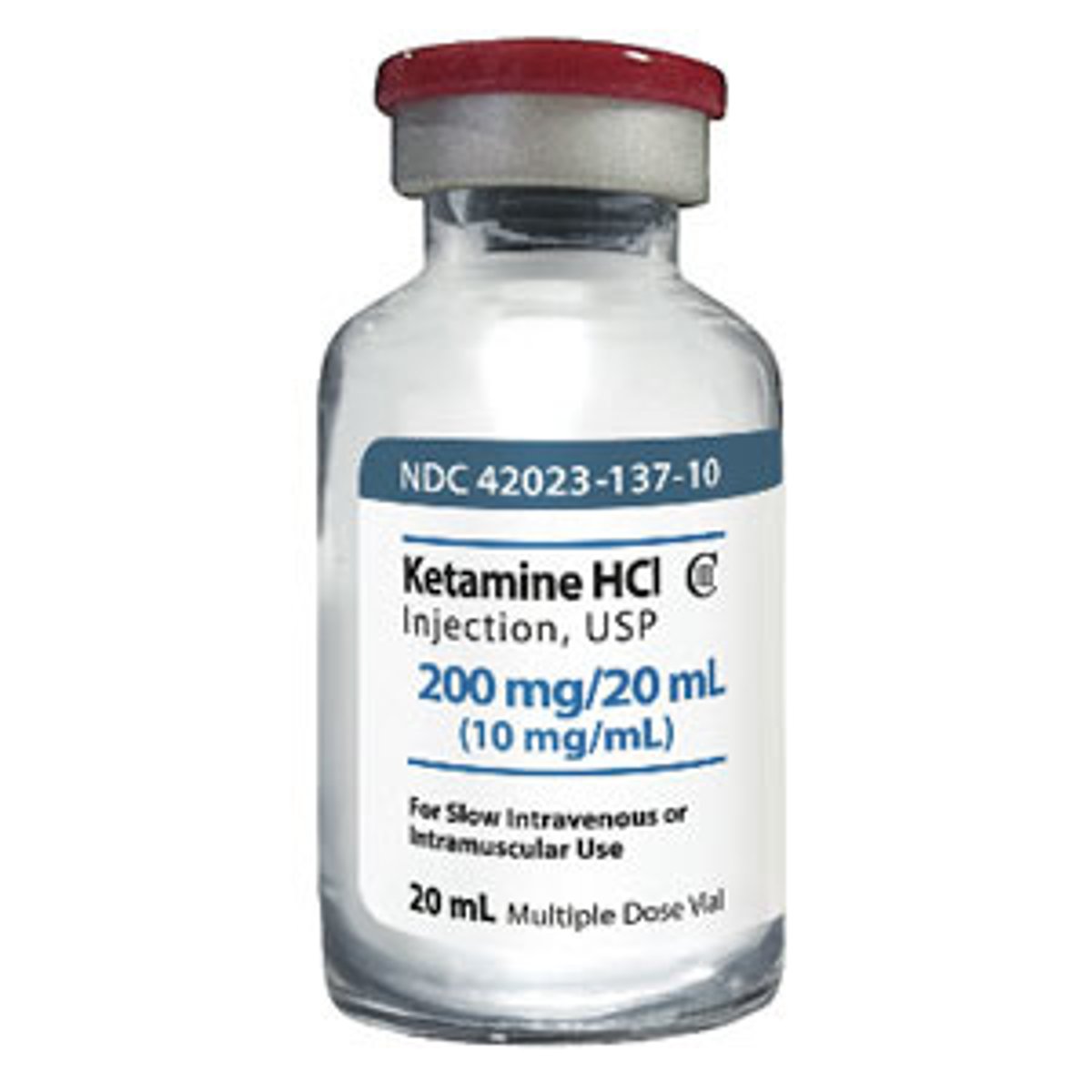
NMDA antagonists
ketamine, methadone
- most often considered in opioid tolerant patients
- sub-dissociative dosing produces strong analgesia
- very quick onset and duration
- too much leads to: "K hole", sedation, severe agitation, death
antidepressants
SNRIs: duloxetine (Cymbalta)
- ADEs: anticholinergic effects, weight gain, sedation, orthostatic HTN
TCAs: amitriptyline (Elavil), nortriptyline (Pamelor)
- ADEs: GI effects
gabapentinoids
gabapentin (Neurontin), pregabalin (Lyrica)
a2∆ blockers
MOA: binds a2∆ subunit of presynaptic voltage gated Ca channels -> destabilization, internalization, and recycling
- commonly used off label for pain management
- dose-related CNS depression
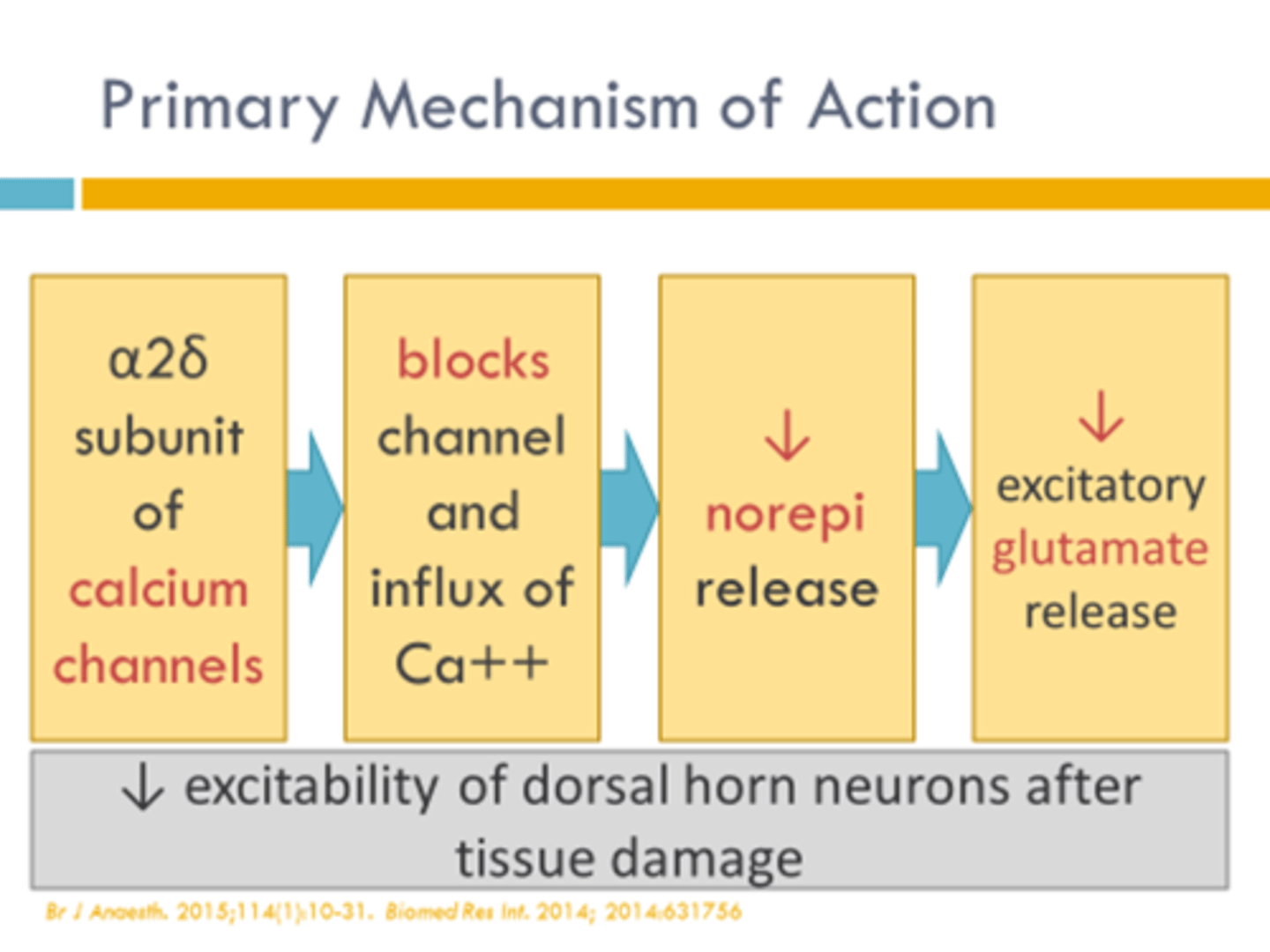
antispastics
used to relax skeletal muscle and decrease muscle spasm (voluntary movement)
- baclofen
- dantrolene
antispasmodics
used to relax smooth muscle (involuntary movement)
- carisoprodol
- cyclobenazaprine
- metaxalone
- methocarbamol
- orphenadrine
- chlorzoxazone
antispastic + antispasmodic
- diazepam
- tizanidine
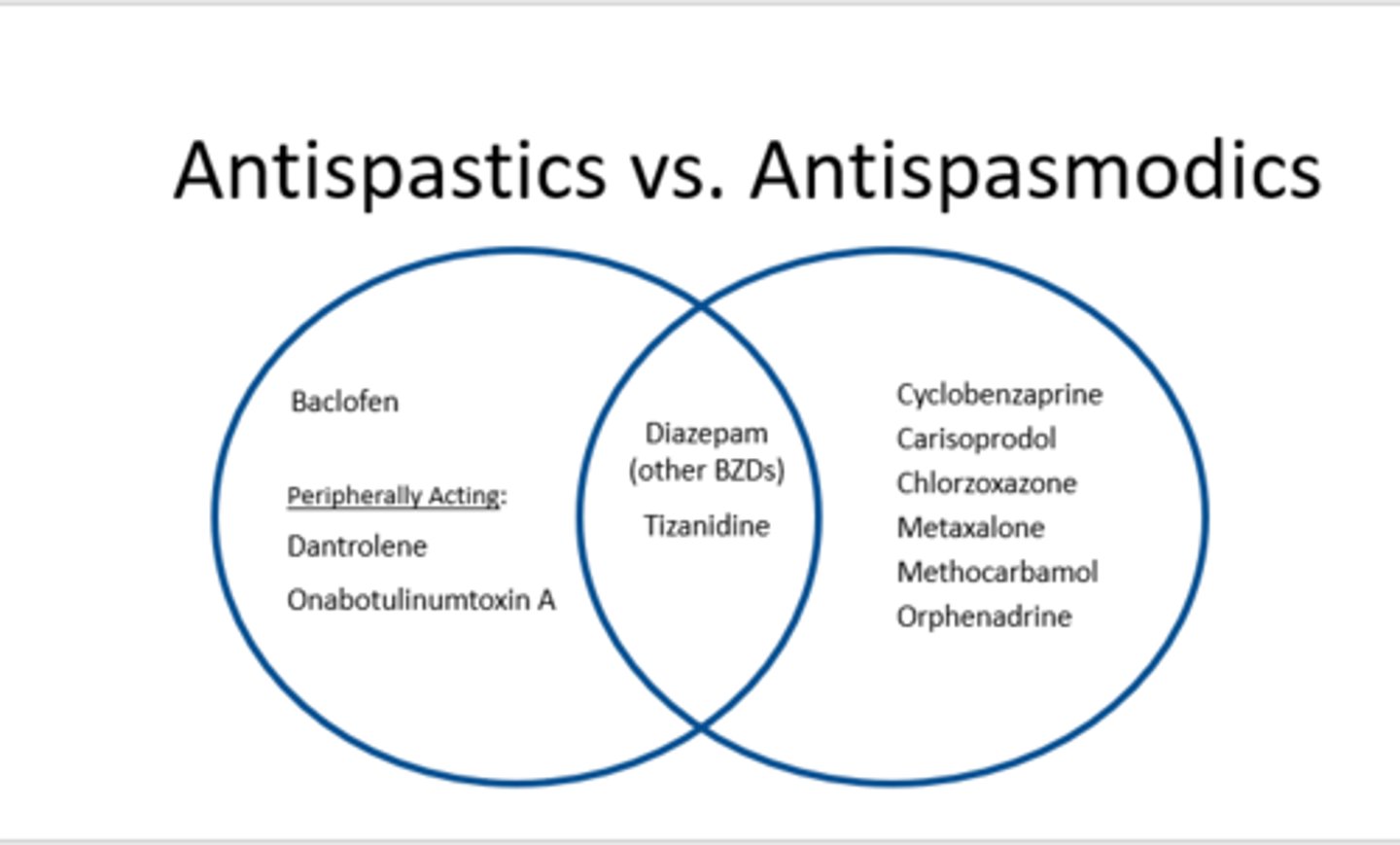
tizanidine
Zanaflex
antispastic + antispasmodic muscle relaxer
