OCAASL S1: Chapter 9 - Substitution Reactions
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Draw SN2 process
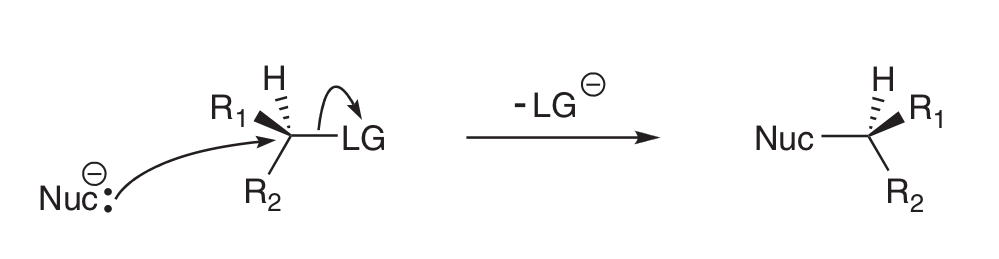
What are the two functions of the LG?
1) Withdraws e- density from C atom it’s attached to → makes C electrophilic
2) stabilises -ve charge after being expelled
Why is SN2 called SN2?
RoR is dependent on concentration of both Nu- and E+ i.e. 2nd order rxn
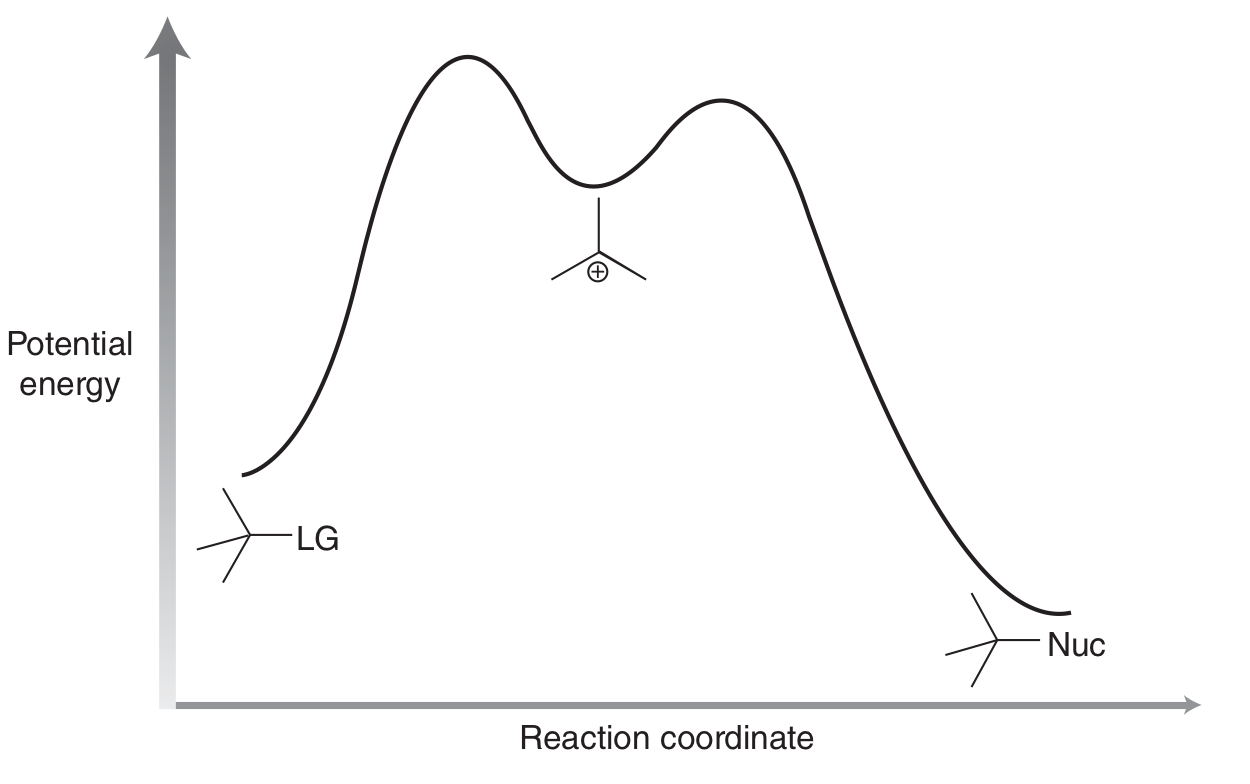
Draw mechanism for SN1.
