UAMS Microbiology Module 1
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
99 Terms
Who made the Gram stain?
Christian Gram
What are the reagents for the Gram stain?
Crystal violet (initial stain placed on bacteria, purple or dark blue look)
Iodine (will mix with the crystal violet present in bacteria to help it stick, makes mordant).
Acetone-alcohol decolorizer (usually 1:1, ethanol 95%:acetone)
Safranin (secondary stain, the pink one).
How do you make slides from a colony on an agar/media
Mix with a drop of saline.
Generally, place the drop if saline on the slide and then take a loopful of bacteria from a colony and swirl it around.
What are the methods to fix slides?
Just think heat.
Generally with a slide warmer at 60C
Pass through flame 2-3 times.
Hold at front of incinerator (this part gets really hot so use tweezers).
Flood smear with methanol and
What can happen due to the cells themselves, and not the process of staining?
Some GP organisms, as they age or from antibiotics, will have weaker cell walls and may appear GN as they can’t hold in the stain.
The best results will be on <24hr old cultures from non-inhibitory media (blood agar, since it’s only nutrients).
Overheating may distort some cells as well.
What are the characteristics of agar media?
Agar media: Polysaccharide melts at 100 C; stays liquid until 45-50C -Media may be formed into plates, slants, or deeps.
Semi-solid: 0.3-0.5 % agar
Solid: 1-2% agar
What does the streak plate method do?
It’s the 3-4 quadrant streaking on the plates.
Separates & semi-quantitatively enumerates
organisms present in patient specimens or mixed cultures.
Principle: Organisms are separated by spreading them over the
surface of the agar plate.
Each successive section has fewer organisms (section 3 < section 2 < section 1) & organisms farther apart.
Colony-forming-units (CFU): Individual or small groups of cells which multiply to form visible colonies. Isolated colonies appear after incubation.
May be unnecessary to sterilize loop between sections when working with patient specimens.
Interpretation:
Growth only in the first section = 1+ = Few, sparse, or light
Growth in the first and second sections = 2+ = Moderate.
Growth into the third section = 3+ = Many or heavy.
What are the types of media?
Nutrient: grows most kinds of fastidious organisms.
Enriched: Supplemented with various substances (blood, blood by-products, vitamins, yeast extract, carbohydrates, etc.). Grow fastidious organisms (special nutritional requirements).
Enrichment broths: Enhance growth of certain MO while inhibiting other organisms.
Selective: Have antimicrobial substances (e.g., dyes, antibiotics, &
chemicals). Inhibit some microbes while allowing the growth of others.
Non-selective/inhibitory: No antimicrobial substances & allow growth of many organisms.
Differential: Metabolic differences distinguish microorganisms. Identification media are differential.
Nutrient media examples
Nutrient agar
trypticase soy broth
Enriched media examples
Sheep blood agar plate (BAP): Most commonly used medium, Grows many microorganisms
Sheep BAP: Nutrient base (e.g., trypticase soy
agar) supplemented with 5% sheep blood.Blood agar made with human, rabbit, or horse
blood is used in some situations
Chocolate agar (CHOC): Grows many organisms including fastidious bacteria such as Haemophilus.
Modified blood agar; made by heating sheep RBC
to release nutrients or by adding nutritional
supplements to a nutrient agar base
Enrichment broth examples
Gram-Negative (GN) broth & Selenite broth: Used to isolate Salmonella and Shigella from fecal specimens.
LIM broth & Trans-Vagil broth Used to isolate Group B
Streptococci from vaginal/rectal specimens.
Selective broth examples
Have antimicrobial substances (e.g., dyes, antibiotics, & chemicals). Inhibit some microbes while allowing the growth of others.
PEA agar is blood agar supplemented with phenylethyl
alcohol.CNA agar is blood agar supplemented with the antibiotics
colistin and nalidixic acid.Gram-positive organisms grow on PEA agar and CNA agar while most gram-negative bacilli do not. (Anaerobic gram-negative bacilli grow on anaerobic formulation of PEA).
Non-selective media examples
No antimicrobial substances & allow growth of many organisms.
Examples: BAP and CHOC
Some medias can be multiple. BAP is both nonselective, enriched, and differential due to growing anything, differing by hemolysis, and containing blood cells.
Differential media examples
Metabolic differences distinguish microorganisms.
Identification media are differential.
Examples: Carbohydrate fermentation media & enteric media.
What is the most common temp used in micro?
35C (though the range is 35-37C)
Other temps are used for specific pathogens, there will either be certain incubators for this or one incubator will be used and the temp changed depending on the organisms that need recovering.
What are the other few temps needed for micro?
Yersinia enterolitica is 22-25C
Campylobacter jejuni is 42C
What are the types of atmosphere conditions needed?
Aerobic: Air approximately 21% oxygen. Obligate aerobes require oxygen.
Anaerobic: No oxygen, O2 toxic for strict anaerobes, some anaerobes tolerate low O2 level.
Facultative anaerobes: Grow both aerobically and anaerobically.
Microaerobic (also known as microaerophilic): Organisms require a decreased level of oxygen (5% -10%).
Capnophilic organisms: require an increased level of CO2 (5% to 10%).
Special CO2 incubators
Candle extinction jars: Place culture plates & lighted, white candle in jar and seal. Colored or scented candles may be toxic. Before the candle burns out, the flame will use some of the oxygen to produce an atmosphere of 1% to 3% CO2.
Humidophilic microbes: require increased humidity (70% to 80%). Some incubators have built-in humidifying systems or water-filled pans to increase humidity.