MOA: activation of presynaptic a2 receptors -> decreased excitatory NT release
- extensive 1st pass metabolism
- ADEs: sedation, dry mouth, N/V, hypotension, hepatotoxicity
- avoid abrupt d/c due to rebound spasticity
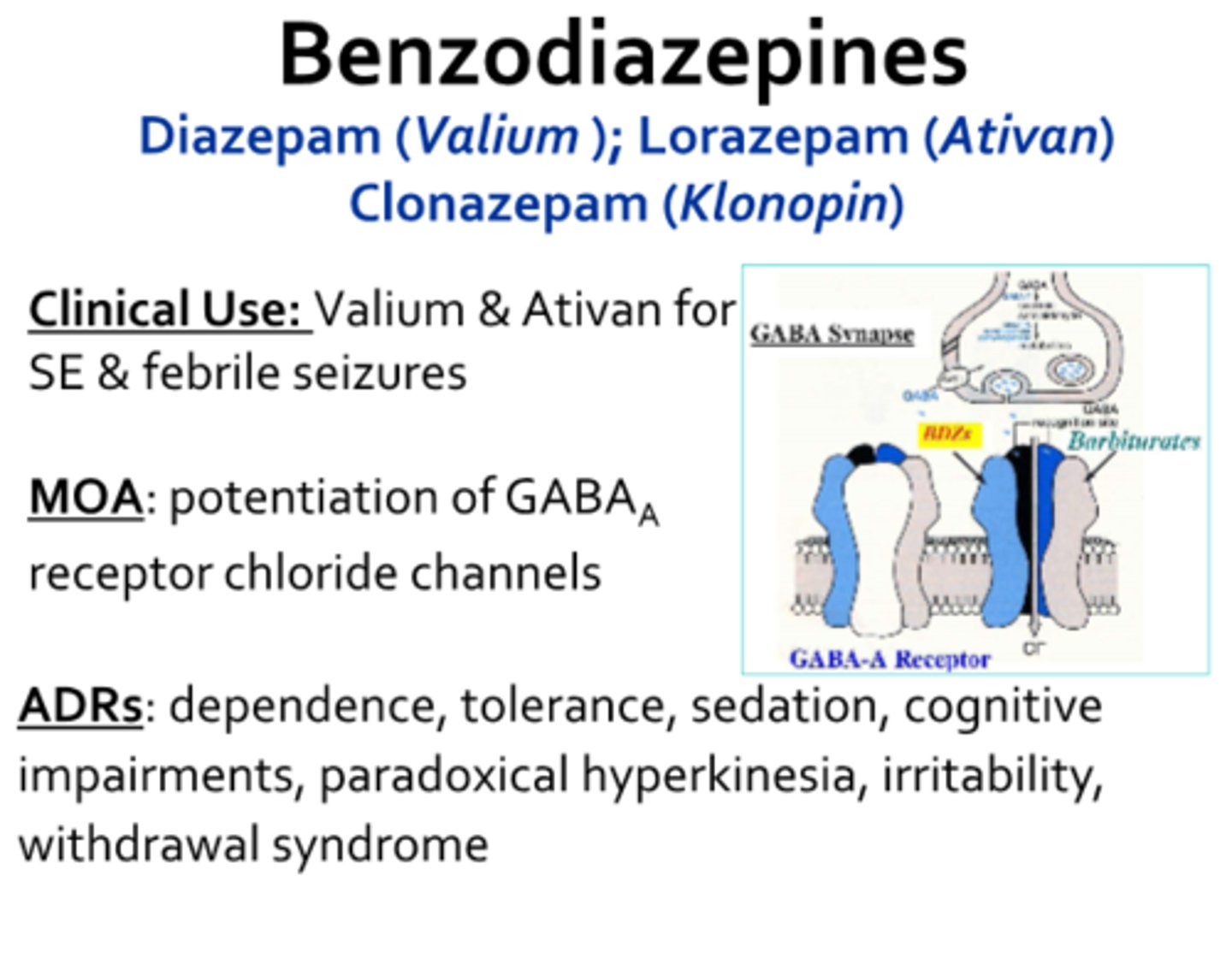
diazepam
Valium
antispastic + antispasmodic
MOA: GABAa receptor agonist (ion channel)
- CYP2C19 and CYP3A4 metabolize to active metabolite
- ADEs: drowsiness, sedation, behavioral changes, dependence potential
- avoid abrupt d/c due to rebound spasticity, seizures, and hallucinations
common approach to antispasmodics
1st line: methocarbamol (less sedating)
- cyclobenazaprine
- tizanidine
- diazepam

carisoprodol
Soma
antispasmodic (carbamate)
MOA: GABAa agonist (like benzos)
- renally excreted
- ADEs: somnolence, euphoria, dependence, withdrawal (life-threatening), transient quadriplegia and vision loss on initiation, anaphylaxis
methocarbamol
Robaxin
antispasmodic (carbamate)
MOA: may decrease nerve transmission in spinal pathways to prolong refractory period of muscle cells
- hydroxylated/demethylated -> conjugation
- ADEs: drowsiness, blurred vision, headache, nausea, rash, weird colored urine (black, blue, green), anaphylaxis
- minimal sedating effects!

