CHAPTER 4- EXAM 2 - BURNS
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
What agency is responsible for administering all parts of the Controlled Substance Act (CSA)?
DEA
The CSA requires a “closed system”. What does that mean?
only registered entities are legally allowed to handle, dispense, or prescribe controlled substances
(basically all activities of controlled substances are regulated)
Goal of the CSA?
prevent diversion of controlled substances
True or False: All CS include a narcotic component.
false—> includes narcotic AND non-narcotics
Controlled substances are placed into how many schedules? Who determines this?
- five schedules (CI, CII, CIII, CIV, CV)
- determined by DEA or Attorney General
Examples of CI, CII, CIII, CIV, and CV drugs:
(just recognize, not memorize)
CI- heroin, marijuana
CII- morphine, codeine, fentanyl (legal version), oxy, methadone, cocaine, etc.
CIII- includes CII combos (codeine w/ APAP), anabolic steroids
CIV- benzos, tramadol, phetermine
CV- antitussives, antidiarrheal products with opium
Definition of CI (abuse potential, medical use):
high potential for abuse, no medical USE TX IN THE US!!!!!!!
Definition of CII (abuse potential, medical use):
high potential for abuse
currently accepted medical use in tx
abuse may lead to severe physical/psychological dependence
Definition of CIII (abuse potential, medical use):
have potential for abuse (less than CI/CII)
currently accepted medical use
abuse may lead to moderate/low physical and/or high psychological dependence
Definition of CIV (abuse potential, medical use):
low potential for abuse
currently accepted medical use
abuse may lead to limited physical/ psychological dependence compared to CIII
Definition of CV (abuse potential, medical use):
low potential for abuse (compared to CIV)
currently accepted medical use
abuse may lead to limited physical/ psychological dependence compared to CIV
All CS commercial containers must be labeled with what?
SYMBOL!!!! (must be prominently displayed, large enough for easy identification!!!)
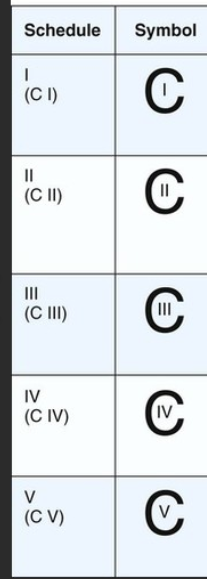
CSA requires registration to engage in activities involving CS.
How often do manufacturers, distributors, and dispensers have to register? Are there any exceptions?
manufacturers AND distributors—> YEARLY
dispensers—> every 3 YEARS
yes, there are exceptions
What are the 3 exceptions for specific people to engage in activities involving CS but do not have to be registered?
1. Agents/Employees of a Registered Entity—> Agent/employee of registered manufacturer, distributor, dispenser when acting within usual course of business/employment
2. common/ contract carrier/ employee in usual course of business/employment (delivery driver)
3. ultimate user legally in possession for lawful purpose (whoever is receiving the CS, patient)
PRACTICE:
Indicate whether the following people would require to be registered to engage in activities involving CS with the DEA:
individual pharmacist working for a chain pharmacy
delivery driver for a warehouse distributer
patient
independent pharmacy
NO
NO
NO
YES
Are individual practitioners required to be registered with the DEA to prescribe controlled substances?
Who is considered an “individual practitioner”? who isn’t?
YES—> individual practitioners ordinarily must be registered
Ex: physician, dentist, vet
EXCLUDES—> PHARMACISTSSSSSSSSSSS
What are the exceptions to the DEA registration requirement for individual practitioners?
Employees who can administer or dispense using their employer's registration
Individual practitioners who are agents/employees of a hospital or other institution can administer, dispense, and prescribe under the hospital's registration (within their scope of practice ofc) AND is designated a specific internal code #
random: those authorized to prescribe/dispense/administer in armed service, public health, or prisons are also exempt from registration (within their scope of practice ofc)
What are some activities with CS that require registration with the DEA?
manufacturing
distributing
reverse distribution
dispensing
conducting research w/ CI-CV
NTP (OTP) programs
chemical analysis
importing/exporting
Does each activity requiring DEA registration need a separate registration?
