Bio 110 Exam 3
0.0(0)
Card Sorting
1/118
Earn XP
Description and Tags
Last updated 8:20 PM on 4/30/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
119 Terms
1
New cards
Metabolism
sum total of all chemical reactions occurring in a biological system at a given time, involve energy changes, involves building up and breaking down
2
New cards
2 types of Metabolism
Anabolic and Catabolic
3
New cards
Anabolic Reactions
complex molecules made from simple molecules (type of metabolism), energy required, often linked to catabolic reactions, driven by energy from catabolic reactions
4
New cards
Anabolic Reaction Example
bringing amino acids together to form polypeptide chains
5
New cards
Catabolic Reaction
complex molecules broken down to simpler ones (type of metabolism), energy released, energy released in catabolic reactions are used to drive anabolic ones, reactions are often linked
6
New cards
Oxidation-Reduction Reaction
electrons transferred between 2 molecules (OIL RIG), one substance transfers electrons to another substance, often associated with transfer of hydrogen ions
7
New cards
If a reaction is reduced…
it gains electrons (oxidizing agent)
8
New cards
If a reaction is oxidized…
it loses electrons (reducing agent)
9
New cards
Energy
capacity to do work, or the capacity for change, can be converted from one form to another, can go from potential to kinetic
10
New cards
Chemical, light, mechanical, electrical, heat
Forms of Energy
11
New cards
Law of Thermodynamics
apply to all matter and all energy transformations in the universe, helps us understand how cells harvest and transform energy to sustain life
12
New cards
1st Law of Thermodynamics
energy is neither created nor destroyed, when energy is converted from one form to another, the total energy before and after the conversion is the same
13
New cards
2nd Law of Thermodynamics
when energy is converted from one form to another, some of that energy becomes unavailable to do work, no energy transformation is 100% efficient, some energy lost to disorder, energy is still equal but not all usable, disorder increases due to energy transformations, disorder increases due to energy transformations
14
New cards
2 types of energy
kinetic and potential energy
15
New cards
Potential Energy (PE)
stored energy, stored as chemical bonds, concentration gradient, or charge imbalance, when ions move across membrane
ex.) cat gearing up for a jump
ex.) cat gearing up for a jump
16
New cards
Kinetic energy (KE)
energy of movement/ motion, can be converted from one form to another (kinetic to potential)
ex.) cat jumping onto table
ex.) cat jumping onto table
17
New cards
Chemical Reactions
occur when atoms collect enough energy to combine or change their bonding partners, energy is stored in bonds and energy s released when bonds are broken
18
New cards
Entropy (S)
measure of disorder in a system, takes energy to impose order on a system, everything moves toward disorder unless energy is applied to the system
19
New cards
Enthalpy (H)
total energy (H=G/TS)
20
New cards
Free Energy (G)
usable energy that can do work,
21
New cards
∆G=∆H-T∆S
Free energy equation
22
New cards
calories or joules
How is change in energy measured?
23
New cards
If ∆G is negative…
free energy is released
24
New cards
If ∆G is positive…
free energy is required
25
New cards
If ∆G is zero…
a reaction does not occur
26
New cards
Exergonic Reactions
release of free energy (-∆G), catabolism (exit), reversible
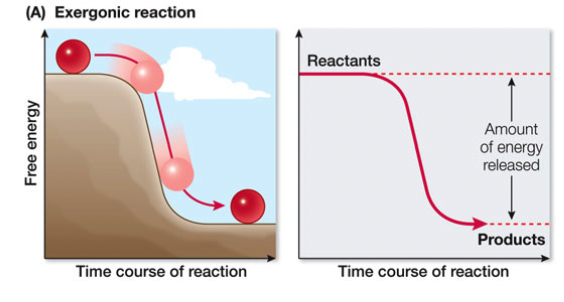
27
New cards
Catabolism
Complexity deceases (generates disorder), complex molecules→free energy+ small molecules (associated with exergonic reactions)
28
New cards
Endergonic Reactions
consume free energy (+∆G), anabolic (enter)
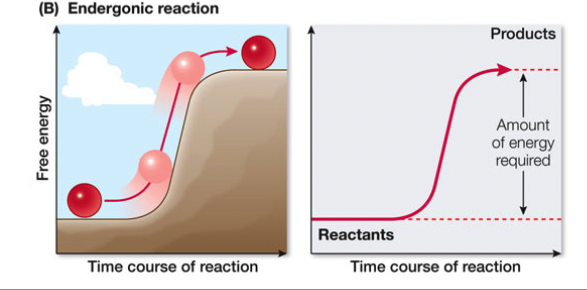
29
New cards
Anabolism
complexity (order) increases, free energy+ small molecules→complex molecules (associated with endergonic reactions)
30
New cards
Chemical Equilibrium
balance between forward and reverse reactions, ∆G=0, every reaction has equilibrium point, ∆G values near zero are characteristics of readily reversible reactions
ex.) state of no net change
ex.) state of no net change
31
New cards
ATP (energy currency)
Where does biochemical energy come from?
