2 - X-ray Diffraction
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
What is an x-ray?
X-ray is a penetrating form of high-energy electromagnetic radiation.
Most X-rays have wavelengths (λ) of 10-12–10-8 m, typically in Å range. Corresponds to frequencies (υ) of 3×1016–3×1019 Hz, and energies (E) of ~0.124–1240 keV.
E = hυ
Energy = Planck’s constant × Frequency
with h = 6.626 × 10-34 J.s
λυ = c
Wavelength × Frequency = speed of light
with c = 3 × 108 m s-1
And 1 eV = 1.6022 × 10–19 J
E = hc / λ → 12.4 keV @ 1 Å
What are the two types of x-ray?
Production of X-rays involves bombardment of target with energetic e-. These e- undergo collisions and scattering during slowing down, resulting in production of Bremsstrahlung and Characteristic Radiation.
Bremsstrahlung (continuous X-ray): result from e- changing direction when travelling in proximity to target nucleus.
· Energy of emitted X-ray photon is subtracted from kinetic energy of incoming e-.
Characteristic X-ray: result from incoming e- knocking out e- from atomic shell of target (KE incoming e- > binding energy shell e-).
· Vacancy filled with e- from outer shell → emission of X-ray photon with characteristic energy, equals to difference in binding energies of shells involved.
What are the atomic shell layers and their corresponding x-ray?
Atom shells: K, L, 1, 2, 3, M, 1, 2, 3, 4, 5
• Kα X-ray is produced due to removal of K shell electron, with L shell electron taking its place. Kβ occurs when K shell electron is replaced by electron from the M shell.
• Lα X-ray is produced due to removal of L shell electron, replaced by M shell electron.
• Mα X-ray is produced due to removal of M shell electron, replaced by N shell electron.
→ characteristics X-ray are specific to each elements.
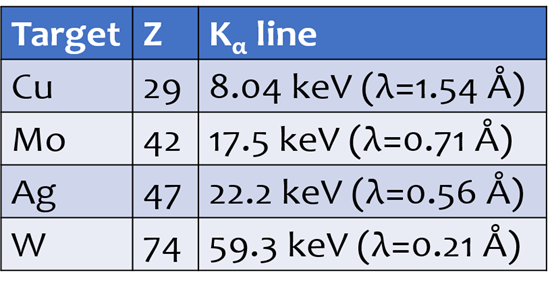
Define diffraction
Diffraction refers to various phenomena that occur when a wave encounters an obstacle or opening.
It is defined as the interference or bending of waves around the corners of an obstacle or through an aperture into the region of geometrical shadow of the obstacle/aperture.
What are the two types of interference?
When 2 sets of waves meet, they combine to produce a new pattern.
Constructive interference: 2 sets of waves meet in phase.
Occurs when path-difference between 2 waves is nλ, with n an integer.
Destructive interference: 2 sets of waves meet completely out of phase.
Occurs when path-difference between 2 waves is (n+1/2)λ, with n an integer.
What equations are associated with single slit diffraction?
Condition for minimum:
Sine = opposite/hypotenuse
→ sin(θ) = (λ/2) / (a/2)
→ (a/2).sin(θ) = (λ/2)
→ a.sin(θ) = λ
Generalised for any integer m
→ a.sin(θ) = m.λ
è Occurs because of path length difference.
What equations are associated with diffraction grating?
Here again, it’s all to do with path length difference.
When path difference between 2 waves is multiple of λ, waves in phase → maximum intensity.
For monochromatic light:
n.λ = t.sin(α)
For example, for red laser light with λ = 650 nm and a grating with t = 1 μm, max. intensity at α = 40.5°.
What equations are associated with crystal lattice planes?
Distances between crystal lattice planes ~ X-rays wavelength ~ Angstrom.
→ sin(θ) = FG/d = GH/d
→ d.sin(θ) = FG = GH
→ 2d.sin(θ) = FG + GH
For constructive interference, total path length change between ABC & DGI (FG+GH) must be integer multiple of λ, or nλ.
So when FG+GH = λ = 2d.sin(θ) → 1st order constructive interference
Generalised to all orders: nλ = 2d.sin(θ) → Braggs’ law
What is the relationship between diffraction order n and lattice planes?
→ 1λ = 2dhkl.sin(θ1)
→ 2λ = 2d2h2k2l.sin(θ2)
→ nλ = 2dnhnknl.sin(θn)
• With monochromatic beam
→ angularly dispersive XRD
• With white light (i.e. synchrotron)
→ energy dispersive XRD
What are the two main applications of X-ray diffraction?
Single crystal X-ray diffraction
Detailed information about internal lattice of crystalline substances (mineral, molecules…).
ID of mineral from its X-ray diffraction pattern if all possible planes diffracted. Need to continuously rotate sample.
Powder X-ray diffraction
Can analyse single crystal or rock powder. Assumes powder contains a lot of small fragments with random orientation. Can get full set of reflections without needing to rotate sample.
How is an x-ray diffractometer used?
X-ray diffractometer
• Powder contains millions of micro-crystals randomly oriented with respect to the incoming beam, which will give rise to all possible planar diffraction events at angles θ.
• In practice, we focus incident X-rays on the target through a Soller slit and collect diffracted intensity by rotating detector, so we record θ the incident angle and 2θ the diffracted angle.
→ download and plot data
→ lookup DIF file
→ identify peaks; they correspond to diffraction on different lattice planes.
Example – Kamargaon meteorite powder
X-ray diffraction on powdered samples, using Cu Kα radiation (λKα ~ 1.54 Å), 2θ range of 15° to 65°, step size of 0.02° 2θ, and 1.2 sec / step.
Allows identifying main phases in the meteorite, which are olivine (forsterite - F), pyroxene (enstatite - E), and iron (kamacite - K).