*The difference between microaerobic and capnophilic organisms is very small. Just remember the key words decreased and increased. (imagine one coming from the bottom to get increased oxy, and the other going down to get decreased oxy).
What are some other considerations for incubating specimens?
Keep lids lightly screwed on unless stated.
Keep plates upside down (agar in the air) so condensation doesn’t drip on the media and colonies.
What is hemolysins and what are the types produced?
It is damage to the RBCs in the agar.
Gamma: No clearing at all.
Alpha hemolysis: green apple, partial clearing of the agar.
Beta: Full clearing, should be able to see through it.
Another called Alpha Prime is alpha hemolysis surrounded by a zone of beta hemolysis (partial clearing and full clearing around that)
What are ways to disinfect the Micro lab?
Sterilization: Destroys all MO including bacterial spores. Exception: Prions (agents that cause transmissible spongiform encephalopathy). Require special methods to destroy.
Disinfection: Pathogenic organisms eliminated. Viable bacterial spores may be present. Antiseptic: Disinfectant used on living tissue (skin)
3) Physical methods: Usually sterilize
Autoclaves: Pressure & steam sterilize at 15 pounds per square inch (psi) with Temperature of 121 C. 15 min exposure time sufficient for most items. 30-60 mins for infectious waste
Dry heat: 160 - 180 C for 1.5 to 3.0 hours. Sterilizes glassware/items not tolerating moist heat
Filtration: Liquids, air, and other gases can be filtered.
Radiation (Ionizing & ultraviolet radiation.
Chemical agents: Usually disinfect:
Alcohols: Ethyl or isopropyl alcohol (60 - 90%)
Aldehydes: Glutaraldehyde
Phenolics (chemically modified phenols)
Quaternary ammonium compounds (“quats”)
Halogens: Bleach (has chlorine, Sodium hypochlorite (NaClO)). 1:10 dilution recommended for blood spills. Clean area first then disinfect (chlorine
inactivated by organic matter)Iodophores (Disinfectants & antiseptics) Iodine compounds/Povidone-iodine.
What are the different biosafety levels?
Level 1: won’t cause disease in normal healthy adults. Wash hands and wipe down things.
Level 2: routinely used by most labs. Human pathogens not transmitted by inhalation. Coats, limited access, and sharps.
Enterics, Herpes simplex virus, Staph. aureus
Level 3: MO transmitted by inhalation, physically separated area, possible respirators, negative pressure, (our labs level).
Level 4: Extremely contagious and dangerous organisms (Ebola).
Mutualism
Both the bacteria and human benefit.
Like intestinal bacteria, they help digest food and make some Vit K for us.
Commensalism
Human is not affected while bacteria benefits.
Normal flora is often this.
Parasitism
Bacteria benefit at the expense of the human host.
Like Grp A strep.
Opportunistic pathogens
Not normally a pathogen unless the body is weakened (immunoompromised, wounds).
Virulence
Due to a variety of microbial factors (toxins, capsules, enzymes). Virulent microorganisms are more likely to cause disease
Carrier
Will carry the disease and can give it to another.
Usually asymptomatic.
Zoonosis
Animal disease in a human.
What are the human defense mechanisms?
Intact skin and mucous membranes
Mucous membrane cleansing activity: Mucus & cilia
Production of antimicrobial substances
Lysozyme: Enzyme disrupts cell walls of gram-positives;
found in tears, saliva, and other body secretions.Fatty acids: Produced by sebaceous glands; reduces skin’s pH, which inhibits microbe growth
Antibodies on mucous membranes. Phagocytosis by PMNs, macrophages, and monocytes
Host susceptibility factors: Nutritional status, age, stress, genetic makeup, and preexisting conditions (surgery, foreign bodies, etc.).
What happens in the invasion of a body by bacteria?
Attachment to host cells:
Some bacteria (N. gonorrhoeae use pili (short, filamentous, surface structures) to adhere to host.
Proliferation: Organisms grow and multiple if they effectively compete with the host and other microbes for nutrients.
Evasion of host defenses:
IgA proteases produced by some organisms & destroy IgA
Inhibition of Phagocytosis by Capsule: Protein/polysaccharide coats bacterial cell
(i) Phagocytes engulf encapsulated MO only
when specific anticapsule antibodies
present. (Example: Streptococcus pneumoniae)Some microbes ingested but not killed by
phagocyte.Antigenic variation: Some MO (Borrelia) change their
surface antigens as host responds to MO. Host has to play immunologic “catch-up”.
What kinds of toxins are made by bacteria?
Exotoxins: Extracellular proteins produced by metabolizing bacteria.
Harm host cells by damaging the membrane or by
interfering with normal metabolic functions.Toxins may be named for their site of action.
Neurotoxins - nervous system.
Enterotoxins - gastrointestinal tract.
Endotoxins: Lipopolysaccharide (LPS) part of gram-negative cell wall.
LPS composed of carbohydrates and lipid A (toxic part).
Once bacteria die, the toxin is released.
Can cause fever, shock, bleeding, and death.
How do normal flora help the human body?
Can interfere with pathogens by:
Producing bacteriocins (antimicrobial substances).
Reducing a body site’s pH (e.g. lactobacilli in female genital tract).
Competing for nutrients.