32
New cards
ATP
energy transfer in biochemical reactions, captures and transfers free energy, can be hydrolyzed to ADP and P¡, releasing a lot of energy for endergonic reactions
33
New cards
ATP+H20→ADP+P¡+free energy, break down with the addition of H20
ATP Hydrolysis Reaction
34
New cards
Formation of ATP is…
endergonic
35
New cards
Characteristics of ATP that allow for release of free energy when hydrolyzed
1) Phosphate groups have negative charges and repel each other
2) Free energy of the P\~O bond is much higher than energy of the O-H bond that forms after hydrolysis
2) Free energy of the P\~O bond is much higher than energy of the O-H bond that forms after hydrolysis
36
New cards
ATP hydrolysis is…
exergonic
37
New cards
ATP coupling reaction
formation and hydrolysis of ATP couple or join endergonic and exergonic reactions
38
New cards
Enzymes
biological catalysts that act as a framework in which reactions can take place, most are proteins, lower the energy barrier by bringing reactants together, lower activation energy, speed up reactions
39
New cards
Function of enzymes
lower the energy barrier by bringing reactants together, 3-D shape of enzyme determines its specificity
40
New cards
1) They do not change final equilibrium
2) They do not change the difference in free energy (∆G) between reactants and products
3) They do not make reactions that wouldn’t otherwise happen
2) They do not change the difference in free energy (∆G) between reactants and products
3) They do not make reactions that wouldn’t otherwise happen
What don’t enzymes do?
41
New cards
Catalyst
increases rate of a chemical reaction
42
New cards
Activation Energy (Ea)
amount of energy required to start a reaction, can come from heating the system, associated with enzymes, heat can start chemical reactions, ∆G=+, requires energy
43
New cards
Transition State
reaction mode of the substrate (aka. reactant) after there has been sufficient input of energy to initiate the reaction, they want to react because they are unstable and want to become stable again (similar to valence shell)
44
New cards
Transition State Intermediates
unstable reactions with higher free energy, added energy to reaction to make it unstable, will become stable quickly and have higher free energy, not in end state, pushing them to react, must become less stable before change is possible, energy required to make unstable, releases free energy to get stable again
45
New cards
Substrates
reactants, molecule(s) on which an enzyme exerts (is working) its catalytic action
46
New cards
Active Site
place on an enzyme where substrate binds (where the enzyme is working)
47
New cards
