Carboxylic Acid Derivatives: Structures, Nomenclature, and Reactions in Organic Chemistry
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
What are carboxylic acid derivatives?
Compounds with functional groups that can be converted to carboxylic acids by acidic or basic hydrolysis.
What is the structure of esters?
Esters are carboxylic acid derivatives where the hydroxy group (—OH) is replaced by an alkoxy group (—OR).
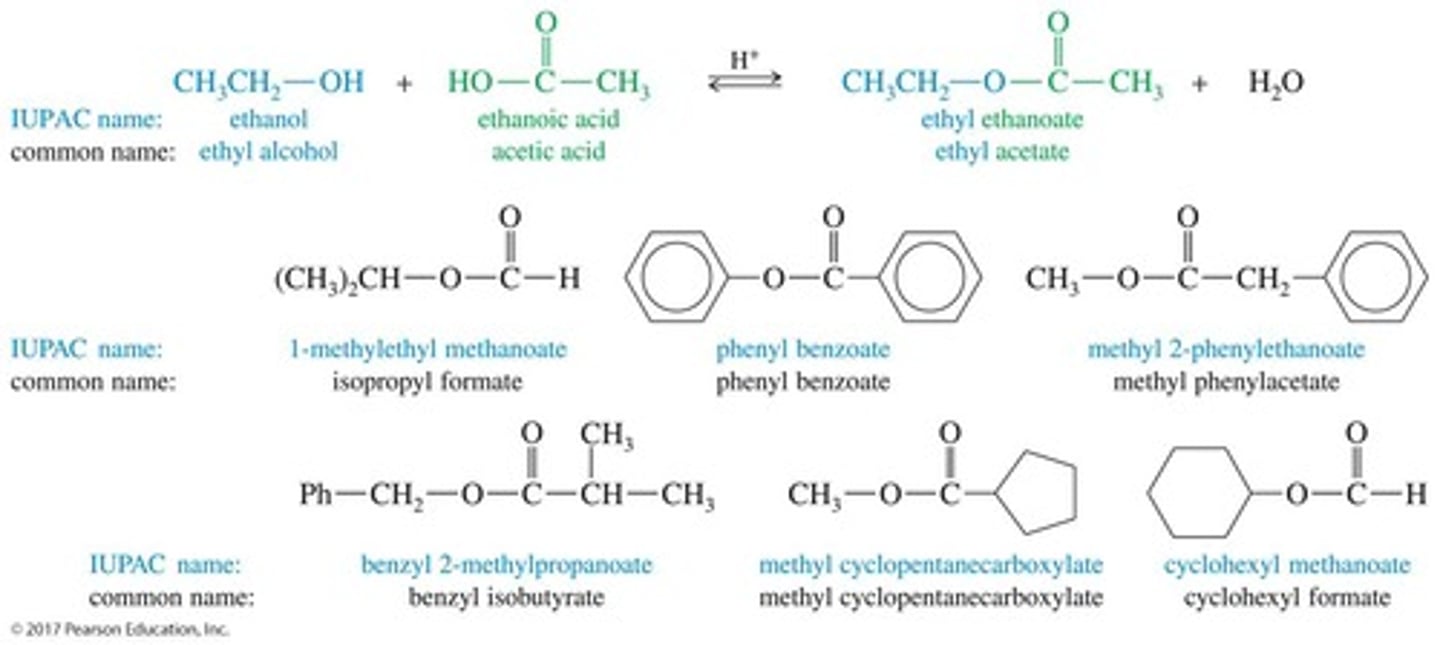
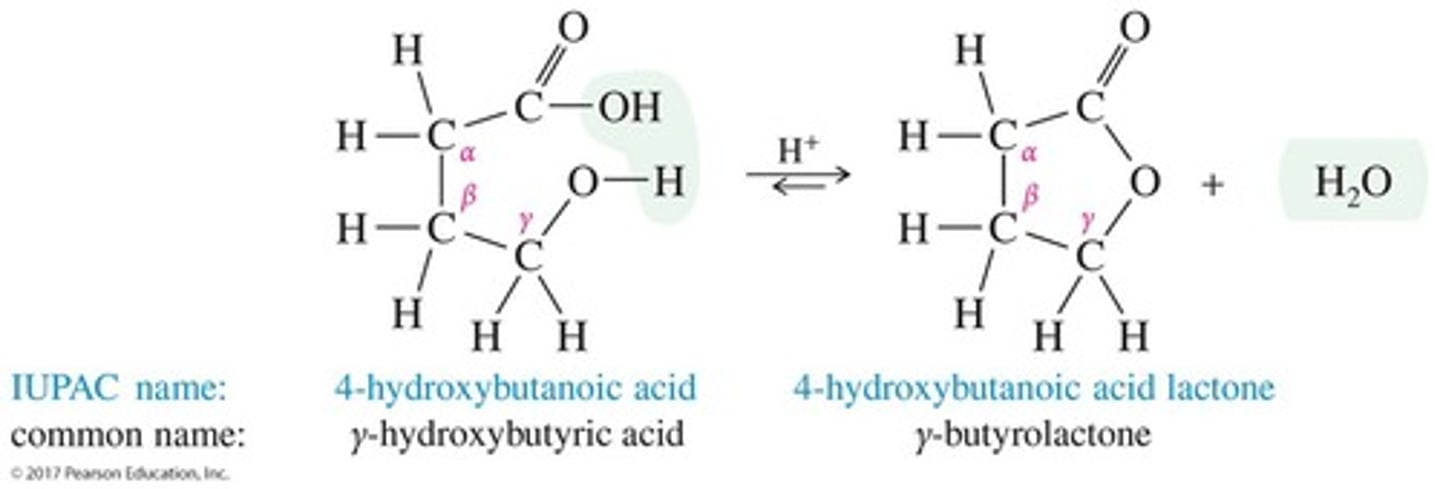
What are lactones?
Cyclic esters, named by adding 'lactone' to the end of the parent acid's name.
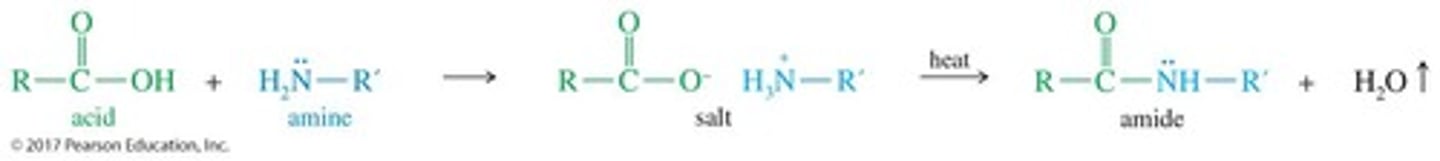
How are amides formed?
Amides are formed from a carboxylic acid and ammonia or an amine, often at high temperatures.
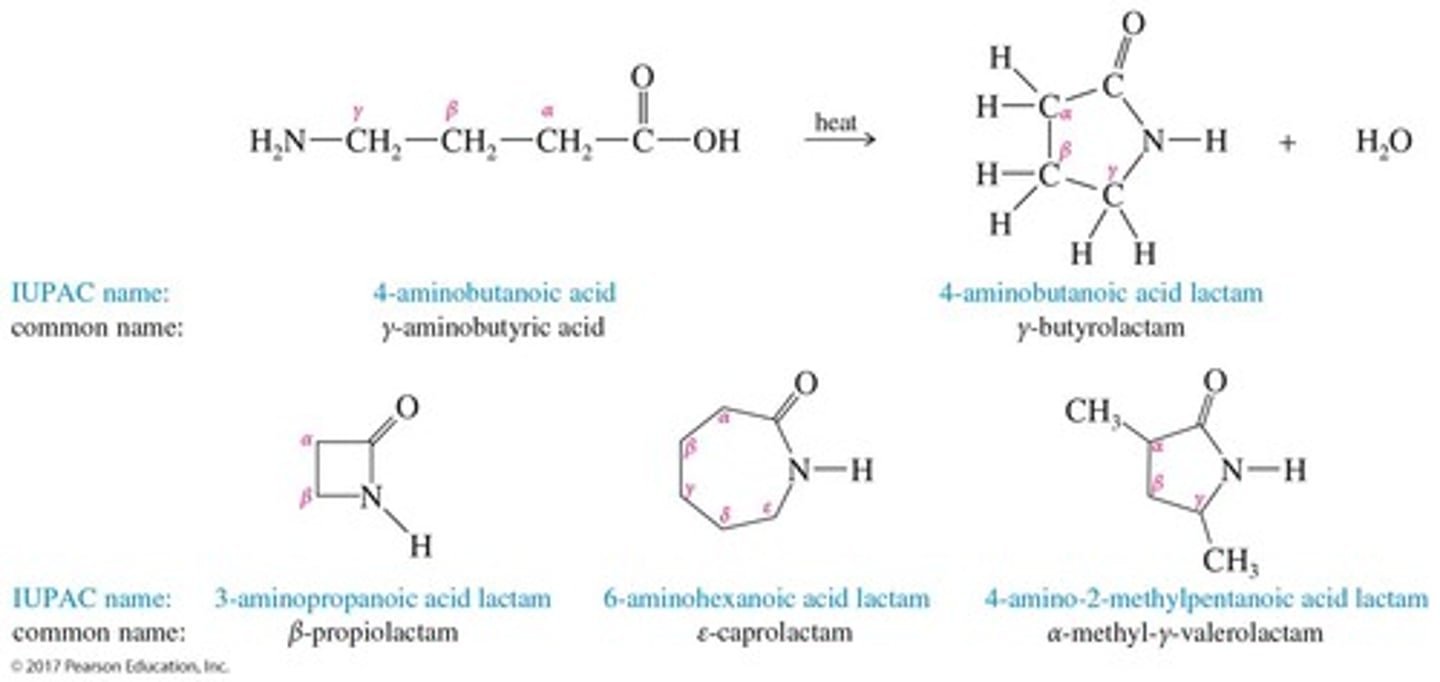