NF of Gastro tract
Anaerobic bacilli (Bacteroides, Clostridium)
Enterococci
Anaerobic cocci
Staphylococcus aureus
Gram-negative enteric bacilli
Yeast (Candida)
Genitourinary NF
Lactobacilli
Diphtheroids
Anaerobic bacilli (Bacteroides, Clostridium)
Streptococci
Anaerobic cocci
Gram-negative enteric bacilli
Staphylococci (S. epidermidis, S. aureus)
Acinetobacter
Enterococci
Yeast (Candida)
Respiratory Tract NF
Staphylococci (S. epidermidis, S. aureus)
Haemophilus
Streptococci (viridans, pneumococci)
Gram-negative bacilli (enterics, nonfermenters)
Enterococci
Anaerobes
Diphtheroids
Spirochetes
Neisseria
Yeast (Candida)
Skin NF
Staphylococci (S. epidermidis, S. aureus)
Diphtheroids
Micrococci
Gram-negative bacilli (enterics, nonfermenters)
Streptococci (nonhemolytic)
Anaerobes
Enterococci
Yeasts and fungi
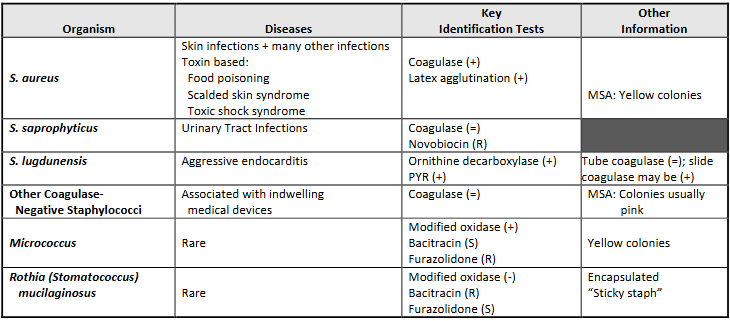
Staphylococcus characteristics
Staphylococcaceae (family): Staphylococcus (genus)
Very common NF, will cause serious diseases.
Two groups:
Coag Negative Staph. Since theres so many, you generally just say coag neg staph until further ID.
Coag Positive Staph: Staph. aureus.
Growth conditions: Faculative anaerobic, will grow on routine media (BAP, CHOC, CNA, PEA).
Normally in anterior nares, mucus membranes and skin.
Staph aureus Characterisitcs (think butter)
Coagulase +
Catalase +
GS: grape clusters.
BAP: Opaque, buttery/smooth, with beta hemolysis. Slight yellow color.
MSA: Yellow, yellow zones. (it fermented the mannitol and produced acid, turning the indicator yellow).
Coag Negative Staph. characteristics (think margarine I guess)
Catalase +
Coagulase -
BAP: opaque white butter, gamma hemolysis (none).
MSA: Red, red zones (doesn’t ferment mannitol).
Diseases caused by Staph. aureus
Invasive diseases
(1) Skin infections (e.g., folliculitis, furuncles, impetigo, and
carbuncles)
(2) Abscesses, wound infections, osteomyelitis, + others
(3) Antibiotic-resistance common.
Toxigenic diseases:
Staphylococcal food poisoning: Improperly stored food contaminated with enterotoxin-producing staphylococci. Symptoms: Diarrhea and vomiting within hours.
Scalded skin syndrome: Usually occurring in neonates and infants due to exfolitin toxins: Skin sloughs.
Toxic shock syndrome (TSS): Toxic Shock Syndrome Toxin - 1 (TSST-1). Symptoms: high fever, hypotension, confusion, diffuse rash, and acute renal failure. Most early cases were associated with menstruating women using hyper absorbable tampons. TSS occurs in men and women as a complication of a number of staph infections.
Diseases caused by Coag-negative Staph.
Once considered to be nonpathogenic
Most infections in Immunocompromised patients, those with indwelling medical devices (catheters).
S. epidermidis: Most commonly isolated species, especially from
cases of prosthetic valve endocarditis.
S. saprophyticus: Urinary tract infections, especially in young,
sexually active women.
S. lugdunensis: Can cause an aggressive form of endocarditis.
important to differentiate from other STPCN because of different
interpretive criteria for oxacillin.
Catalase test principle
Catalase (enzyme) breaks down hydrogen peroxide
to water and oxygen with bubbles formed by the released oxygen.
catalase
H2O2 ⎯⎯⎯⎯→ H2O + O2 (bubbles)
Procedure: Place colony of test organism onto a glass microscope slide. Add a drop of 3% H2O2.
Interpretation:
Positive: *Immediate production of bubbles.
Negative: No bubbles.
False-positive reactions:
If test performed in reverse order with an iron or nichrome
loop.Carefully remove colonies growing on BAP (RBC have small amount of catalase).
*Pseudocatalase: Some bacteria (e.g., enterococci)
produce pseudocatalases which decompose H2O2. These false-positive results may be avoided by reading the test within 20 to 30 seconds.
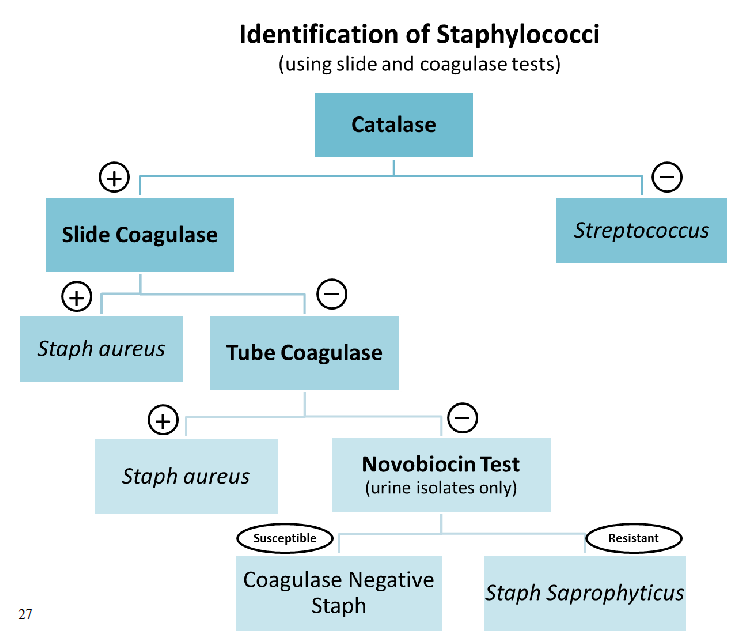
Coagulase test principle.