1. Enzymes can orient substrates so they react (orientation) (substrates fit well into enzymes)
2. Enzymes can indue strain by stretching substrate (physical strain) (stretches and breaks up bonds, makes bonds unstable and more reactive)
3. Enzymes can temporarily add chemical groups (chemical charge) (adding a positive or negative charge can make enzyme unstable)
3 Mechanisms of Enzyme Action
48
New cards
1. regulation of gene expression- how many enzyme molecules are made (turn on gene in DNA)
2. regulation of enzyme itself- enzyme shape may change, or enzyme can be blocked by regulators (cells can turn enzymes off and on when needed)
2 ways to regulate enzymes
49
New cards
Reversible Inhibition
inhibitor bonds covalently to the active site, preventing substrate binding, doesn’t allow enzyme to bind (competitive, uncompetitive, noncompetitive)
50
New cards
Irreversible Inhibition
inhibitor covalently (strong) bonds to side chains in active site and permanently inactivates the enzyme
51
New cards
Competitive Inhibitors
compete with natural substate for binding sites, if substrate doesn’t get there first, it cant bind because of the inhibitor, competition for binding to active site, degree of inhibition based on concentration of substrate vs. inhibitor
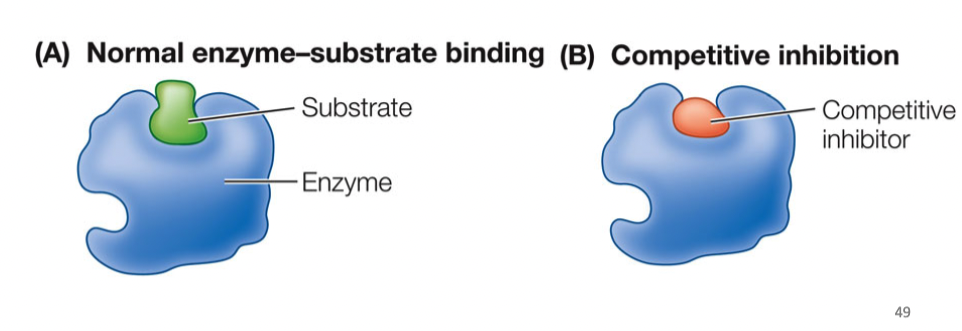
52
New cards
Noncompetitive Inhibitors
bind to enzyme at site other than the active site, enzyme changes shape and alters active site (allosteric), changes shape of active site
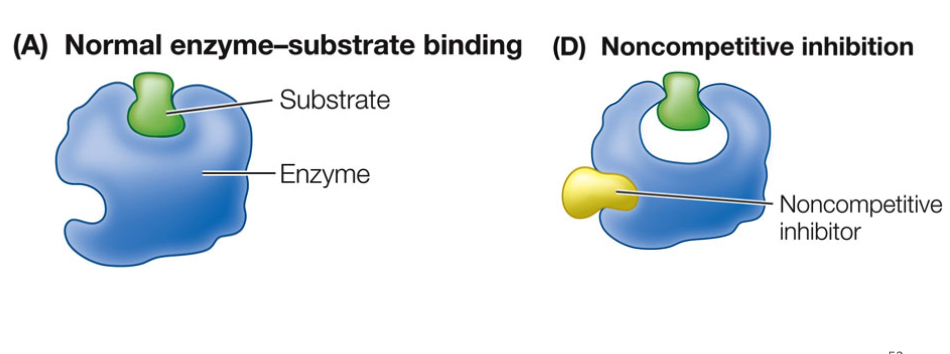
53
New cards
Allosteric Regulation
an effector binds an enzyme at a site different from the active site, changing the enzyme’s shape, doesn’t allow enzyme to bind properly because shape of site is different, enzyme will function or not function based on new shape (tuns on or off) (active and inactive form)
54
New cards
Uncompetitive Inhibitors
bind to enzyme-substate complex, preventing release of products, cannot be overcome by adding more substate, holds substrate to enzyme (locks enzyme in place), not competing for active site
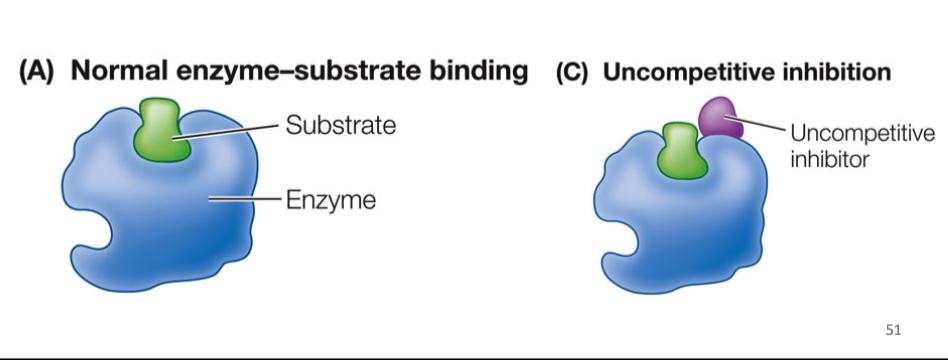
55
New cards
Commitment Step
first reaction, followed by other reactions in sequence, cell is committed to following pathway
56
New cards
Feedback Inhibition
final product acts as noncompetitive inhibitor of the 1st enzyme, shutting down the pathway
57
New cards
Ribozymes
catalytic RNAs that speed up reactions involving their own nucleotides
58
New cards
Induced Fit
enzyme changes shape when it binds substrate, altering shape of active site
59
New cards
every enzyme is most active at a particular pH, which influences ionization of functional groups
How does pH affect enzymes?
