LIQUIDS | 3.3
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Last updated 11:53 AM on 10/4/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
1
New cards
Vapor Pressure
> pressure exerted by the gas that is in equilibrium with its liquid form at a definite temperature
> stronger IMFA, lower VP
2
New cards
high VP
evaporates more quickly
3
New cards
low VP
does not evaporate easily
4
New cards
Volatile
liquid that readily evaporates
5
New cards
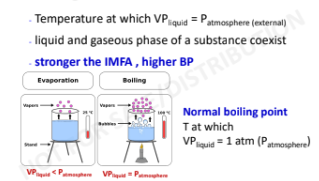
Boiling point
6
New cards
Stronger IMFA, Lower VP
Stronger IMFA, **** VP
7
New cards
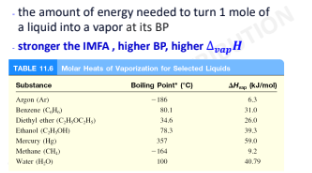
Heat or Enthalpy of Vaporization
8
New cards
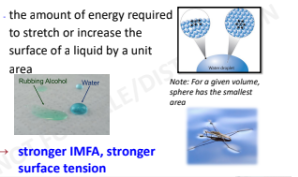
Surface Tension
9
New cards
Capillary Action
spontaneous rising of a liquid in a
tube of slender bore (capillary)
10
New cards
Adhesive Forces
IMFA between unlike molecules (liquid and the capillary wall)
11
New cards
Cohesive Forces
IMFA between like molecules (with its own)
12
New cards
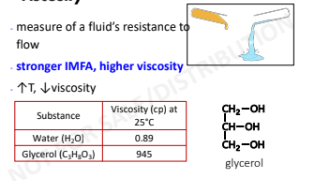
Viscosity