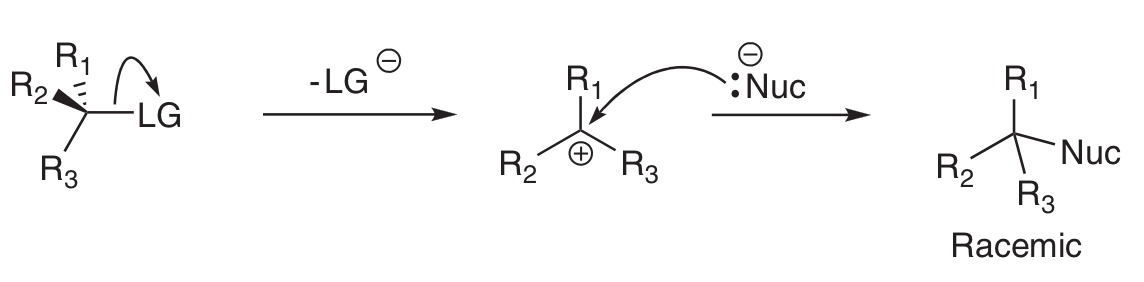
Why does SN1 reaction result in both enantiomers?
Occurs via trigonal planar intermediate - Nu can attack from both sides (unlike SN2).
Why is SN1 called SN1?
Rate only dependent on the E+
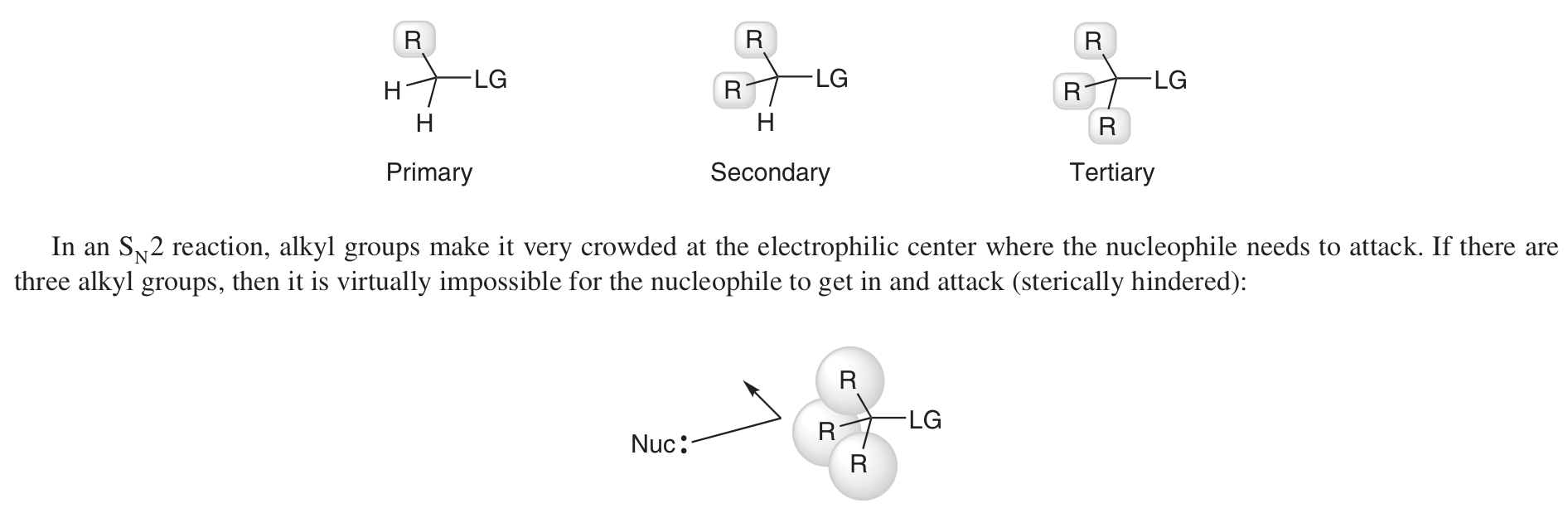
What is referred to as the substrate in substitution and elimination reactions?