60
New cards
every enzyme has an optimal temp., at high temp, noncovalent bonds begin to break and enzymes lose tertiary structure and denature, enzymes adapted to higher temps do not denature because of covalent bonds in their tertiary structure, at low temps reactivity slows down
How does temperature affect enzymes?
61
New cards
Aerobic Respiration
the process of using glucose and oxygen to produce ATP, more efficient than anaerobic respiration
62
New cards
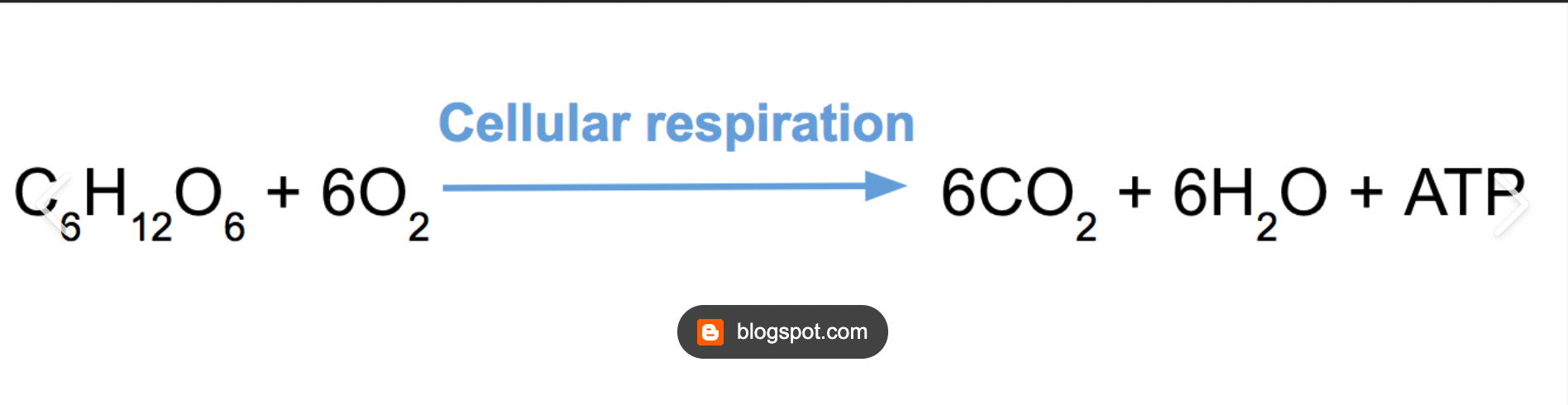
\
Cellular Respiration Formula
63
New cards
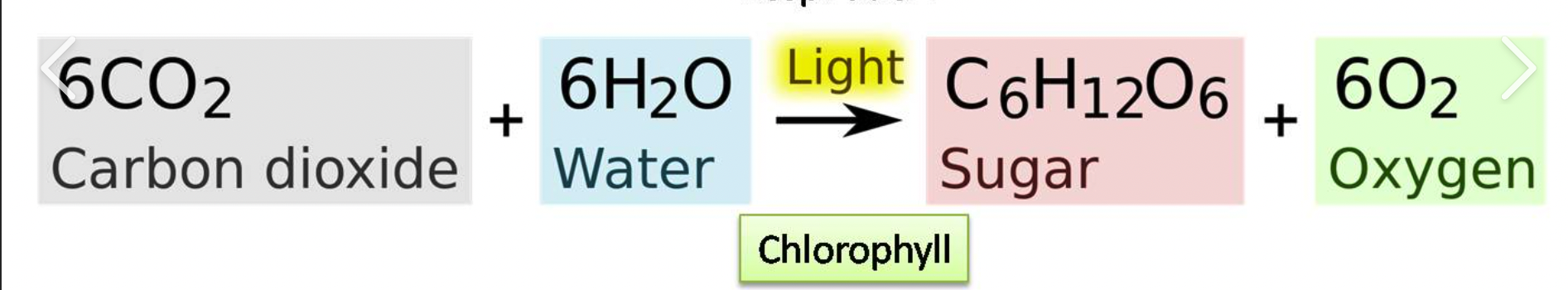
opposite of cellular respiration (except light energy and ATP), linked to cellular respiration
Photosynthesis Formula
64
New cards
Photosynthesis
produces oxygen gas and glucose (potential energy)→cellular respiration→produces CO2 and H2O, opposite of cellular respiration
65
New cards
1. Complex transformations occur in a series of separate reactions (multiple step process)
2. Each reaction is catalyzed by a specific enzyme (need different enzymes in different pathways, reactions must happen quickly)
3. Many metabolic pathways are similar in all organisms
4. In eukaryotes, metabolic pathways are compartmentalized on specific organelles (different compartments for photosynthesis and cellular respiration makes it easier for all compartments to be in each compartment efficiently)