Coagulase converts fibrinogen > fibrin
The reagent is just plasma containing coagulase.
There are a few different versions: the slide and tube coagulase tests.
Slide coagulase (bound coagulase, clumping factor) test Principle: Bound coagulase attached to the cell wall (hence the name). When S. aureus is mixed with plasma, visible clumps of cells appear as fibrin strands are formed.
Procedure: Use microscope slide
Mix organism in saline - heavy smooth suspension
Mix in a drop of plasma with applicator stick
Interpretation:
Positive: Appearance of clumps in 10 to 15 seconds. Report organism as S. aureus.
Negative: No clumping.
Invalid: MO autoagglutinates (clumps in saline).
Perform tube test: On slide coagulase-negative isolates since not all S.aureus strains are slide coagulase positive. And on strains which autoagglutinate
Tube coagulase test Principle: Extracellular (free) coagulase forms complex with coagulase-reacting factor (CRF), in plasma.
Complex converts fibrinogen to fibrin.
Procedure: Test organism emulsified in plasma in a tube. Tube incubated at 35 C for four hours.
Interpretation: Gently tilt tube to detect clot formation (+ result). Reexamine negative tubes after overnight
incubation at room temperature.
Sources of error:
Read slide test within 10 secs to avoid false-(+) results.
False (+) or false (=) results with nonsterile plasma
False-negative tube tests may occur with S. aureus strains that lyse clot after prolonged incubation.
Methicillin-Resistant S. aureus (MRSA): Often slide coagulase negative and tube coagulase positive
Colonies from media with a high salt content (MSA) more
likely to autoagglutinate. Best to use colonies from BAP.
Latex agglutination Test principle
Latex agglutination (LA)test.
Many clinical laboratories replaced slide and tube coagulase tests
with commercial LA test kits.
Detects clumping factor, protein A (a S. aureus cell wall antigen), and possibly capsular polysaccharides.
False (=): possible with MRSA.
False (+): possible with other Staphylococcus/Micrococcus species.
Passive hemagglutination/fluorogenic methods also available
Novobiocin susceptibility test principle
Used to presumptively ID S. saprophyticus in urine cx.
Principle: Agar plate inoculated for confluent growth with test MO. Paper disk with novobiocin (an antibiotic) placed on agar. Novobiocin diffuses into the agar and produces a gradient
of changing drug concentrations. If test organism inhibited by drug, a zone of no growth (zone of inhibition) occurs around the disk.
Procedure:
Make broth suspension (0.5 McFarland turbidity standard).
Test MO swabbed onto agar plate (BAP or Mueller-Hinton).
Place paper disk with 5-g novobiocin onto inoculated
area.Incubate plate incubated for 18-24 hours at 35 C.
Interpretation:
Susceptible: Zone of inhibition > 16 mm in diameter.
Resistant: Zone < 16 mm (presumptive S. saprophyticus).
Source of error: Rarely other staph species resistant.
Ornithine decarboxylase test principle
Perform on coag-neg (tube coagulase) staphylococci isolated from blood cultures; presumptively ID S. lugdunensis.
Principle: Checks if MO can degrade ornithine (amino acid).
Decarboxylases cut carboxyl (COOH) from amino acids → amines.
Amines make environment alkaline
pH indicators detect pH change
Procedure:
Inoculate organism to Moeller’s broth with ornithine.
Control tube (containing Moeller’s base medium) is
inoculated.Cover each broth with sterile mineral oil (produces
anaerobic conditions).Incubate tubes.
Interpretation:
Positive: Amino acid tube = purple & control tube = yellow (presumptively S. lugdunensis).
Negative: Yellow in both tubes.
What are some other ID tests?
Automation (PCR) with rapid multi test systems.
Used for genetics and getting the ID down to the species.
Micrococcus Characteristics
Catalase +
Coagulase -
Large GPC (often tetrads/four).
Media growth: lemon yellow color common.
Very similar to Staph (skin, mucus membranes, environment).
Will rarely cause disease, normal contaminant.
Bacitracin: S
Mod Oxidase test principle
Detects cytochrome oxidase.
Enzyme oxidizes tetramethyl--phenylenediamine (oxidase
reagent) to form indophenol.
Modified by adding dimethyl sulfoxide (DMSO) which makes
bacterial cells more permeable to the oxidase reagent.
Perform by rubbing test MO onto paper disk with reagent.
Positive: Blue color within 30 seconds
Rothia (Stomatococcus) mucilaginosus characteristics
Normally in oral cavity.
GPC that form clumps and tetrads. Is encapsulated.
Catalase -
Media growth: sticky staph (strongly adheres to agar surface), gray-white mucoid.
Is an opportunist, not very commonly seen.
Cheat sheets for Staphs

Big cheat sheet.
Flow chart of staphs
Streptococci characteristics
GPC in pairs and chains
Growth conditions: Faculative anaerobe.