The ELECTROPHILE!
Which substrates work better for SN2?
1º - less steric hindrance for the Nu- to attack at electrophilic centre
What’s most important in SN1 reactions?
Stability of CC (formed in rds)
Which substrates work best (and which don’t) for SN1?
3º - alkyl groups (EDGs) stabilise the CC
1º substrates are usually unreactive.
Which rxn will occur with 1º or 2º substrate?
Most likely SN2
Which rxn will occur with 3º substrate?
Most likely SN1
Other than alkyl groups, how else can CCs be stabilised?
Resonance!
Look out for LGs in benzylic or allylic positions → will generate resonance-stabilised CCs after LG leaves.
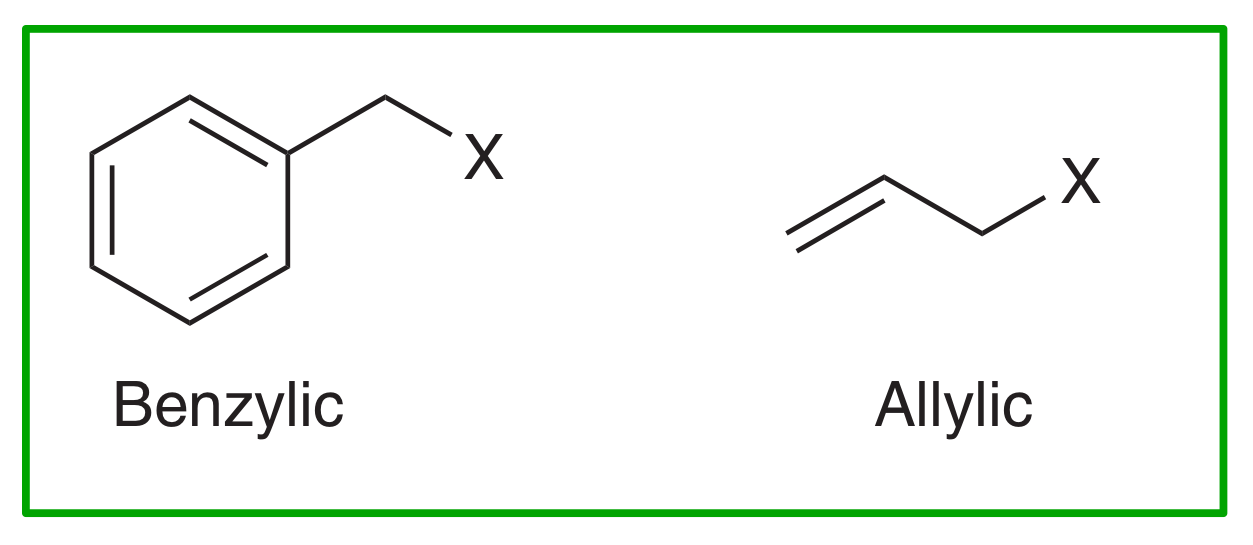
Which type of Nu- favours SN2?
Strong Nu- favours SN2.
(Weak one disfavours it.)
Which type of Nu- favours SN1?
None. SN1 is only dependent on substrate conc - not Nu-.
How does Nu- type affect competition between SN1 and SN2?
Strong Nu- favours SN2.
Weak one disfavours SN2 → allows SN1 to compete successfully!
Which factors affect Nu- strength?
1) Charge
2) Polarisability (more important)
Why are I- and HS- particularly strong Nu-s?
I and S are highly polarisable atoms (large + lots of e-s) → stronger
Does LG identity affect SN1 and SN2?
Yes! If LG is bad, neither can operate.
SN1 is more sensitive to LG identity.