What are lactams?
Cyclic amides, named by adding 'lactam' to the end of the IUPAC name of the parent acid.
What is the structure of nitriles?
Nitriles contain the cyano group.
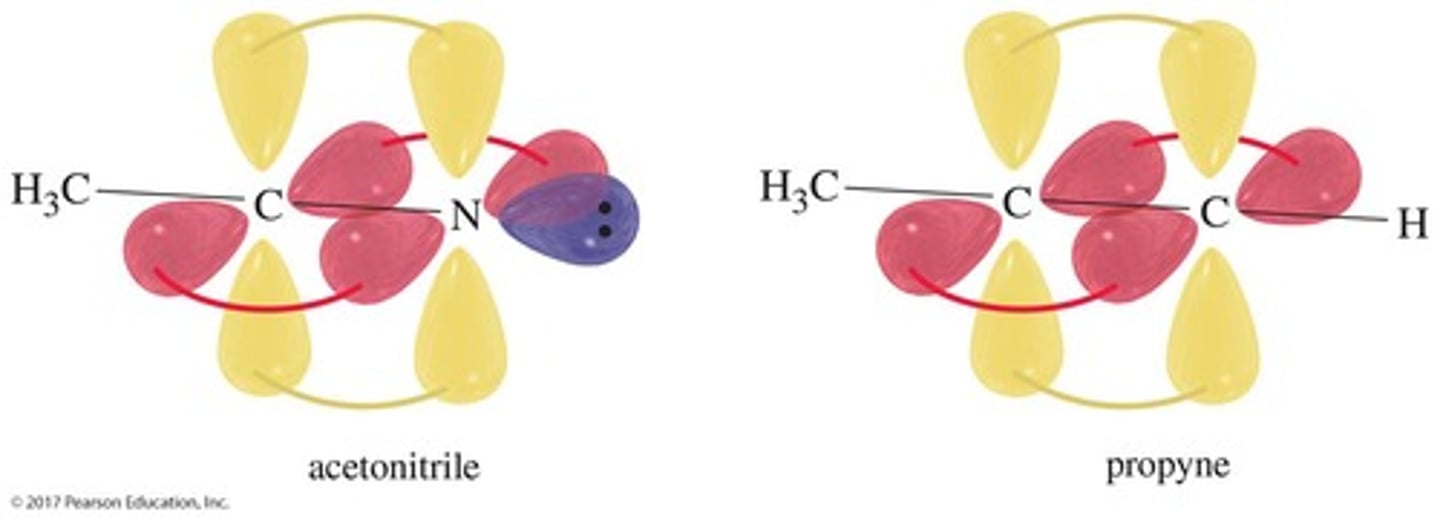
What is unique about the electronic structure of nitriles?
The atoms at the ends of the triple bonds are sp hybridized with a bond angle of 180°.
What are acid halides also known as?
Acyl halides.