cyclobenazaprine
Flexeril
antispasmodic (tricycloc dimethlpropamine)
MOA: centrally acting a2 agonist + %-HT2 antagonist
- ADEs: dizziness, drowsiness, anticholingeric effects, QTc prolongation, seizure potential
- largest amount of evidence for improvement

metaxalone
Skelaxin
antispasmodic (oxazolidinone)
MOA: unknown, reduces COX1/2, NF-kB, TNF-a, IL-6, PHE2
- avoid in liver failure
- ADEs: dizziness, drowsiness, N/V, hemolytic anemia, jaundice, leukopenia
- take with food
- less sedating than other SMRs (methocarb is still best)
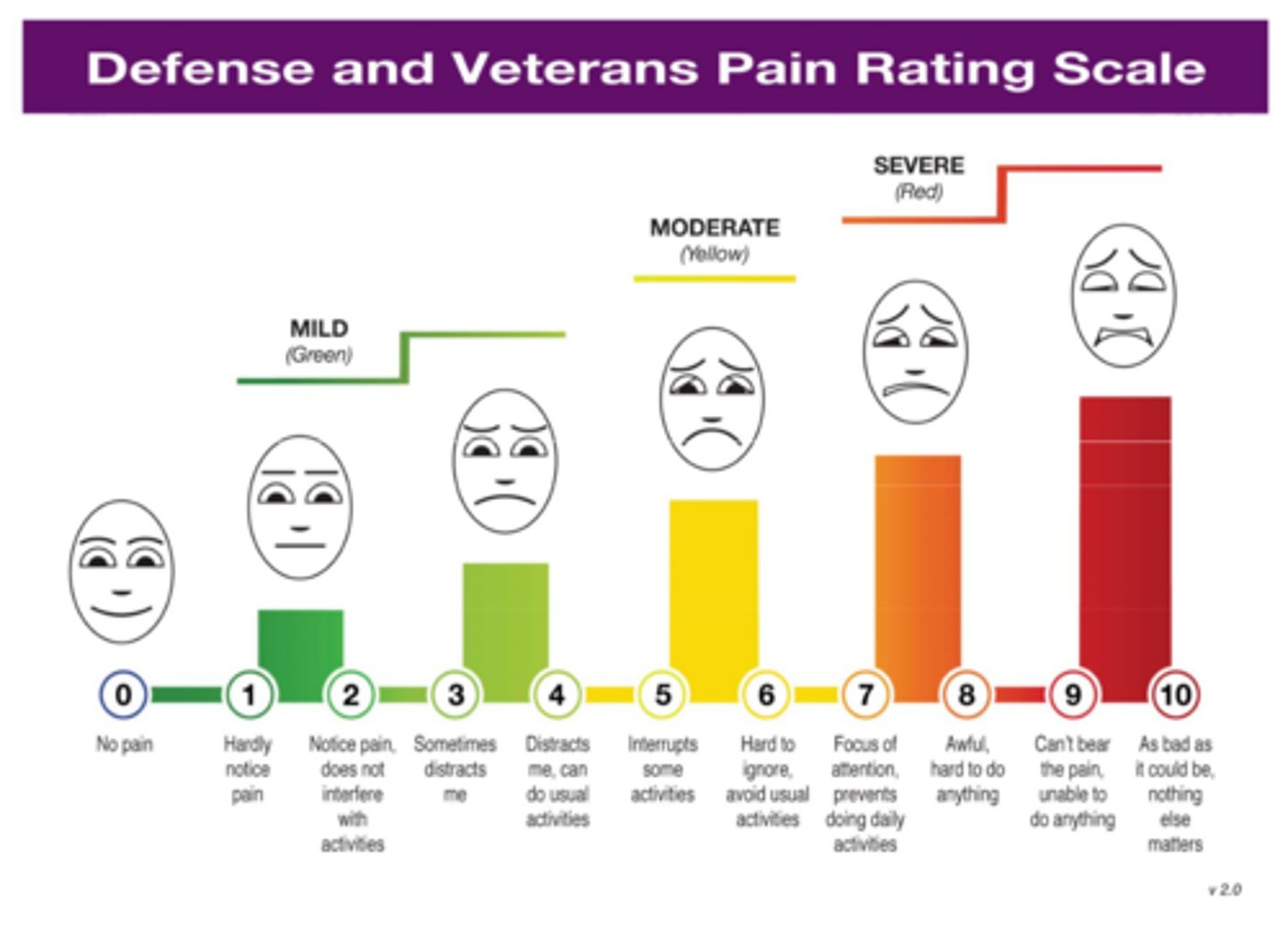
unidimensional pain assessment
visual analog scale: no pain - pain as bad as it could possibly be
numeric pain intensity: numeric rating scale 0 - 10
verbal descriptors: mild, moderate, severe
faces scale: :) - :(
defense & veterans pain scale rating: combines all of these
functional pain assessment: how does the pain affect your daily life, sleep, work
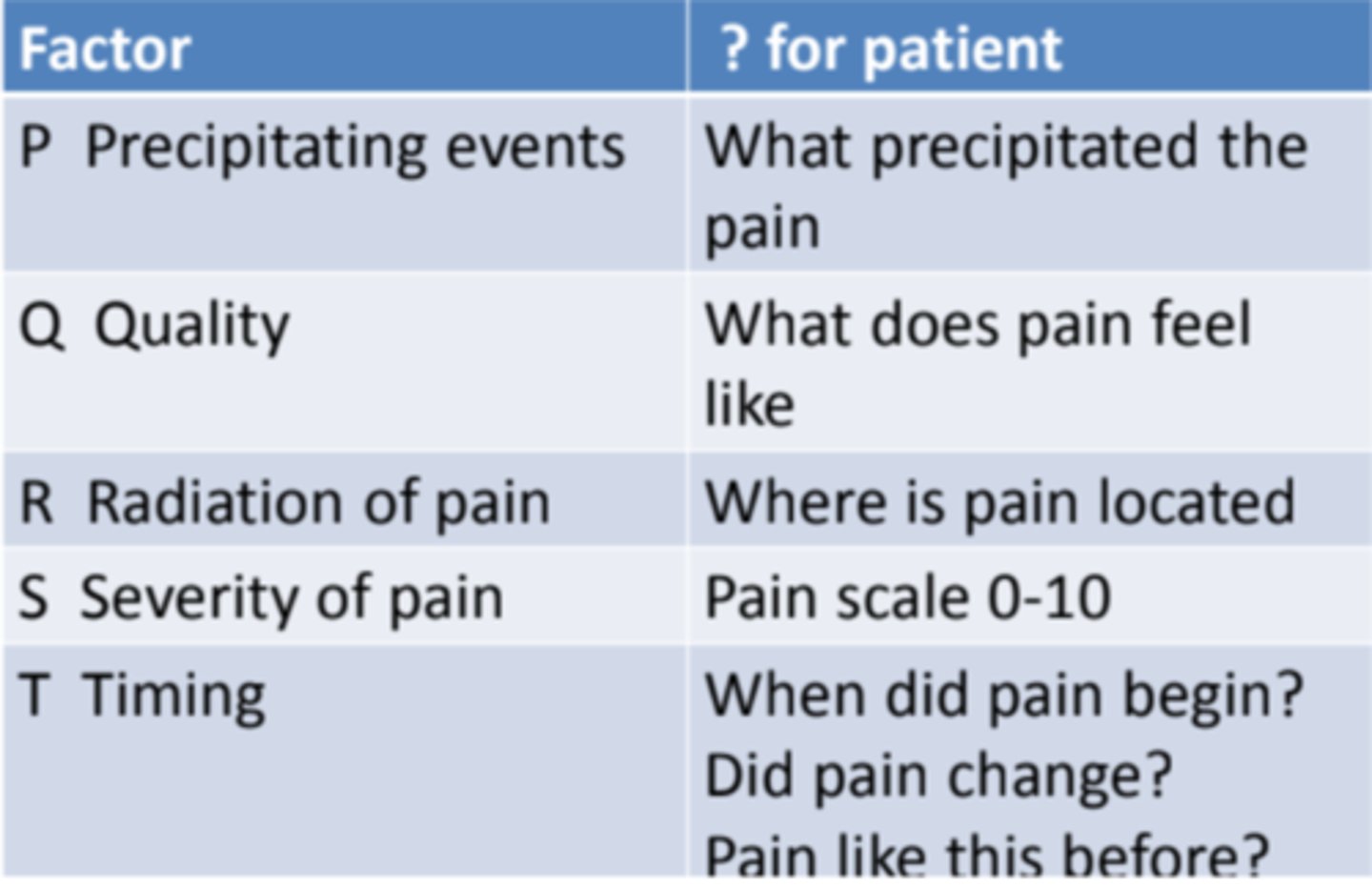
multidimensional pain assessment
PQRSTU
P = precipitating & previous therapy
Q = quality of pain (aching, throbbing, stinging)
R = region & radiation (where is it? does it spread?)
S = severity
T = temporal (onset, does it change?)