YES NEED SEPERATE REGISTRATIONS!!!!—> there are some exceptions (ex: the activities are coincidental and permitted under a single registration like a pharmacy that dispenses may do a little distribution)
What does "dispensing" mean under the Controlled Substances Act (CSA)?
Delivering a controlled substance (CS) to an ultimate user by lawful order, including prescribing and administering.
Referring to dispensing—> who qualifies as a "practitioner" under the CSA? (don’t confuse with individual practitioner with registration)
Physicians, dentists, vets, scientific investigators, pharmacies, hospitals, or other licensed/registered individuals authorized to distribute, dispense, research, or administer CS.
Does a pharmacy have to register to be a dispenser?
Do physicians have to register to be a dispenser?
YES & YES (definition of dispensing includes prescribing so physicians also have to register)
Does DEA registration as a dispenser automatically allow a pharmacy to prescribe CS?
no—→ refer to STATE law on who can prescribe
Do pharmacists engaged in the ordinary practice of pharmacy need to register as manufacturers?
NO!!!! (however if engaged in compounding, repackaging, or relabeling should be aware of the possible consideration)
Is manufacturing defined the same in the FDCA and the CSA?
NO
Can a pharmacist manufacture and distribute certain narcotic preparations to other practitioners without registering as a manufacturer under the CSA? What about the FDCA?
under the CSA—> YES!!!! —→ a pharmacist can manufacture and distribute aqueous or oleaginous solutions or solid dosage forms containing a narcotic substance in preparations not exceeding 20% of the complete product without registering as a manufacturer under the CSA.
under the FDCA—> NO!!!!—> would VIOLATE THE FDCA
ultimately—> YOU CAN’T DO THIS/ NOT ALLOWED
What is the definition of Distributing? Examples of distributors?
the delivery of a CS (other than by administering or dispensing)
ex: wholesalers, reverse distributors
How can DISPENSERS distribute to other practitioners without registering as a distributor?
other practitioner is registered to dispense
DEA Form 222 used for CI/CII distribution
total # of dosage units distributed does not exceed 5% of the total units of CS distributed and dispensed in 1 year
(distribution to ADS and reverse distributors don’t count towards 5%)
Dispensers can conduct research of C__-C__ without registering separately as a researcher. C__ requires separate registration.
(not that important)
CII-CV without registering separate. CI requires separate registration.
Does each place of business or professional practice where CS are manufactured, distributed, or dispensed require a separate registration?
YESSSS!
What are some exceptions to when each place of business doesn’t require a separate registration? (idk how important)
warehouses that store CS at separate warehouses for a registrant
prescribes that prescribe from more than one office only need one registration
For dispenser registration a DEA Form ____ is required. Form ____ is required for renewal.
224, 224A
When a pharmacy is sold to another as an ongoing business…
Who approves the transfer of registration?
What must be done to the inventory?
What form must be filled out?
Record?
DEA must approve any transfer of registration
Complete inventory of CS must be taken on date of transfer
DEA Form 222 MUST be used for any transfer of CI or CII
ALL records must be transferred on date of transfer
PRACTICE:
Does each CVS pharmacy require a registration to distribute, or can they just apply for 1 registration?
EACH PHARMACY!!!!!!!!!!
Registrations may be suspended or revoked by the AG (via DEA). What are some things that must be determined prior to denial or suspension/revocation? (idk how important)
denial- state board recs, conviction record, compliance concerns, other things threatening the public
suspension/revocation- falsified application, convictions, state disciplinary actions, excluded participation in Medicard/Medicare
What does FEDERAL law say about pharmacies and institutional practitioners storing CII—> CV?