How are acid halides named?
By replacing the -ic acid suffix with -yl and adding the halide name.
What is an acid anhydride?
An acid anhydride contains two molecules of acid with the loss of a molecule of water.
How are acid anhydrides named?
By replacing the word 'acid' with 'anhydride' in the name.
What factors influence the physical properties of carboxylic acid derivatives?
Their polarity and hydrogen-bonding properties.
What does the resonance picture of an amide indicate?
It shows the strongly polar nature of amides.
In what solvents are carboxylic acid derivatives soluble?
Common organic solvents.
What is the range of C═O stretch frequencies for most acid derivatives?
Between 1700 cm-1 and 1800 cm-1.
How do proton chemical shifts in acid derivatives compare to those in other compounds?
They are close to those of similar protons in ketones, aldehydes, alcohols, and amines.
What is nucleophilic acyl substitution?
A reaction where a nucleophile adds to the carbonyl, forming a tetrahedral intermediate, followed by elimination of the leaving group.
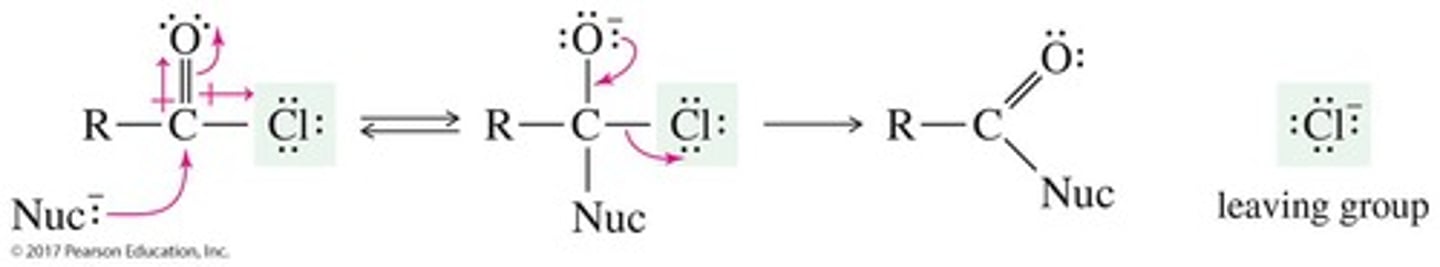
What are the two steps in the mechanism of acyl substitution?
Step 1: Addition of the nucleophile forms the tetrahedral intermediate. Step 2: Elimination of the leaving group regenerates the carbonyl.
What is the reactivity trend of acid derivatives?
More reactive derivatives can be converted to less reactive derivatives.
What is the significance of nucleophilic acyl substitutions?
They are also called acyl transfer reactions because they transfer the acyl group to the attacking nucleophile.
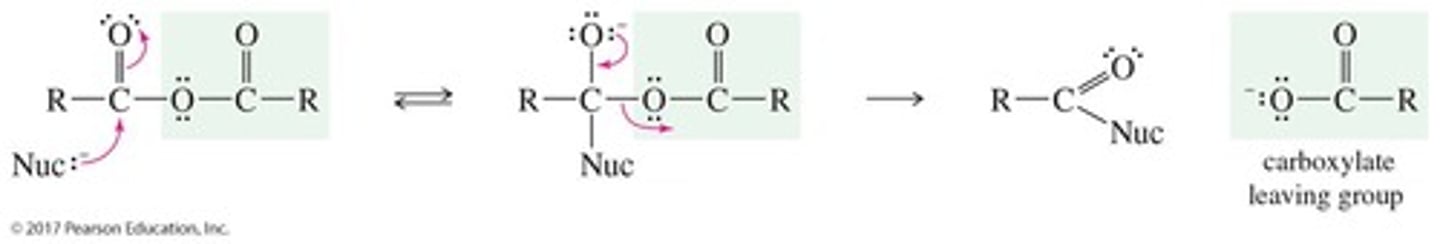
What is formed when a carboxylic acid attacks an acyl chloride?
A tetrahedral intermediate, followed by the formation of an anhydride after chloride ion leaves and deprotonation occurs.
What product results from the reaction of an acyl chloride with alcohol?
An ester is formed after the alcohol attacks the acyl chloride, forming a tetrahedral intermediate, followed by chloride ion leaving and deprotonation.
What types of amides can be formed from the reaction of ammonia and acyl chlorides?