U = you (how does it affect daily life?)
considerations of pain assessments
- communicative ability of patient (children, cognitive impairment, alertness)
- push towards user-friendly functional pain assessments
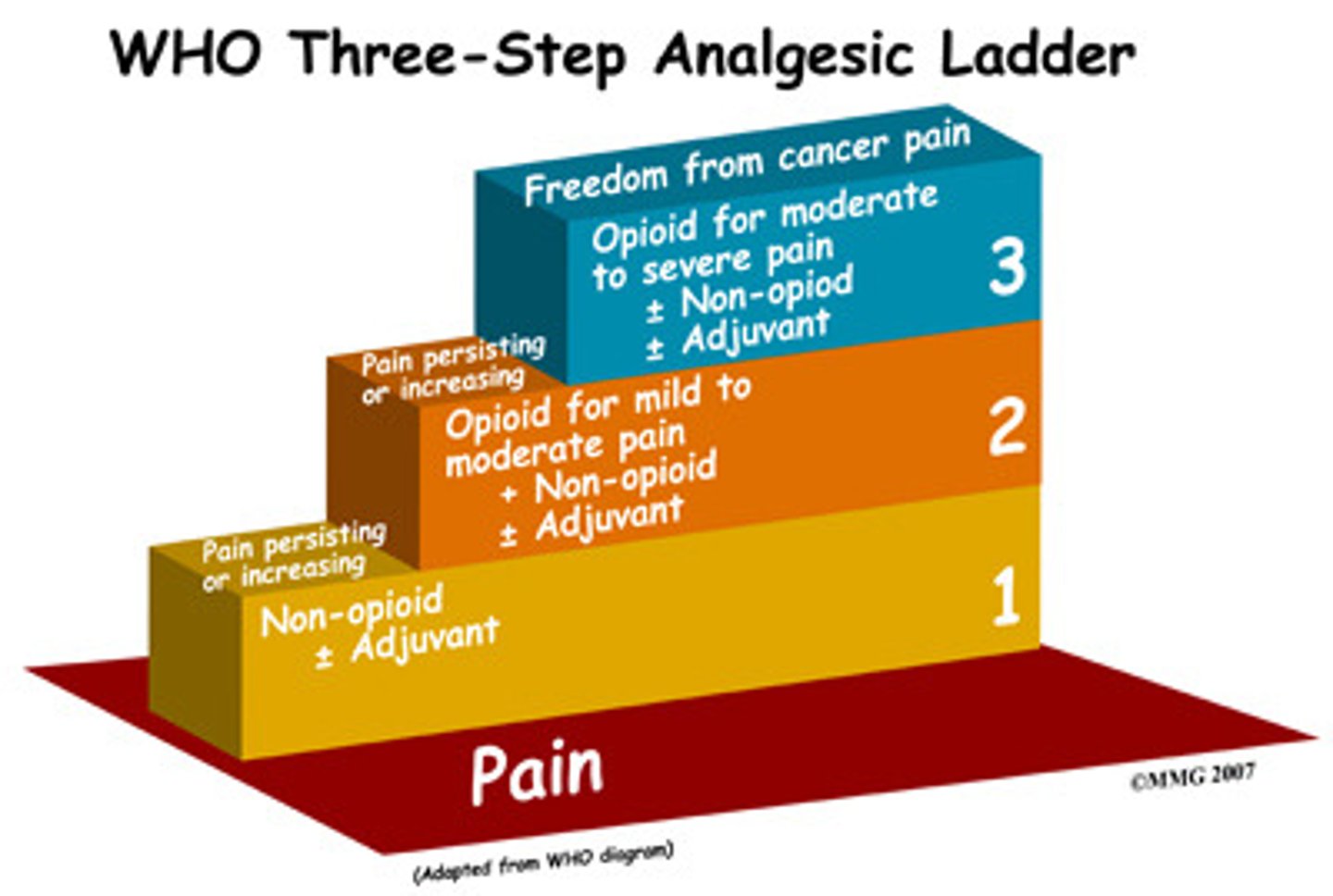
1st line for acute, mild-moderate pain
ibuprofen 400 mg PO Q6H
or
acetaminophen 1000 mg Q6H
+
non-pharm therapy
- RICE (rest, ice, compress, elevation)
- MEAT (movement, exercise, analgesia, treatment)

1st line for severe, acute pain
IV or IN: NSAIDs (ketorlac), opioids, or ketamine
- need quick onset
prescribing opioids
- generally limit to <3-7 days
- review PDMP
- conduct risk assessment
- educate on proper storage and disposal
- avoid XR products
- set function-based expectations before prescribing
who is at risk for opioid adverse outcomes?
overdose risk:
- 65+
- sleep-disordered breathing (sleep apnea)
- depression
- overdose hx
- high daily dose (>100 MME)
- combo with other CNS depressants
- ER/LA
OUD risk:
- adolescent/ young adult
- depression
- SUD (including alcohol)
- high daily dose
- long term use (3+ months)
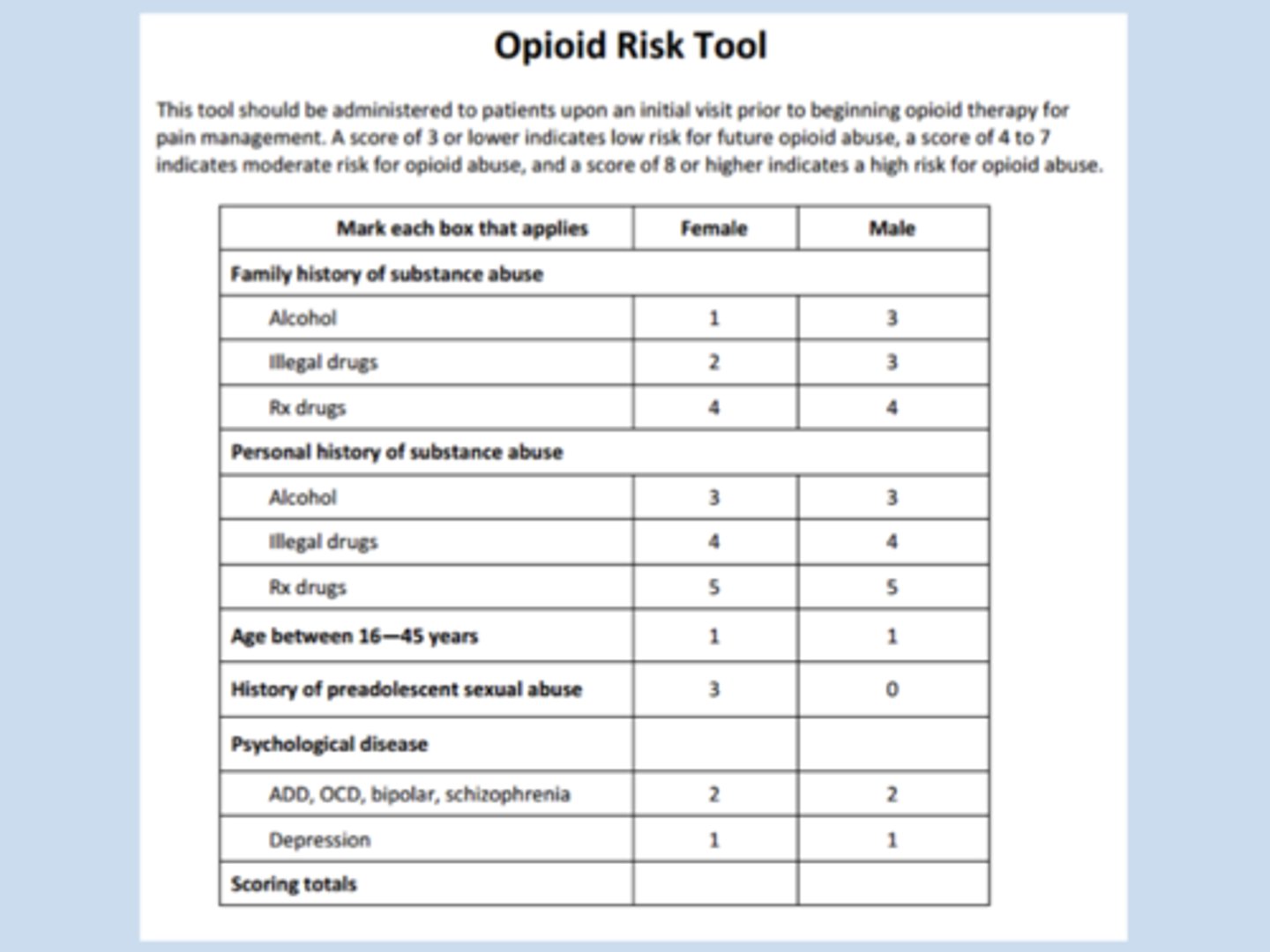
ORT-OUD
a simplified risk opioid risk screening tool
- low risk = ≤2
- high risk = >2
asks about: family hx of use, personal hx of use, age, and psych diseases
- 1 point for each "yes"
opioid storage & disposal counseling
- store in original packaging
- keep in a locked cabinet/ lockbox
- keep out of reach of children
- count medicine to know if any goes missing
- 193+ take back locations in KY
- DEA takeback day
- Deterra safe disposal system

postoperative pain management
pain <30 days with identifiable cause
- goal: shortest course + lowest dose to improve function, minimize pain, and avoid ADRs
- do NOT want to under-treat pain -> reduced QOL, increased surgical complications, prolonged rehab, development of chronic pain
what does multimodal analgesia include?
- patient education
- expectation management!!