Catalase -
What is the main way to ID the streps?
Serogrouping: helps identify many strep (but not all):
Based on C carbohydrate in cell wall (Lancefield grouping).
Latex agglutination, EIA or coagglutination technology.
Human serogroups: A, B, C, D, F, & G.
May have multiple organisms in same group, thus additional testing may be necessary for clinically significant isolates.
Physiologic properties:
Biochemical reactions
Organism given a genus and species name.
Hemolytic activity: gamma, alpha, beta.
Common names (lab jargon): beta strep, alpha strep, and pneumococci.
Strep pyogenes (Grp A strep)
aka GAS
Hemolysis: Beta (fullc learing). Stab the plate to see this more clearly.
Toxins:
Streptolysin S: oxygen stable,
Streptolysin O: oxygen labile (needs no oxy or won’t be seen)
This is why all of the suspected strep plates are put in the CO2 incubator.
Colony morpho: Small, translucent, large beta hemo.
No normal habitat.
A disk S: (once this is known you can prelim it as GAS).
SXT disk: R
PYR:
Think of how a forest fire can clear a lot of land for the hemolysis.
Diseases:
Suppurative (pus forming) and toxic diseases:
Pharyngitis (“strep throat”), scarlet fever, bacteremia.
Skin infections/pyoderma (e.g., erysipelas, cellulitis & impetigo).
Streptococcal toxic shock syndrome (tsmpons).
Nonsuppurative sequelae:
Rheumatic fever.
Acute Glomerulonephritis.
When testing is done for detection, always verify negative results with a culture.
What are the Beta hemolytic streps?
S. pyogenes,
S. agalactiae
Grps C, F, G
Bacitracin (A disk) test priniciple
(“A” disk identifies group A strep)
Principle: Low concentration bacitracin (antibiotic) inhibits GAS
Procedure:
Inoculate BAP for confluent growth.
Paper disk (0.04 units of bacitracin) placed onto plate.
Incubate overnight..
Interpretation: Susuceptible (S) = Any zone of inhibition
Reporting results: Bacitracin susceptible -hemolytic strep may be reported as “Presumptive group A streptococci”.
Sources of error (SOE):
Test only Beta-hemolytic strep; some alpha-hemolytic strep (S).
False (+) with some group B, C, & G streptococci.
GAS rarely bacitracin resistant.
Many labs have replaced the A disk with PYR & serogroup antigen tests.
Trimethoprim-sulfamethoxazole (SXT) disk susceptibility test principle
Principle:
Groups A and B streptococci (R).
Groups C, F, and G are (S).
Some labs use media containing SXT to select for GAS.
Procedure: Same as A disk
Interpretation: S = any zone
PYR (L-pyrrolidonyl--naphthylamide) hydrolysis test principle
Principle
L-pyroglutamyl aminopeptidase
L-pyrrolidonyl--naphthylamide ⎯⎯⎯⎯⎯⎯→ Free -naphthylamine
Free -naphthylamine + N,N dimethylaminocinnamaldehyde → Red color
Procedure:
Place test MO onto disk with PYR; wait a few minutes.
Add developing reagent.
Interpretation:
Positive: Red.
Negative: No color change.
Source of error:
Variety of other MO PYR (+).
Interpret in conjunction with MO’s gram stain, colonial morphology, type of hemolysis, & catalase reaction.
S. agalatiae (Grp B strep).Think of clouds with the hemolysis being the rain directly underneath.
Hemolysis: Beta, some gamma. The hemo is normally directly indernesth the MO.
Colony morpho: small, flat, cramy white.
NF of genitourinary tract.
Very dangerous in babies (can cause meningitis/sepsis). This is why the GBS is done in prenatal care so it can be treated.
A disk: R
SXT: R
PYR: +
How is the GBS screening test done?
Use enrichment broth:
LIM Broth (Todd Hewitt broth w/ colistin & nalidixic acid)
Trans-Vag Broth (Todd Hewitt broth w/ gentamicin & nalidixic acid + the addition of 5% defibrinated sheep blood).
If growth, subculture to BAP at 24 hrs; hold broth for 48 hours.
CAMP test principle
Principle:
Some S. aureus produce “beta-lysin” (a toxin).
GBS produce “CAMP factor”.
CAMP factor + beta-lysin = arrowhead-shaped area of
enhanced hemolysis.
Procedure:
Streak S. aureus down the middle of a BAP.
Streak test MO perpendicular to staph streak, incubate.
Interpretation:
Positive: Arrowhead-shaped hemolysis (GBS).
Negative: No enhancement (Most other streptococci).
Sources of error:
False (+): Some GAS (10%) CAMP positive.
False (=): Approximately 2% of GBS CAMP negative.
Not all S. aureus strains produce beta-lysin.
Run (+) & (=) controls each time test performed.
Hippurate Hydrolysis test principle
Principle: Can detect the glycine and sodium benzoate.
hippuricase hippurate ⎯⎯⎯⎯⎯→ sodium benzoate + glycine
Sodium benzoate detection: Special broth + MO incubated overnight
(2) Test supernatant by adding 7% ferric chloride
(3) Positive: Precipitate after 10 minutes
(4) Negative: Precipitate clears within 10 minutes
sodium benzoate + 7% Ferric chloride ⎯⎯⎯→ precipitate.
Glycine detection:
Place disk with hippurate in tube with water.
Add test MO, incubate for 2 hours, add ninhydrin.
Positive : Blue/purple color after 10 mins.