A 1° amide is formed from ammonia, a 2° amide from a 1° amine, and a 3° amide from a 2° amine.
What occurs when alcohol attacks an anhydride?
A tetrahedral intermediate is formed, and the other acid unit is eliminated as the leaving group.
What is the result of ammonolysis of an ester?
The nucleophile must be NH3 or a 1° amine, and prolonged heating is required to form an amide.
What is a characteristic of leaving groups in nucleophilic acyl substitution?
A strong base, such as an alkoxide (-OR), is not usually a leaving group, except in an exothermic step.
What is the energy change associated with the elimination of an alkoxide in nucleophilic acyl substitution?
The elimination is highly exothermic, converting the tetrahedral intermediate into a stable molecule.
What is transesterification?
The process where one alkoxy group is replaced by another, typically using an acid or base catalyst and a large excess of the desired alcohol.
How does hydrolysis of acid chlorides and anhydrides occur?
Hydrolysis occurs quickly, even in moist air, without the need for acid or base catalyst, but reagents must be protected from moisture.
What is saponification?
The base-catalyzed hydrolysis of esters, commonly referred to as soap-making.
How are soaps produced?
By heating NaOH with a fat (triester of glycerol) to produce the sodium salt of a fatty acid.
What happens to amides during hydrolysis?
Amides are hydrolyzed to carboxylic acids under acidic or basic conditions.
What is the mechanism of basic hydrolysis of amides?
The hydroxide ion attacks the carbonyl, forming a tetrahedral intermediate, followed by elimination of the amino group and proton transfer to the nitrogen.
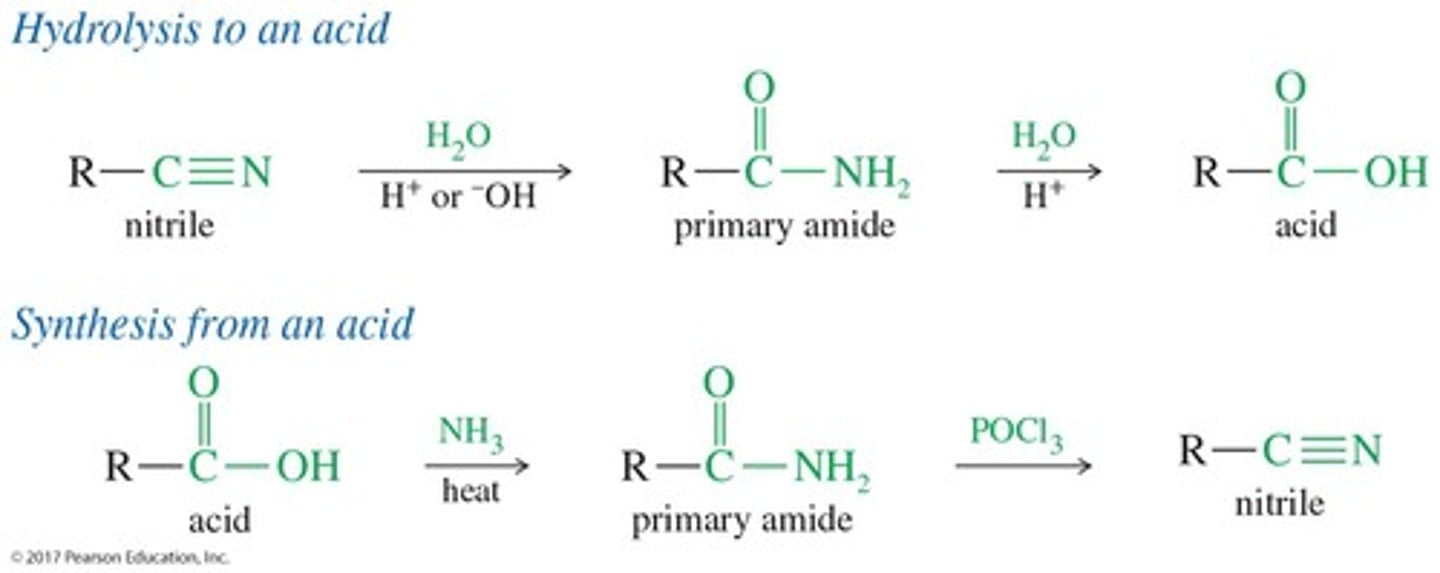
How are nitriles hydrolyzed?