- non-pharm therapy (RICE, physical therapy)
- NSAIDs/APAP
- opioids (oral is preferred)
- ketamine
- lidocaine (mayhaps)
perioperative NSAIDs
in pre-op:
- consider bleed risk
- avoid ketorolac and indomethacin (higher bleed risk, renal impairment)
- consider celecoxib (lower bleed risk)
in post-op:
- change any IV NSAIDs to PO when tolerating diet
- ketorolac IV can be given at skin closure
perioperative opioids
- continue chronic opioids (avoid opioid withdrawal)
- avoid new pre-op ER/LA opioids
- IV opioids are used in the PACU
- transition to PO when diet is tolerated
- begin bowel regiment ASAP (PEG, senna)
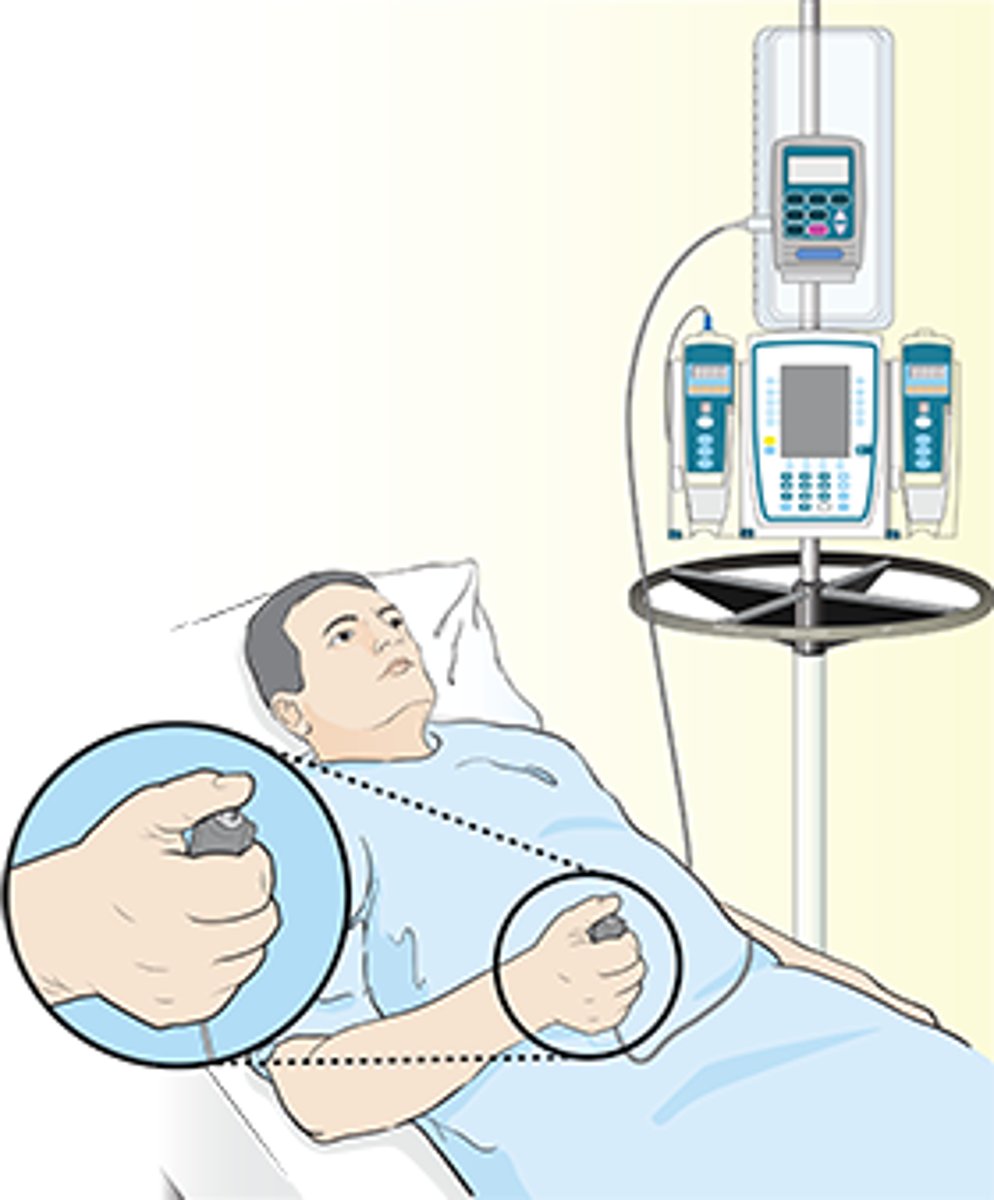
patient-controlled analgesia (PCA)
allows patients to self-administer pain relief with a button
- has lockout to only allow every ~10 minutes
- best for high-intensity, short duration pain
- patient MUST be responsive, without cognitive impairment
perioperative ketamine
consider in:
- painful, major procedures
- surgeries w high risk of chronic pain (amputation)
- chronic opioid use, high tolerance
AVOID in:
- coronary artery disease, vascular disease, elevated intracranial/ intra- ocular pressure
- pregnancy
- active psychosis

are opioids 1st line for post-op?
no
- if necessary though, use shortest course, lowest effective dose, pair with risk assessment + education
chronic pain
pain lasting >3 months
high-impact chronic pain
pain that limits at least one major life activity
(going to school/ work, household chores)
more common in:
- african americans, native americans, asian
- elderly
- high school or less education
- divorced/ widowed, never married
increases risk of:
- depression
- anxiety
- fatigue
- cognitive problems
considerations when initiating opioids for chronic pain
- maximize non-pharm and non-opioid therapy for
- discuss risks/ benefits of opioid therapy
- set functional goals
- develop plan for d/c if goals aren't met
- avoid ER/LA on initiation
- use lowest effective dose
- caution when changing doses/ therapies