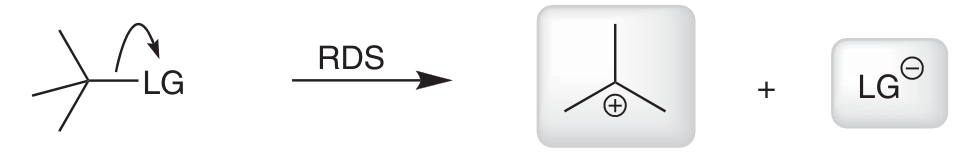
Why is SN1 more sensitive to LG identity?
RDS is the loss of LG to form a CC and LG.
RDS is sensitive to stability of CC and LG → LG must be highly stabilised.
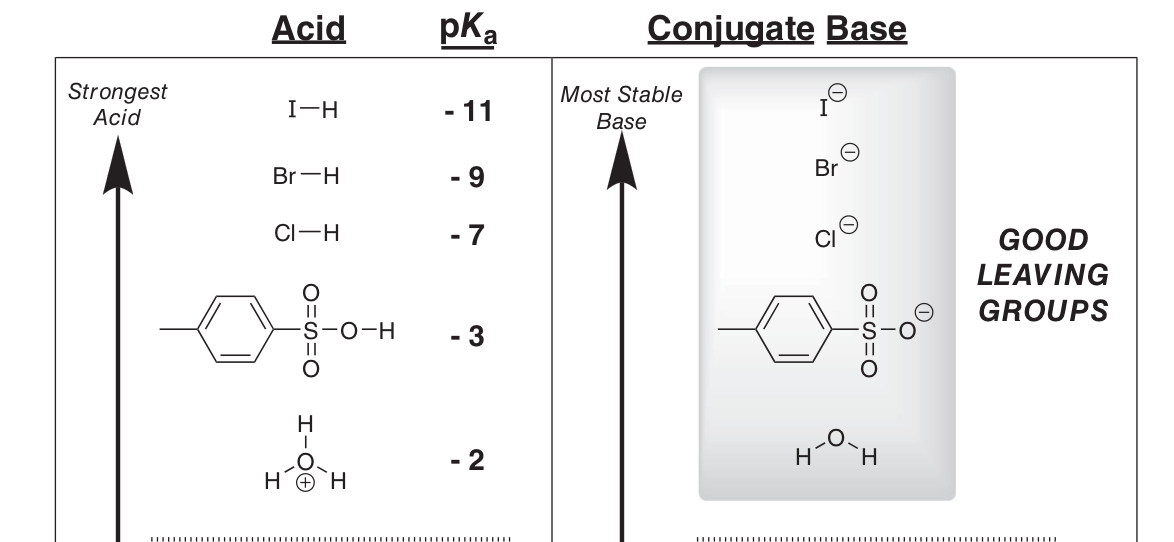
What’s a good LG like?