Negative: No color change
glycine + ninhydrin ⎯⎯⎯→ blue/purple color.
Sources of error (SOE):
Sodium benzoate method: Reading too soon.
Ninhydrin method: Wrong media
Other MO hippurate (+); Interpret in conjunction with MO’s gram stain, catalase, & colonial morphologly.
Grp C, F, and G streptococci
Made of several species.
Hemolysis: Beta
NF: Resp, gastro, vag.
Many diseases (Grp C + G can cause pharyngitis).
Grp D streptococci (
Hemolysis: Alpha, gamma.
NF in gastrio.
Split into two groups: Enteroccus and non-enterococcal grp d strep.
Enterococcus is much more resistant to antibiotics.
S. gallolyticus (previously s.bovis) found in humans. (is non-enterococcal GDS). Is associated with colon cancer.
Bile-esculin test principle
Principle: Determines ability to hydrolyze esculin if 40% bile present. High bile concentration inhibits many organisms
Agar has ferric ions:
esculin ⎯40% bile→ esculetin + glucose
esculetin + ferric ions ⎯⎯→ black color
Procedure: Inoculate agar (tube or plate) & incubate
Interpretation:
Positive: Black (Nonenterococcal GDS & enterococci)
Negative: No color change
SOE: some viridans strep > faint black color if heavy inoculum.
6.5% bile esculin test principle
Principle: Determines MO’s ability to grow in 6.5% NaCl (Some manufacturer’s include pH indicator).
Procedure: Inoculate broth tube with 6.5% Na Cl, incubate.
Interpretation:
Positive: Turbidity or acid pH.
Negative: No growth or pH change.
Enterococci: Bile-esculin (+); 6.5% salt (+)
*Nonenterococcal GDS: Bile-esculin (+), 6.5% salt (=).
Source of Error:
Some other gram (+) MO may grow in 6.5% salt.
Do bile-esculin test at same time as the 6.5% salt test.
Enterococcus
E. faecalis and E. faecium are most common isolates.
Hemolysis: gamma or alpha, rare beta.
Colony morpho: small grey, shiny.
NF in oral/gastro tract.
Disease: variety, antimicrobial R is a big concern.
Catalase -
Bile esculin + 6.5% salt +
PYR hydrolysis +
S. pneumoniae
Pneumococcus
GS: lancet shaped GPC in pairs, kinda like 2 footballs.
Growth needs: CO2 optimal.
Hemolysis: Alpha.
Colony morpho:
Mucoid: Large amounts of polysaccharide capsule (> 80 types).
Umbilicated: Depressed center w/ raised edges, indented.
Due to autolytic enzymes.
Young colonies = small mounds. Depressed center as age.
NF in upper resp tract.
Most common cause of community acquired bacterial pneumonia. Other variety of diseases (meningitis, pneumonia, otitis media).
Used to ID with the capsule.
Optichin S test principle
P disk (P disk is used to identify pneumococci).
Principle: Ethylhydrocupreine hydrochloride (optochin) inhibits pneumococci.
Procedure: Same as A disk; incubate in CO2 overnight.
Interpretation:
Zone depends on disk size (6 mm or 10 mm).
Susceptible: Zone > cutoff size.
Resistant: No zone.
Questionable results:
Zone present but < cutoff size
Do bile solubility test
P disk (S) alpha-hemolytic strep = “Presumptive pneumococci”
Bile solubility test principle
Principle: Bile salts (sodium deoxycholate and sodium taurocholate) accelerate autolysis.
Broth method:
Turbid suspension of MO prepared in broth or saline.
Divide equally into 2 tubes; Add bile to 1 tube.
Incubate both tubes at 35 C for 2-3 hours.
Positive: Clearing (lysis of the cells) S. pneumoniae (+).
Negative: Turbidity (no lysis).
Plate method:
Add bile to colonies on BAP; incubate.
Positive: Lysed colonies (pneumococci).
Negative: Intact colonies.
Sources of error:
(1) Mixed cultures.
(2) Nonpneumococcal colonies may be lifted off the plate.
Quelleng reaction test principle
Principle:
IDs isolate as pneumococcus & determines its capsular type.
Specific capsular ABY + encapsulated cells → ABY-AGN
reaction - gives appearance of swelling (optical illusion).No “swelling” if ABY does not match capsular antigen.
Polyvalent ABYs react with many capsule types.
Procedure:
Capsule antibody + suspension of pneumococci.
Second (control) suspension is left untreated.
Examine wet mounts of each suspension.
Interpretation:
Positive
(a) “Capsular swelling” in ABY treated cells and
(b) No “swelling” in the untreated cells.Negative: No “swelling”
Strep. viridans
several species
hemolysis: alpha or gamma.
NF in Resp tract, gastro, skin.
Diseases: vary, endocarditis in pts with damaged/artificial heart valves, called subacute bacterial endocarditis.
Usually ID by rule out.
Alpha or gamma-hemolytic strep = viridans streptococci if they are NOT enterococci,
GDS not enterococci, pneumococci, or group B streptococci.
Strep anginosus
Formerly S. milleri.
Included with 3 names: S. anginosis, S. constellatus, & S. intermedius.
Hemolysis: all of them. Will be minute colonies.
Microaerophilic, use increased C02.
NF in oral cavitiy, gastro, vag.
Satelliting streptococci
Other names: Nutritionally deficient, Thiol requiring, Nutritionally variant, Pyridoxal dependent, Vitamin B6 dependent, Symbiotic streptococci.
Resemble viridans streptococci, but genetically distinct.