- do not force taper unless absolutely necessary
- follow up within 1-4 weeks + regularly after
- continually assess and mitigate risks
- review PDMP
- caution opioids + benzos combination (additive CNS depression)
- for OUD: offer/arrange treatment, avoid detox alone
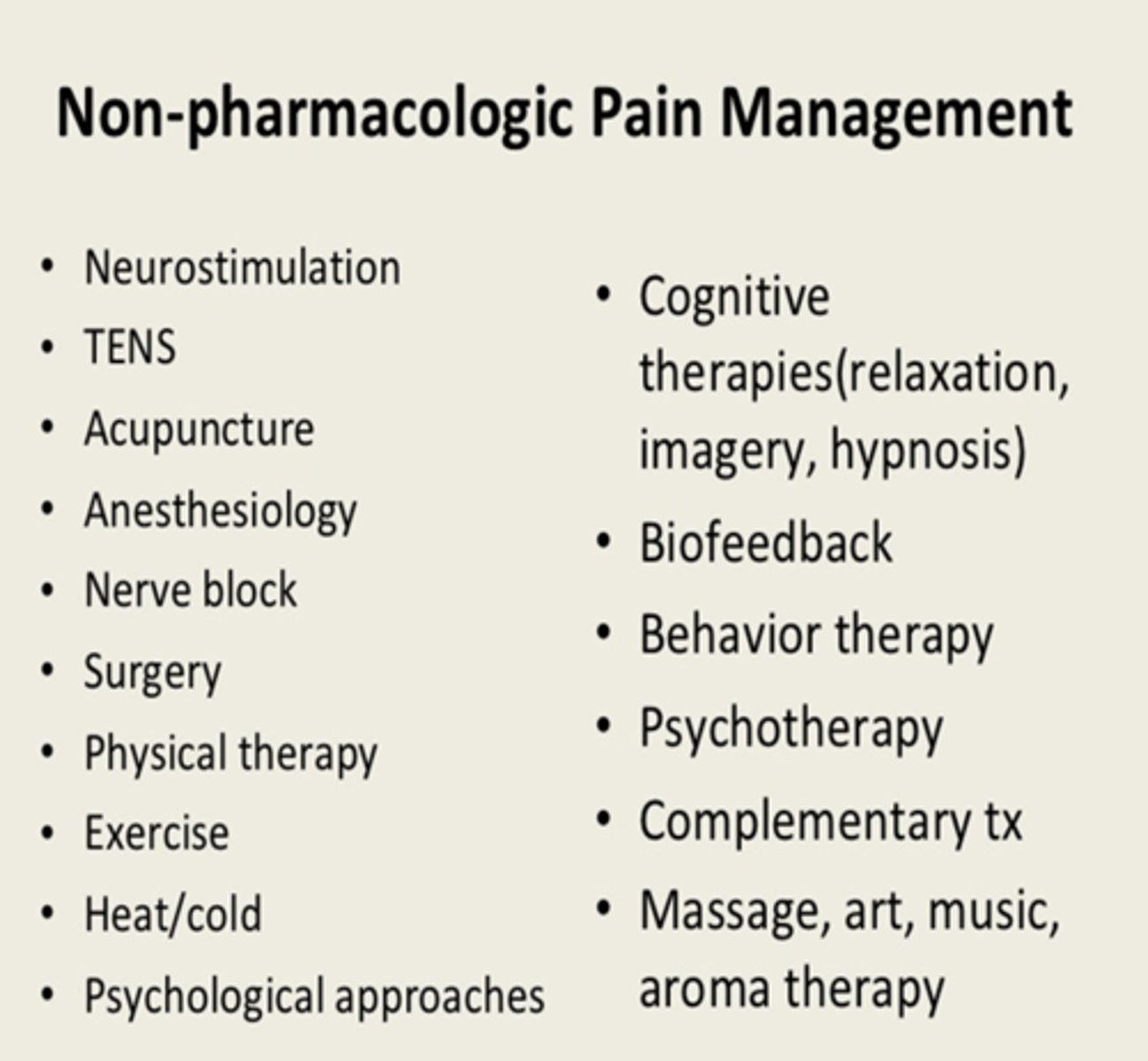
chronic pain non-pharm therapies
1. physical therapies
- PT, exercise, massage
- consider for function-limiting conditions, MSK pain
2. psychological therapies
- CBT, mindfulness, biofeedback
- consider for anxiety, maladaptive beliefs, sleep issues
3. complimentary therapies
- yoga, nutrition, peer support
- consider based on pt preference, culture, and as low-risk adjuncts
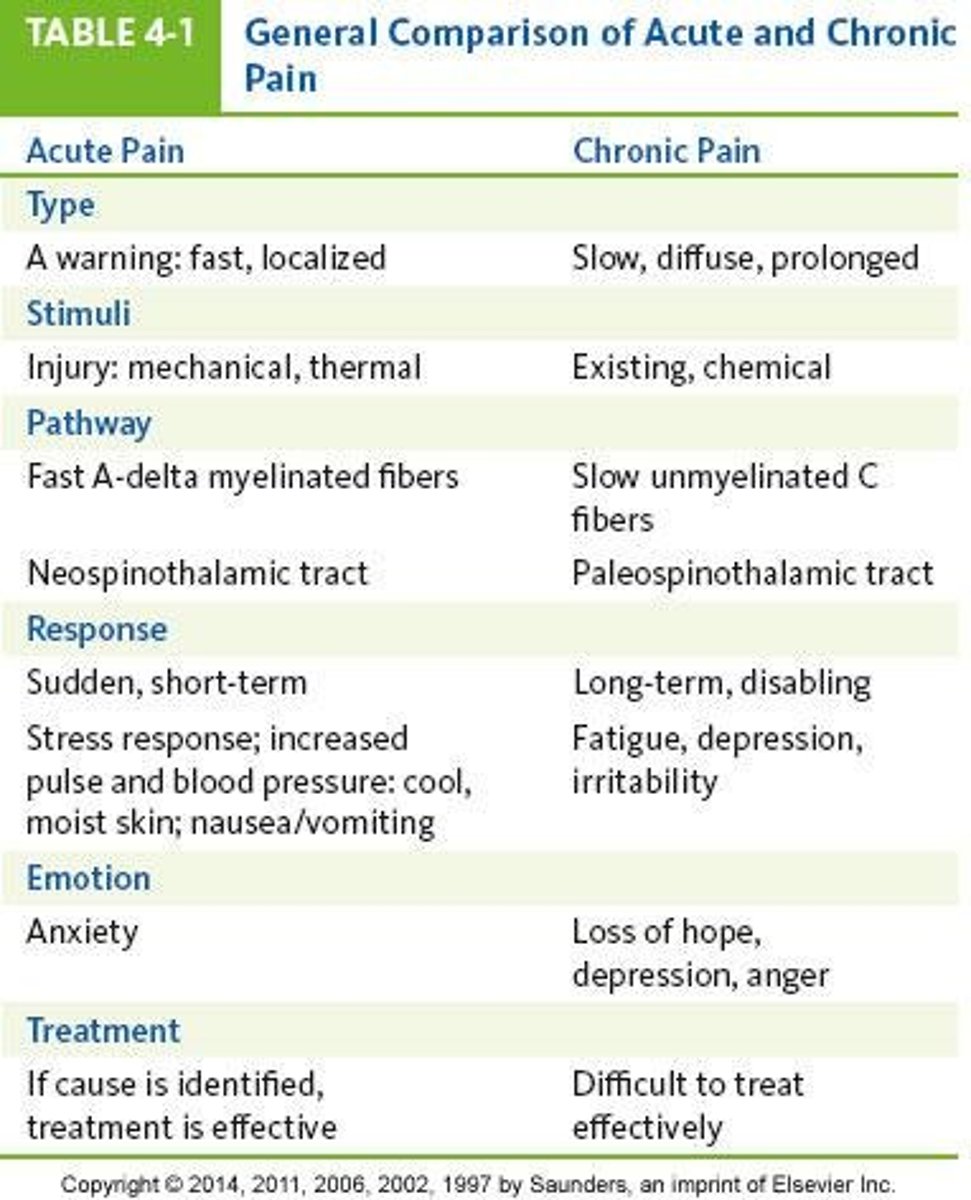
differences in acute and chronic pain pharm-therapies
acute:
- short-term (hours-days)
- goal = resolve pain and return to baseline function
- 1st line = simple analgesics +/- opioids
- opioids are common for moderate-severe pain
- risk of ADRs (GI, sedation), low risk or misuse
- counseling focuses on expected length of use, ADR prevention, stopping pharm therapy
chronic:
- long-term (week-years)
- goal = manage pain, improve function and QOL
- 1st line = non-pharm + adjuvants, opioids sometimes
- opioids have limited role (individualized, trial-based, time-limited)
- risks of dependence, tolerance, opioid-induced hyperalgesia, social/functional harm
- counseling focuses on goal-setting, risk mitigation, self-management
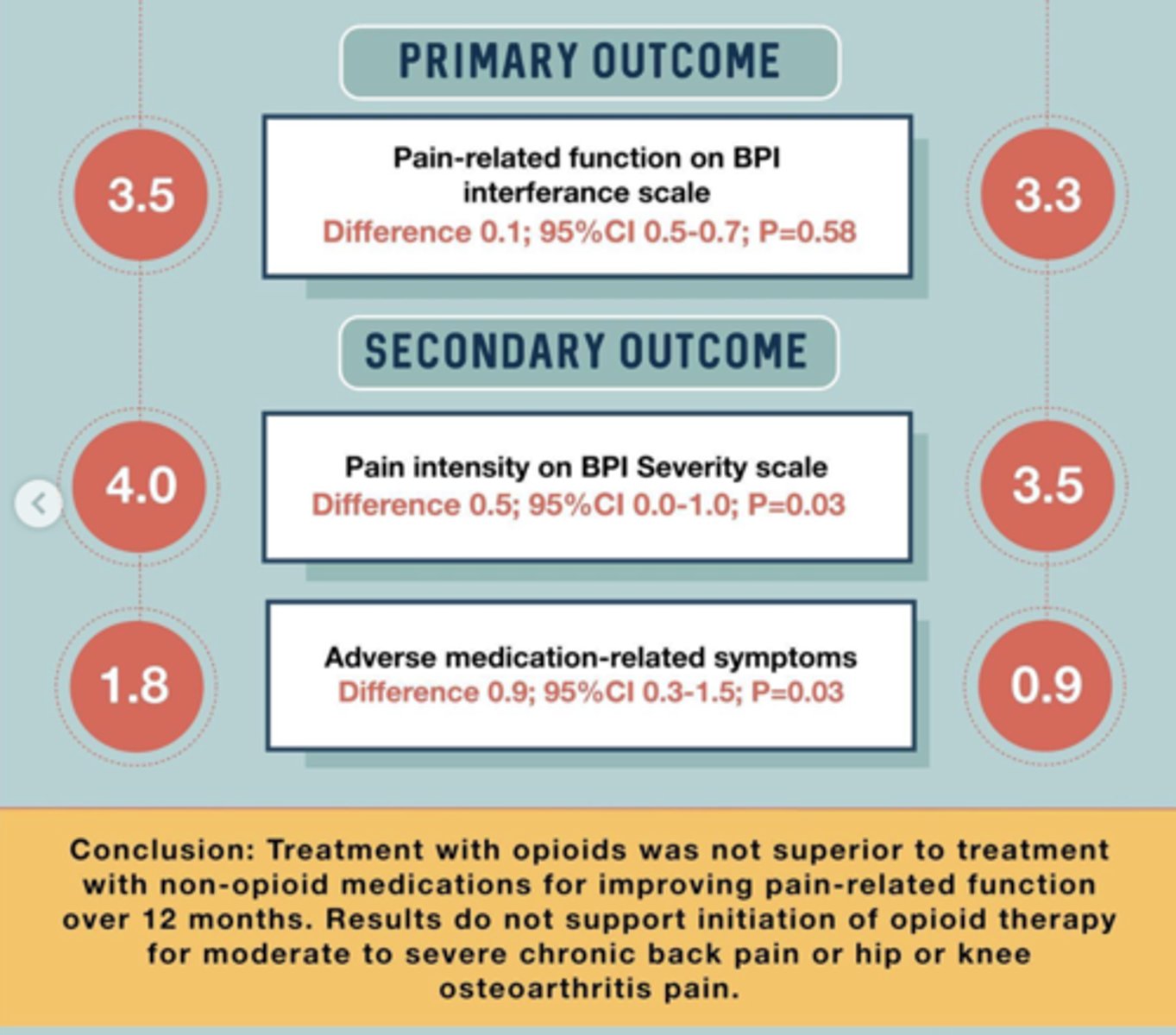
what did the SPACE trial say about opioids?
non-opioids work about as well as opioids, but opioids have a much higher ADE profile
toxicology testing
immunoassay using confirmation with GC-MS (Gas Chromatography-Mass Spectrometry) and LC-MS (Liquid Chromatography-Mass Spectrometry)
- false positives (diphenhydramine -> opioids; PPIs -> cannabinoids)
- false negatives (synthetics such as fentanyl, buprenorphine, tramadol, and alprazolam)
opioid-benzodiazepine combinations
AVOID!!!