Heating with aqueous acid or base will hydrolyze a nitrile to a carboxylic acid.
What reduces esters to alcohols?
Lithium aluminum hydride (LiAlH4) reduces esters, acids, and acyl chlorides to primary alcohols.
What is the role of lithium tri-tert-butoxyaluminum hydride in reductions?
It is a milder reducing agent that reacts faster with acyl chlorides than with aldehydes.
What is DIBAL and its function?
Di-isobutylaluminum hydride (DIBAL or DIBAL-H) is a mild reducing agent that can reduce esters to aldehydes.
How are amides reduced to amines?
Amides are reduced to the corresponding amine by lithium aluminum hydride (LiAlH4).
What is the outcome of reducing nitriles?
Nitriles are reduced to primary amines by catalytic hydrogenation or lithium aluminum hydride reduction.
What do Grignard and organolithium reagents do in reactions with acid chlorides and esters?
They add twice to acid chlorides and esters to give alcohols after protonation.
What happens when esters react with Grignard or organolithium reagents?
Esters react with two moles of these reagents to produce an alcohol, with a ketone intermediate reacting with a second mole to form the alcohol.
What do dialkylcuprate reagents react with to form ketones?
Acid chlorides react just once with dialkylcuprates (Gilman reagents) to give ketones.
What do Grignard reagents form when they react with nitriles?
A Grignard reagent or organolithium reagent attacks the cyano group to form an imine, which is hydrolyzed to a ketone.
What are the most convenient reagents for synthesizing acid chlorides?
Thionyl chloride (SOCl2) and oxalyl chloride (COCl2) are the most convenient because they produce only gaseous side products.
What is the general method for synthesizing anhydrides?
The reaction of an acid chloride with a carboxylic acid or a carboxylate salt.
How can pyridine be used in the synthesis of anhydrides?
Pyridine is sometimes used to deprotonate the acid and form the carboxylate.
What is a unique feature of using cyclic anhydrides in Friedel-Crafts acylation?
Using a cyclic anhydride allows for only one of the acid groups to react, leaving the second acid group free for further reactions.
What is acetic formic anhydride primarily made from?
Sodium formate and acetyl chloride.
Why is the formyl group in acetic formic anhydride more electrophilic?
Because of the lack of alkyl groups.
What is favored in the formation of lactones?
Formation is favored for five- and six-membered rings; for larger rings, water removal shifts equilibrium toward products.
What can strong dehydrating agents do to primary amides?
They can eliminate the elements of water from a primary amide to give a nitrile.
What are two examples of dehydrating agents used for amides?
Phosphorus oxychloride (POCl3) and phosphorus pentoxide (P2O5).
What types of lactams can form from heating or adding a dehydrating agent to amino acids?
Five-membered lactams (γ-lactams) and six-membered lactams (δ-lactams).
What are β-lactams and where are they commonly found?
Unusually reactive four-membered ring amides found in antibiotics like penicillins, cephalosporins, and carbapenems.
What is the mechanism of β-lactam acylation?
The nucleophile attacks the carbonyl of the four-membered ring amide, forming a tetrahedral intermediate, followed by nitrogen elimination and carbonyl reformation.
How do β-lactam antibiotics function?
They interfere with the synthesis of bacterial cell walls, rendering the acylated enzyme inactive for cell wall protein synthesis.
What is a thioester and how is it formed?
A thioester is formed from a carboxylic acid and a thiol.
What are thioesters also referred to as?
Thiol esters, to emphasize that they are derivatives of thiols.
How does the resonance overlap in thioesters compare to that in esters?
The resonance overlap in a thioester is not as effective as that in an ester, making thioesters more reactive toward nucleophilic acyl substitution.
What is Coenzyme A (CoA) and its role?
CoA is a thiol whose thioesters serve as biochemical acyl transfer reagents.
What is the mechanism of action of Acetyl CoA?
Acetyl CoA transfers an acetyl group to a nucleophile, with coenzyme A serving as the leaving group.
What is the primary use of thioesters in living systems?
Thioesters are common acylating agents due to their selective acylating properties.
What are polycarbonates and how are they synthesized?
Polycarbonates are polymers bonded to the carbonate ester linkage, synthesized using bisphenol A.
What is a common reaction to produce polyurethanes?
The reaction of toluene diisocyanate with ethylene glycol.