(REMEMBER: state/company policy is PROBABLY stricter)
you can store CII—> CV throughout the pharmacy, BUT they must be dispersed between other drugs
What does FEDERAL law say about individual practitioners storing CII—> CV?
must be LOCKED
What does FEDERAL law say about storing CI’s?
(us pharmacists won’t deal with this really—> mainly applies to research)
must be LOCKED
Can you employ someone who has been convicted of a felony related to a CS or has had their application denied, revoked, or surrendered related to a CS?
NO
Any theft or significant loss of CS MUST be reported to the DEA in writing within what time frame?
WHAT form must also be filled out?
in WRITING within 1 business day of discovery
FORM 106
What penalties are possible for CSA violations?
a. civil penalties
b. criminal penalties
c. state board discipline
d. all of the above
d
The CSA allows the DEA to enter and inspect any place where CS/ records kept or persons are registered.
Prior to inspection, what must the inspector state/say? consent?
purpose of inspection
present credentials
a WRITTEN notice of inspection—> NEEDS CONSENT!!!!!
The CSA allows the DEA to enter and inspect any place where CS/ records kept or persons are registered.
What are the normal components of an inspection?
(not that important AT ALL—> just FYI)
DEA inspector can…
examine and copy all record/reports
inspect premises
take inventory of CS (compare to records)
if consent—> can look at financial data
STATE inspectors are different then DEA inspectors… what’s a major difference?
state inspectors might not need consent—> DEA inspectors do
(ofc, varies upon state law)
What’s an exception to when consent is NOT needed for a pharmacy inspection?
if there’s an administrative inspection warrant (AIW) or search warrant—> NO CONSENT
(FYI: there are some exceptions, but not that imp)
There are 2 main pathways for treating disorders related to opioids… what are they?
1. Opioid Treatment Programs (OTPs)
2. OUD treatment in community setting
What are the components of a traditional OTP?
facility requirements?
patients do what?
current drugs available?
separate registration required for facility
patients to go to facility for treatment
current drugs for OUD (opioid use disorder)—> methadone, buprenorphine, and combo products
(think of this like an outpatient/methadone clinic—> not a pharmacy)

________ enforces and certifies practitioners for OTP
SAMHSA (FYI: some mobile OTPs approved by DEA)
Can methadone be dispensed in a community pharmacy?
ONLY FOR ANALGESIC PURPOSES!!!!!!!! CANNOT BE PRESCRIBED FOR OUD/ DETOX OR ANYTHING RELATED TO THAT!!!!!!!!!!!!!!
What law expanded treatment of OUD to community settings and allowed for office-based tx of patients and dispensing of tx from pharmacy?
DATA 2000
In order to be able to prescribe tx for OUD, prescribers used to have to register with SAMSHA and have an X waiver… what is the new requirements?
as long as your state authorized and have a DEA # you can prescribe now!!!!!!!!!!!!!!! (got rid of all that b.s.)
What is the only medication assisted treatment for OUD a prescriber can prescribe outside of OTPs?
buprenorphine/ buprenorphine-naloxone (CANNOT PRESCRIBE METHADONE!!!!!!!! ONLY IN CLINIC FOR OUD)
What did the Anabolic Steroids Act of 2004 do?
made anabolic steroids a CIII drug (even though it doesn’t fit the defintion)
What did the Controlled Substance Registration Protection Act do?
(idk how important)
mandates federal investigation for robberies involving CS in certain circumstances—> gives severe penalties
The Combat Methamphetamine Epidemic Act of 2005/ Methamphetamine Prevention of 2008 regulated sales of products used to make meth… including pseudoephedrine.
WHAT ARE THE QTY LIMITS AND THEIR DAY PERIODS? (KNOW THIS)
for OTC products:
3.6g per day OR more than 9g in a 30 day period
7.5 g for MAIL ORDER
Where must products used to make meth, like pseudoephedrine be stored?
BEHIND A COUNTER
Are products used to make meth, like pseudoephedrine only stored behind pharmacy counters?
NOOOO!!!! can be any counter