- increased overdose and suicide risk
- worse treatment outcome
- increased health-service use
recommendations:
- use alternatives
- if necessary, limit dose and duration + monitor closely
- taper/ d/c when possible
- provide naloxone
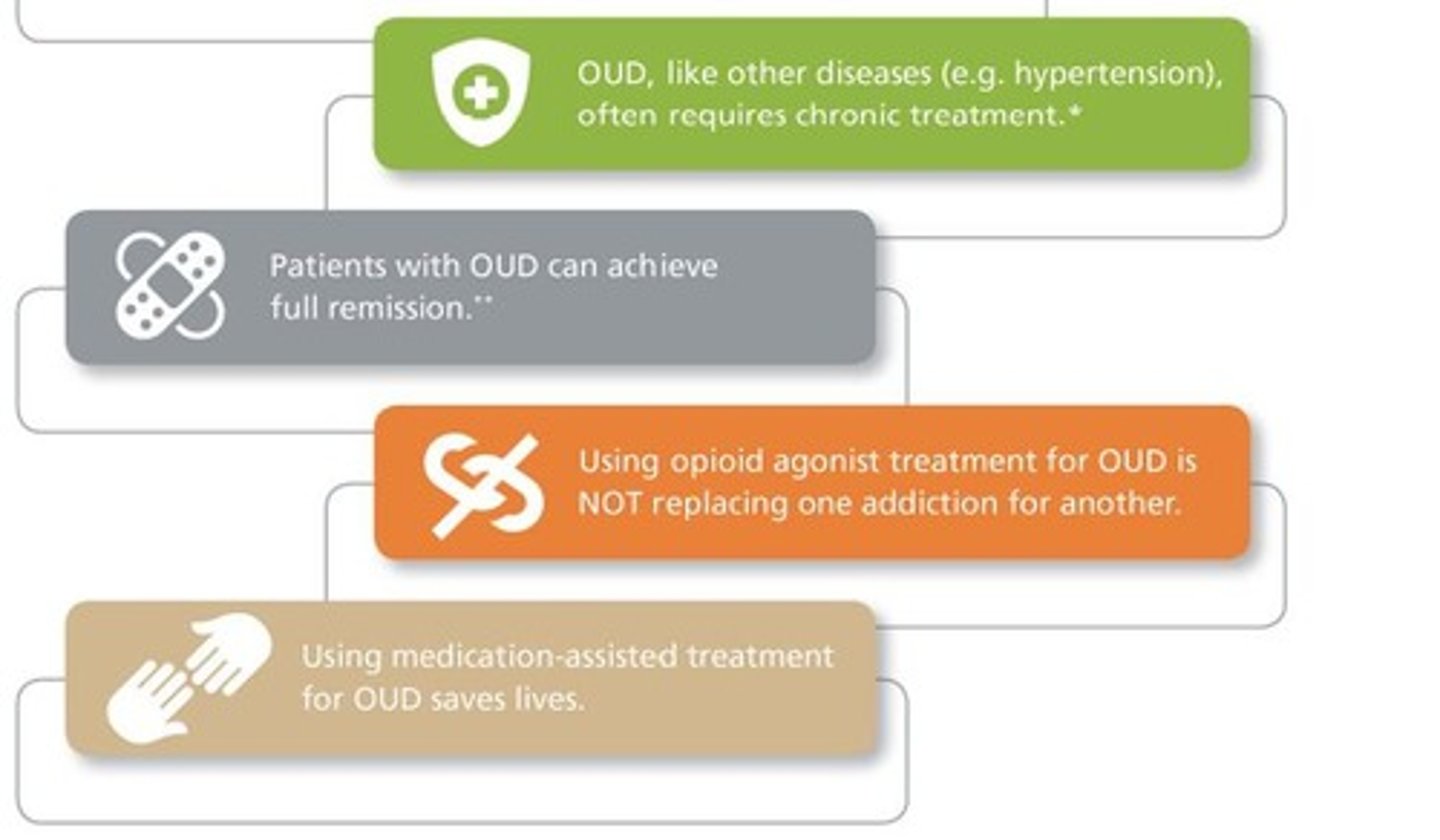
opioid use disorder (OUD)
a problematic pattern of opioid use that causes significant impairment or distress
- chronic medical illness
treatment:
- agonist medications - buprenorphine, methadone
- comprehensive plan
when to consider opioid tapering
- if pain improves (or doesn't!)
- patient requests
- evidence of misuse
- serious ADRs
- other medications/ disease state increase overdose risk
- long-term treatment with unclear risk-benefit
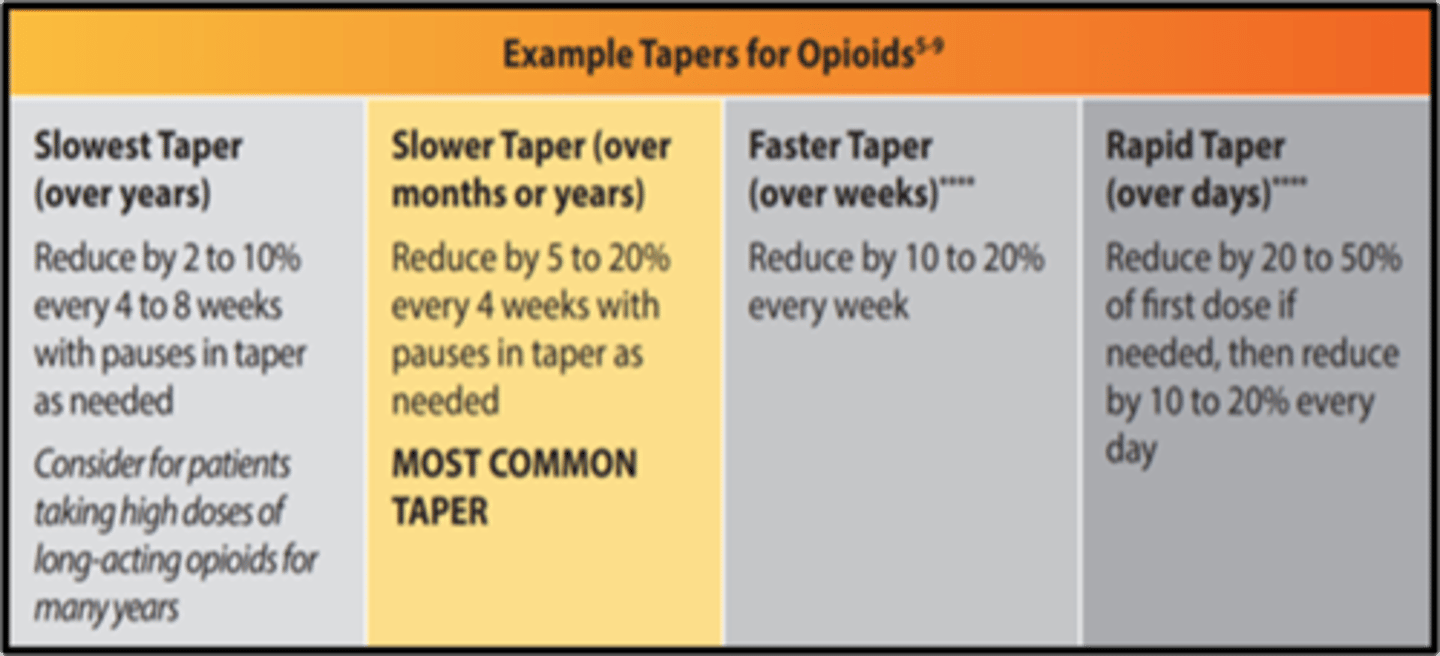
opioid tapering
- assess benefits and risks to decide when to taper
- work with the patient!!! + other experts (OB, addiction)
- counseling
- do not force/ abrupt taper (unless medically necessary)
- taper may be slow (years) to rapid (days)
- most common taper: reduce 5-20% every 4 weeks
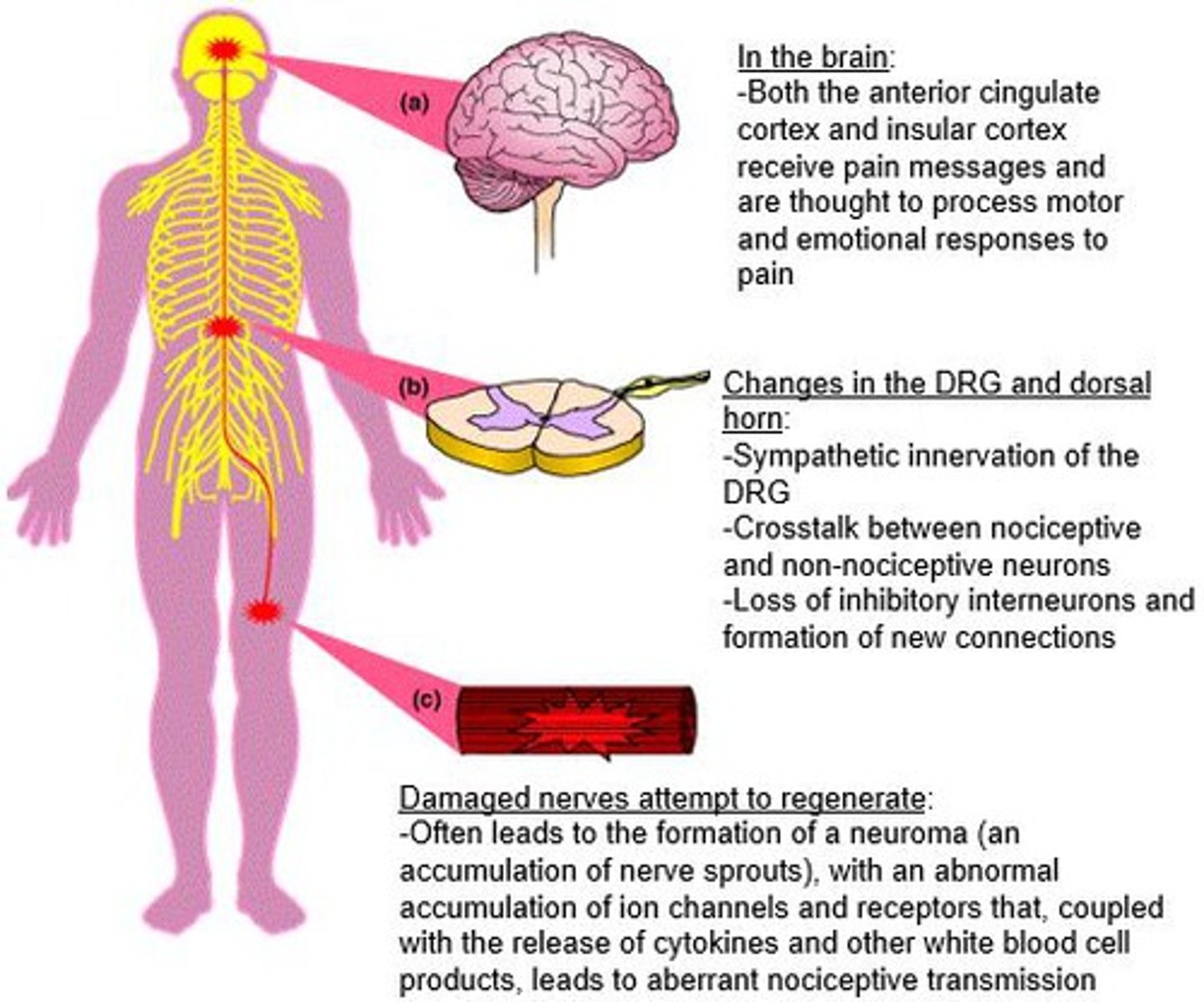
neuropathic pain
pain from damage to neurons of either the peripheral or central nervous system
- pain pathway is dysfunctional
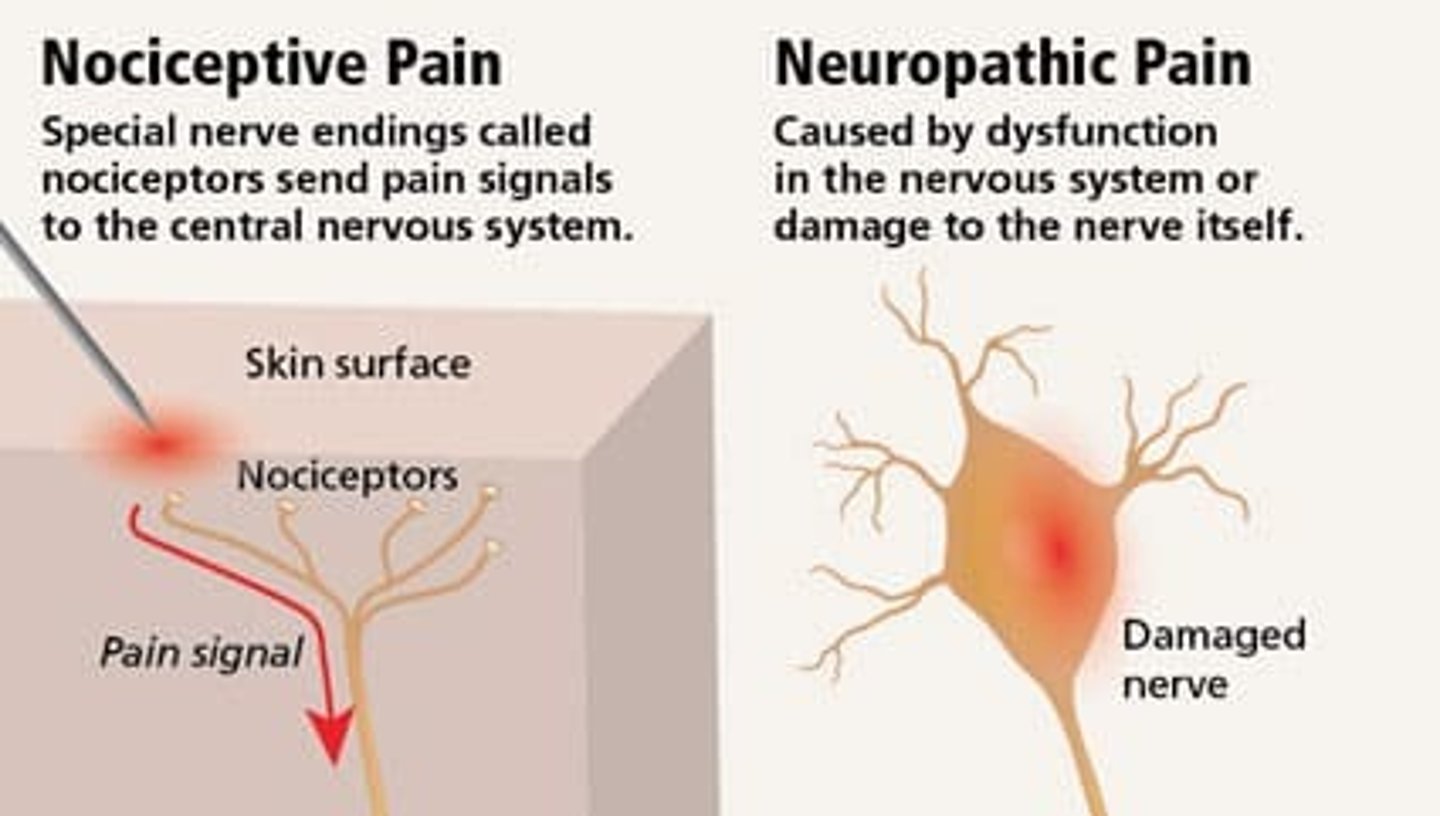
neuropathic vs nociceptive pain
neuropathic:
- from lesion or disease of somatosensory system
- central (brain SC) or peripheral
- does NOT respond well to conventional analgesics
- feels like: burning, stinging, shooting, electrical
nociceptive:
- from ongoing inflammation (signal of injury)
- visceral (gut) or somatic (pinpoint)
- responds to conventional analgesics (APAP/NSAIDs)
- feels like: pressure, stabbing
epidemiology of neuropathic pain
7-10% of population
more common in women
more common age 50+