Conjugate base of strong acid (i.e. weak base since they’re highly stabilised e.g. I-)
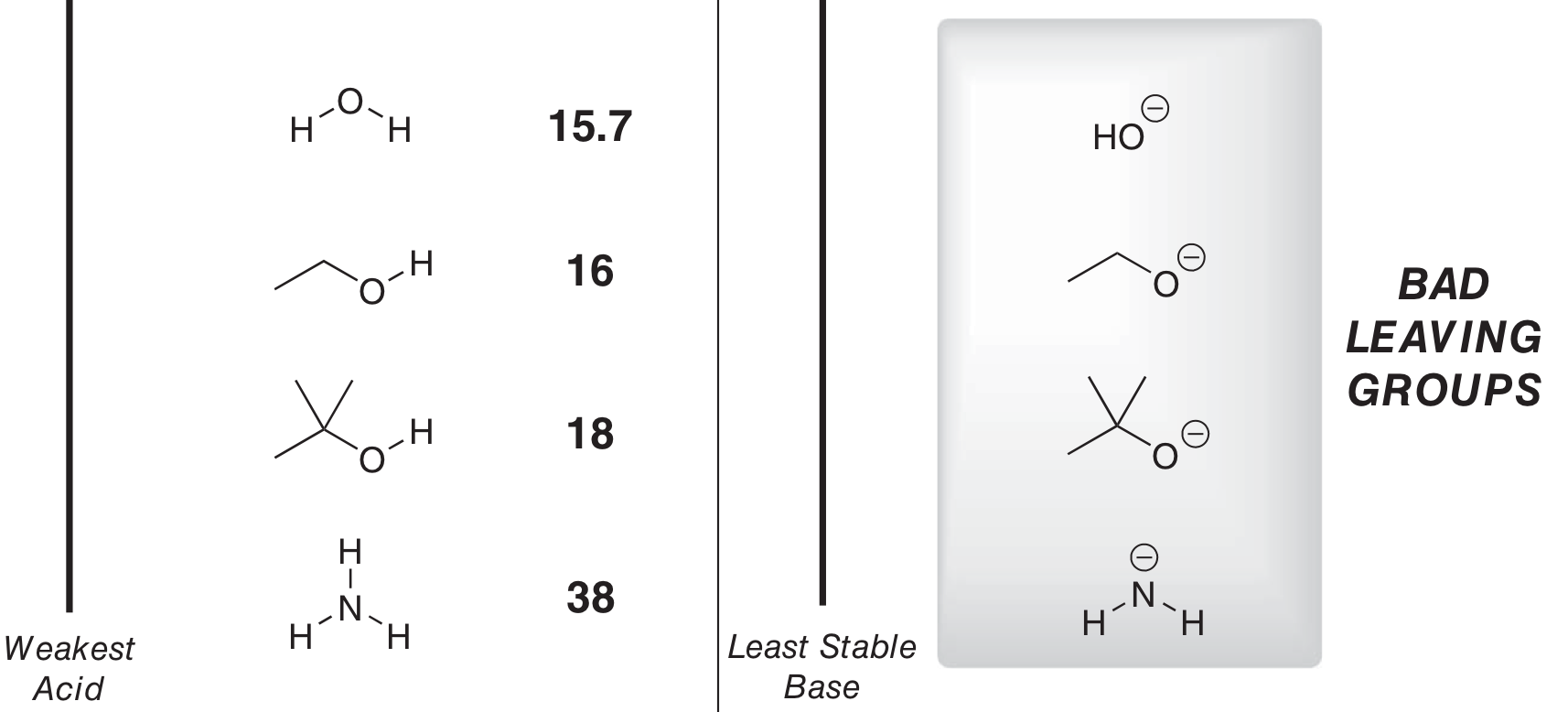
What’s a bad LG like?
Stabilised base
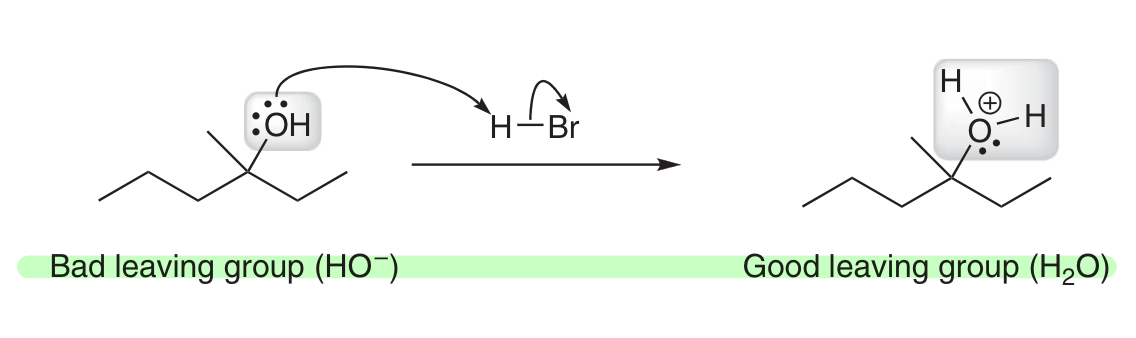
How can you convert -OH into a good LG?
Protonate it w/ a strong acid!