5. Key enzymes can be inhibited or activated to alter the rate of the pathway (pathway can be turned on and off)
5 principles of Metabolic Pathways
66
New cards
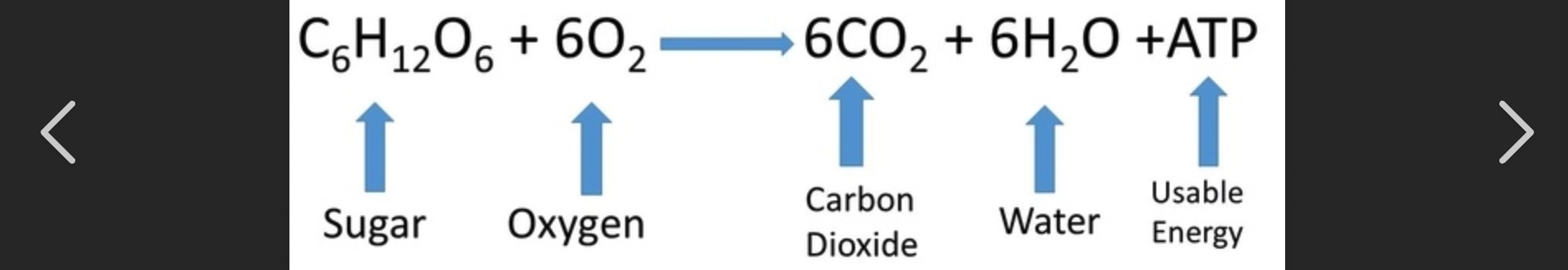
Burning/ Metabolism of Glucose Formula
67
New cards
Metabolism of Glucose
cells OBTAIN energy from glucose, reaction is extremely exergonic (-∆G value), drives endergonic formation of many ATP molecules
68
New cards
How cells break can break down glucose from energy:
1) Glycolysis (anaerobic, oxygen absent) (always start with glycolysis followed by cellular respiration or fermentation depending on presence of oxygen)
2) Cellular Respiration (aerobic, oxygen present)
3) Fermentation (anaerobic, oxygen absent)
1) Glycolysis (anaerobic, oxygen absent) (always start with glycolysis followed by cellular respiration or fermentation depending on presence of oxygen)
2) Cellular Respiration (aerobic, oxygen present)
3) Fermentation (anaerobic, oxygen absent)
3 Catabolic processes that harvest energy from glucose
69
New cards
Transfer of electrons is associated with…
transfer of hydrogen ions
70
New cards
When a molecule loses H atoms…
it becomes oxidized
71
New cards
Electron carrier molecules
NADH, FADH2, NADPH
72
New cards
Electron Carrier Molecule Definition
garbs electrons from one molecule and moves to another molecule, shuttles electrons from one place to another
73
New cards
NADH and FADH2
Which electron carrier(s) are used for cellular respiration?