most commonly cervical/ lumbar radiculopathy (but we're not covering this bc it is acute, treatment is usually short, and involves surgery, PT)
neuropathic pain pathophysiology
excitatory neurons rule:
- loss of GABA interneurons
- 2nd order neuron hyperexcitability
- ion channel changes
inhibitory neurons:
- noradrenergic dysfunction and CPM loss
- altered thalamocortical projections
general neuropathic pain management
1. antidepressants (TCAs, SNRIs) and gabapentinoids
2. topical lidocaine/ capsaicin +/- tramadol/ tapentadol
3. botox, opioids, dibenzazepines

1st line in neuropathic pain
TCAs and SNRIs
- both take ~4 weeks to see effect
TCAs: amitriptyline, nortriptyline
- start with low dose and titrate weekly to effect
- metabolized by CYP2C19
- significant ADEs
SNRIs: duloxetine, venlafaxine
- better tolerated
- ADE: GI effects
gabapentin
Neurontin
Horizant - prodrug, increases F
Gralise - gradual release
- used in neuropathic pain, but does not have great efficacy
- need a high dose, only 30-40% of people get 50% pain relief
- has saturable absorption → reduced bioavailability
- ADEs: dizziness, drowsiness, edema, gait instability
pregablin
Lyrica
- similar net benefit to gabapentin, not great
- has some more evidence in pts with mixed neuropathy, trauma-related neuropathy, or central pain post-stroke
- ADEs: dizziness, somnolence
- for every 2 people helped, 1 person d/c due to side effects
neuropathic pain topical therapies
lidocaine patches
- consider for localized pain
- low quality of evidence
- may be good for elderly pts already on lots of meds
- well-tolerated
capsaicin
- 8% Rx patch is effective for postherpetic neuralgia (PHN)
- poorly tolerated though

opioids for neuropathic pain
tramadol (Ultram) or tapentadol
- preferred over conventional opioids
- poorly tolerated + loses efficacy over time