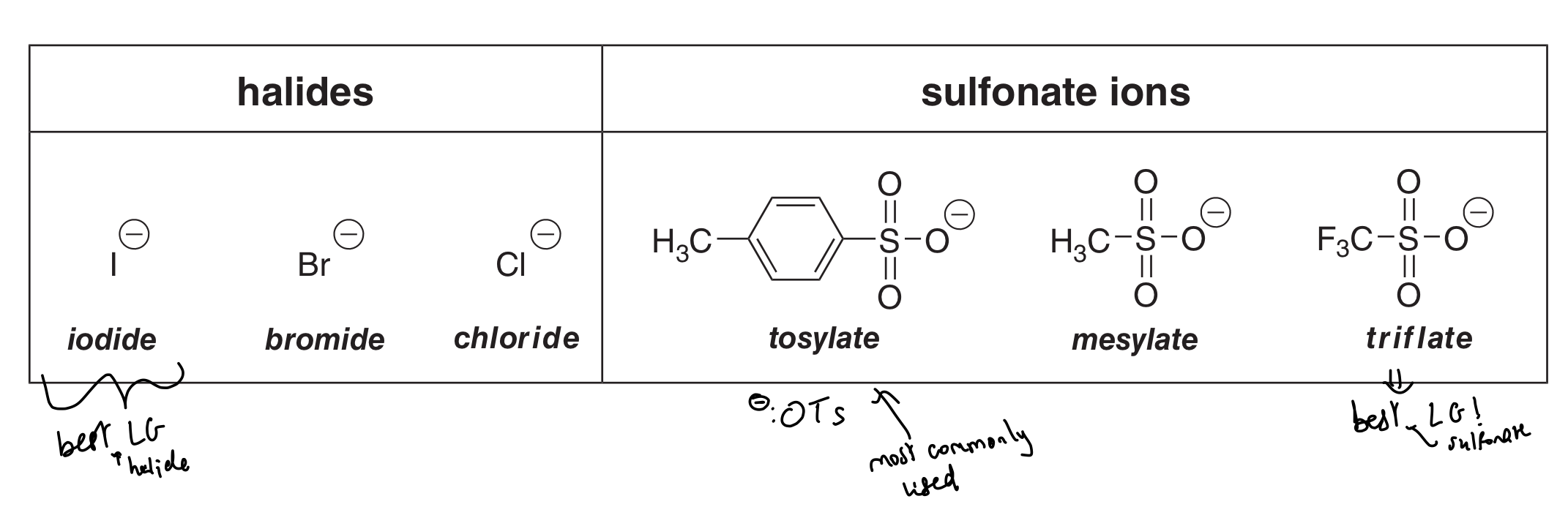
State + draw the 6 most common LGs
Halides: I-, Br-, Cl-
Sulfonate ions: tosylate (-OTs), mesylate and triflate
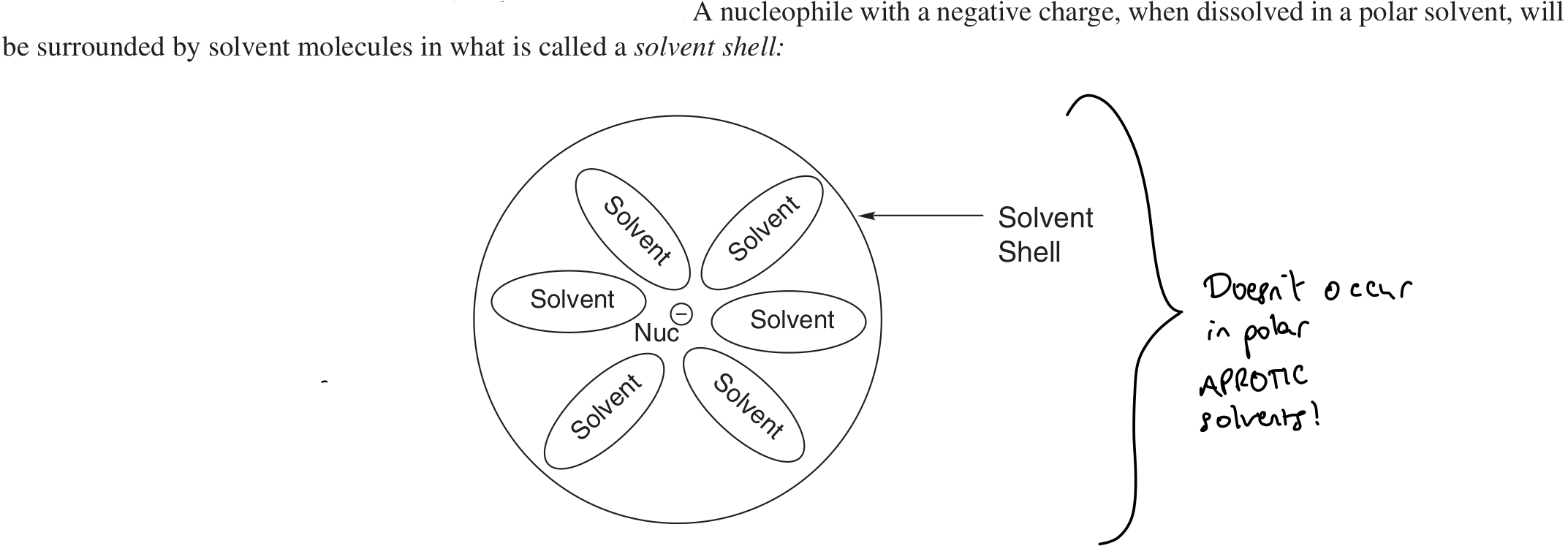
Which solvent greatly affects competition between SN1 and SN2?