Current terminology: Abiotrophia and Granulicatella.
NF in Upper resp, urogenital, gastrointestinal tract.
Diseases: Endocarditis + others.
How are Satelliting strep grown?
Nutritional requirements:
Require vitamin B6 (pyridoxal)
b) Will not routinely grow on BAP or chocolate agar (CHOC)
c) Can add pyridoxal to growth medium or
d) Can be supplied by another organism growing on the agar plate
Satellite procedure
a) “Deficient” organism spread onto the surface of an agar plate.
b) Single streak of 2nd organism (usually a staph) made through area
c) Incubate overnight incubation CO2
d) Faint growth or tiny colonies near the “staph streak”
e) Staph makes excess nutrients which allow “deficient” strep to grow.
Clinical specimens: Mixed cultures; tiny colonies near big colonies'.
Haemophilus: GNB
Satelliting strep: GPC
Blood cultures: BC media contains pyridoxal & allows satelling strep to grow.
Perform “staph streak” procedure when:
Blood culture broth shows GPC &
No growth when subcultured onto BAP or CHOC.
Identification:
Report of “satelliting streptococci” is usually sufficient.
Complete identification by reference labs.
Differentiate from other strep because they may be more R to penicillin.
What are the streptococcus like spp.?
Genera:
Aerococcus
Gemella
Lactococcus
Pediococcus
Leuconostoc
Resemble viridans streptococci and enterococci.
Hemolysis: Alpha or gamma
NF: Human mucous membranes and the environment.
Diseases: Rare, but variety do occur
Catalase =, but a few weak +/
Vancomyocin R: Pediococcus, leuconostoc.
Vancomyocin S: Aerococcus, Gemella, Lactococcus.
There are Nucleic acid tests for further ID of them.
What are the most commonly isolated streps?
GAS
GBS
Grps C, F, G
Enterococci
GDS
Pneumococci
Viridans strep
What are the alpha hemolytic strep? (or the most likely Bacteria) And what are the tests to ID?
Most likely organisms are:
Enterococci
Group D streptococci
Pneumococci
Viridans streptococci
Appropriate tests include:
P disk or bile solubility
Bile-esculin hydrolysis
6.5% salt tolerance
PYR
What is the most likely bacteria and what test would you perform for Beta hemolytic strep?
Most likely organisms are:
GAS
GBS
Groups C, F, or G streptococci
Appropriate tests include:
A and SXT disks
PYR
CAMP or hippurate hydrolysis
Serogrouping
What is the most likely bacteria and what test would you perform for Gamma hemolytic strep?
Most likely organisms are:
GBS
Enterococci
Group D streptococci
Viridans streptococci
Appropriate tests include:
CAMP or hippurate hydrolysis
Bile-esculin hydrolysis
6.5% salt tolerance
PYR
Bacillus spp. characteristics.
Spores are heat and chemical resistent.
Gram positive rods/bacilli
The spores don’t stain in the GS, will appear as holes in cells or refractile.
Culture conditions: Aerobic, faculative anaerobics.
Media: BAP, possible PEA.
Bacillus anthracis and cereus are most important.
Bacillus anthracis
Found in soil, plant material. Spores viable for decades.
GS: large gram positive bacilli. Won’t normslly see spores in direct ID from specimen.
Hemolysis: gamma
Motility: nonmotile
Catalase: positive.
Colony: rapid aerobic growth on BAP.
Medusa head (filamentous growths).
Ground glass look.
Tenacious-look like egg whites when lifting edge of colony and it stays upright.
String of pearls (chains of spherical baccilli), forms when incubated for several hours on agar with penecilli.
Must be on alert for B. anthracis with healthy pts that rapidly decline with resp illness or cutaneous ulcer.
If presumptive ID of B. anthracis, must notify public health staff and be worked up to isolation and full ID.
Bacillus anthracis diseases
Anthrax usually in herbivores. Human infection rare in U. S. Associated with imported animal products (wool, hair, or hides).
Cutaneous: most common form; black eschar skin lesion
Two other clinical forms are inhalation & gastrointestinal
Specimens: Skin lesions (eschar), sputum, feces, oropharyngeal ulcer,
CSF, and blood.
Laboratory hazard:
Wear PPE (gowns and gloves).
Work in a biosafety cabinet.
Decontaminate work areas.
BSL-3 recommended for activities w/ high potential for aerosol
or droplet production.
What is the laboratory response network?
Consortium & partnership of laboratories
b) Provide immediate & sustained laboratory testing &
commununication in support of public health emergencies
c) Particularly in response to acts of bioterrorism
LRN laboratories are designed at sentinel, reference & national:
Sentinel labs: Function at the local front line (e.g.,
hospitals); provide “presumptive identication – recognize,
rule out and refer”.Reference labs: Includes local local & state public health;
provide confirmatory testing.National labs are Federal labs that have BSL-4
containment facilities such as the CDC; work w/ agents
such as Ebola, smallpox virus, etc.; provide definitive
characterization.
Packaging critical biologic agents: Requires “triple” packaging:
primary receptacle.
water tight secondary packaging.
durable outer packaging.
Appropriate labeling of the package.
Documentation of hazardous contents.
NAAT ID also available.
Bacillus spp (other than B. anthracis)
Found in the environment.
Usually a infectious oppurtunistic.
B. cereus causes food poisining and tissue infections (from toxins).
GS: large boxy GPR.
Colony: large irregular flat, beta-hemolytic.
Spore forming under aerobic conditions, must use biochemical tests for spp. ID.
Hemolysis: Beta
Motility: motile
Catalase: variable.