74
New cards
NADPH
Which electron carrier(s) are used for photosynthesis?
75
New cards
glycolysis, pyruvate oxidation, citric acid cycle, respiratory chain/ATP synthesis, fermentation
5 energy-yielding metabolic pathways
76
New cards
Aerobic
oxygen is present
77
New cards
Anaerobic
oxygen is absent
78
New cards
In the cytoplasm
Where does glycolysis occur in eukaryotic cells?
79
New cards
In the cytoplasm
Where does fermentation occur in eukaryotic cells?
80
New cards
In the matrix of the mitochondria
Where does the citric acid cycle occur in eukaryotic cells?
81
New cards
In the matrix of the mitochondria
Where does pyruvate oxidation occur in eukaryotic cells?
82
New cards
In the inner membrane of the mitochondria
Where does the respiratory chain/ ATP synthesis occur in eukaryotic cells?
83
New cards
In the cytoplasm
Where does glycolysis occur in prokaryotic cells?
84
New cards
In the cytoplasm
Where does fermentation occur in prokaryotic cells?
85
New cards
In the cytoplasm
Where does the citric acid cycle occur in prokaryotic cells?
86
New cards
On the cell membrane
Where does pyruvate oxidation occur in prokaryotic cells?
87
New cards
On the cell membrane
Where does the respiratory chain/ ATP synthesis occur in prokaryotic cells?
88
New cards
Glycolysis, Pyruvate Oxidation, Citric Acid/ Krebs Cycle, Electron Transport chain/ATP synthesis
Which pathways occur when oxygen gas (O2) is present?
89
New cards
Glycolysis and Fermentation
Which pathways occur when oxygen gas (O2) is absent?
90
New cards
Glycolysis
takes place in the cytoplasm and has to move to the mitochondria, converts glucose into 2 molecules of pyruvate, produces 2 ATP and 2NADH (to be used in electron transport chain), occurs in 10 steps: first 5 require ATP energy input, last 5 yield NADH and ATP
91
New cards
Oxidative Phosphorylation
ATP is synthesized by reoxidation of electron carriers in the presence of O2
92
New cards
2 Stages of Oxidative Phosphorylation
1) Electron transport-add phosphate to ADP to make the remaining 28 ATP molecules (electrons dumped, occurs in inter membrane space
2) Chemiosmosis
2) Chemiosmosis
93
New cards
Chemiomosis
protons (hydrogen ions) flow back across the membrane (mitochondria) through a channel protein with the concentration gradient, from the matrix to the inter membrane space and back, energy released is uses to make ATP
94
New cards
ATP synthase
couples the diffusion with ATP synthesis, producing ATP
95
New cards
NADH and FADH2 donate their electrons to the electron transport chain
Electron transport chain- Where do the electrons come from?
96
New cards
Energy from electrons are used to produce many ATP
Electron transport chain- What are electrons used for?
97
New cards
As electrons travel through the transport chain, carrier molecules use the potential energy of the electrons to transport hydrogen ions into the inter membrane compartment
Electron Transport chain- Importance of proton/H+ gradient
98
New cards
Proton-motive force
force generated across a membrane having 2 compartments- a chemical potential plus an electric potential due to the electrostatic change on a proton
99
New cards
At the end of the transport chain, electrons are donated to an oxygen atom, which combines with hydrogens to form H2O
Electron Transport Chain- Final electron acceptor
100
New cards
If oxygen isn’t present, electrons can’t be donated and everything gets “backed up”- FAD and NAD+ cant be produced, which are required for the Krebs cycle
Why are pyruvate oxidation and Krebs cycle considered aerobic?