Polar aprotic solvents!
Why do SN reactions need polar solvents?
‘Like dissolves like’
SN1 needs it to stabilise CC (needs polar more than SN1)
SN2 needs it to dissolve the Nu-
Will rarely see a substitution rxn in a non-polar solvent.
What’s a polar protic solvent?
Solvent that has proton connected to an EN atom (e.g. H2O or EtOH)
Protic since solvent can serve as source of protons.
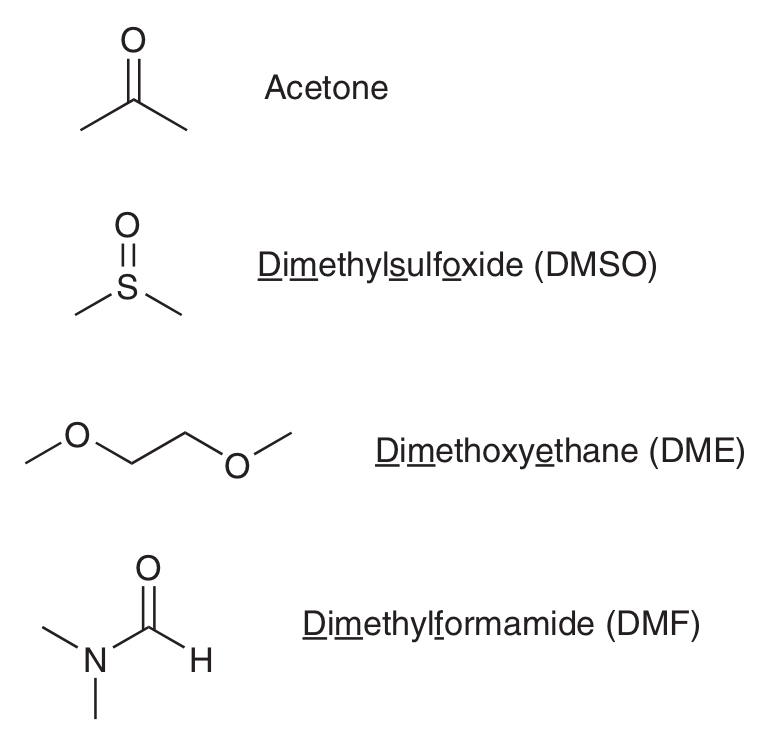
What’s a polar aprotic solvent? Show 4 common e.g.s
Solvent doesn’t have proton on an EN atom
(Solvent can still have H atoms, but they won’t be connected to EN atoms.)
e.g. acetone, DMSO, DME and DMF
Why do polar aprotic solvents favour SN2?
Polar aprotic solvents aren’t good at forming solvent shells around Nu-.
→ Frees up Nu- to react (doesn’t have to shed solvent shell)
Which of SN1 and SN2 are susceptible to CC rearrangements, and why?
SN1!
SN1 involves CC intermediate — SN2 doesn’t.
Which two factors should you consider in each problem?
Steric and electronic.
Electronic effects (e.g. Nu-. LG and solvent effects) are usually more complicated.