When will you test a gram negative rod for actually being a gram positive rod.
Some strains confused with nonfermentative GNR:
Stain gram-negative.
Colony morphology not typical.
Spores may not be present.
Some grow slightly on EMB or MAC.
Suspect Bacillus when a “GNR” :
does not fit ID schemes
Encourage spore formation by extended incubation
Vancomycin susceptibility
(a) Most Bacillus (S).
(b) Most gram-negative nonfermenters (R).
Corynebacterium spp.
GS:
GP non-spore-forming pleomorphic rods (tapered, curved, club shaped. coryne is greek for club).
Chinese letters (V, L, Y, palisades).
Diptheroids or coryneforms: includes Corynebacterium, Aacronobacterium, Rothia.
Note: “corynebacteria” refers to the genus Corynebacterium; “coryneform” refers to
irregularly shaped GPR. Corynebacterium one of several coryneforms.
Growth conditions: aerobic, faculative anaerobics. small colonies on bap at 35C.
NF: Skin, resp tract, GI.
Non-motile.
Catalase: +
Corynebacterium diptheriae diseases
Disease: Diptheria.
Usually infects upper respiratory tract; sometime skin. Has toxigenic strains (toxin produced).
Infected with beta-phage (a virus) which has toxin gene. Causes upper respiratory tract and systemic damage.
Pseudomembrane may block airway.
Nontoxigenic: Pharyngitis or a less severe diphtheria.
Prevent through immunization with toxoid.
Specimens: Throat, nasopharyngeal, and wound material.
Corynebacterium diptheriae
Has typical coryneform morphology, is not specific for C. diptheriae.
Can do the methlyene blue stain: Stain smears from specimens or cultured MO
Observe for metachromatic granules.
Reddish purple to deep blue, intracellular granules.
May be in other Corynebacterium species.
Media: BAP, tellurite, Loeffler/Pai agars.
Elek test: positive for the toxin.
What are the agars used for C. diptheriae and why?
BAP: General purpose, nonselective medium grows C. diphtheriae, Group A, C & G strep & Arcanobacterium.
Tellurite media: Cystine-tellurite medium and Tinsdale agar.
Contain tellurite; Selective and differential.
Tellurite inhibits many normal flora MO.
Most corynebacteria not inhibited; gray to black colonies.
Other MO can also make black colonies.
Tinsdale: C. diphtheriae: Black colonies with brown halo. A few other Corynebacteria also have this colony.
Loeffler medium:
Serum + other factors.
Promotes C. diphtheriae growth & metachromatic granules.
Pai agar, an egg-based medium, may be used instead of Loeffler’s.
Elek test principle
Tests C. diphtheriae for toxin production.
Principle: Immunodiffusion test. Line of precipitate forms in test agar when diphtheria toxin interacts with diphtheria antitoxin.
Procedure:
Saturate strip of filter paper with diphtheria antitoxin
Embed in special agar medium.
Test MO, (+) & (=) control strains streaked onto agar.
Streaks parallel to each other & perpendicular to filter
paper.Incubate overnight at 35 C.
Interpretation:
“Arc of identity”indicates test MO is toxigenic.
No “arc” if nontoxigenic
Crynebacterium other than C. diptheriae
Is a NF of human, mostly is a contaminant. But can cause serious infections.
Presumptively ID with:
Catalase +
GS: small GP, pleomorphic rods.
Colony: small, white, grey or yellow.
Hemolysis: usually non-hemolytic.
What are the other 2 spp. of Corynebacterium that are watched?
C. jeikeium: Formerly known as CDC Group JK.
Serious infections in immunocompromised patients.
Presence of catheters/prosthetic devices contribute to
infection.Causes septicemia and endocarditis – most common cause of diphtheroid prosthetic endocarditis in adults.
Resistant to many antibiotics.
C. urealyticum:
Resistant to antibiotics.
Associated with urinary tract infections.
MO hydrolyzes urea & cause stones in urinary tract.
Listeria monocytogenes
GS: small gpr, no spores. might even look coccobaccillary or coccoid.
Culture: faculative anaerobic, will grow on routine media.
NF: environment, vagina, intestines of healthy humans.
Diseases: in pregnant women to the baby from meats and dairy. Also immunocompromised and elderly.
Specimens: CSF, blood, vaginal secretions.
Hemolysis: Beta, remove colony to see.
Catalase +
Bile esculin: Positive.
Motility: motile at RT (25) (Umbrella) and reduced motility at 35 (tumbling appearance).
This can be confused with GBS and Corynebacterium.
Erysipelothrix rhusiopathiae
GS: GP, pleomorphic, no spores.
Culture: Faculative anaerobic, grows on routine media.
Found: soil, water, animals (swine).
Disease: Mostly animal pathogen and animal contact. Forms Erysipeloid (red skin lesion). May disseminate.
Specimens: skin and infected sites.
Catalase =
Hemolysis: Alpha, gamma
Motility: non-motile.
H2S production on TSI agar: +
Lactobacillus
GS: GPR, no spores, long rods in chains.
Culture: BAP, CHOC. atmosphere varies with strains.
Hemolysis: Alpha, gamma (just like listeria).
NF: oral cavity, GI, Female genital tract.
Diseases: rare.
Catalase =
Motility: non-motile
Use anaerobic media and procedures for full ID.
Arcanobacterium haemolyticum
Diseases: may cause pharyngitis, wound infections, bacteremia.
GS: GPR
Hemolysis: Beta.
Catalse =
Complete ID with Biochemical testing.