Biochem Lec Ch3 (Ang Ganti ng Api)
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
What is an Enzyme?
biological catalysts that are protein in nature except ribosomes typically ending in the suffix -ase
ex: myosin ATPase causes ATP to split into ADP and P—also triggering myosin head to switch to high activation state making it bind to the actin filament then pull causing muscle shortening
Creatine Kinase MB as a cardiac marker for myocardial infarction
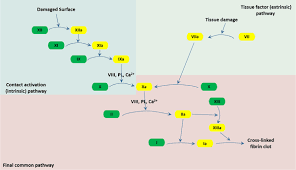
In the Coag cascade, most enzymes are serine proteases (like factor VIIa, IXa, Xa, and thrombin) that activate other proteins by limited proteolysis.
Are enzymes changed in the process? To what degree does it increase reactions?
No, 10^ 20
What do you call the protein part of an enzyme?
Apoenzyme
The inactive protein part of an enzyme without its cofactor. Coagulation enzymes circulate as inactive zymogens (apoenzymes) that require activation by cleavage
What is the non-protein part of an enzyme necessary for catalytic function? What are some molecules that act like this?
Cofactor; Zn2+ and Mg2+
In Coag Cascade Factor V and factor VIII serve as protein cofactors that form complexes with activated proteases to greatly enhance their activity.
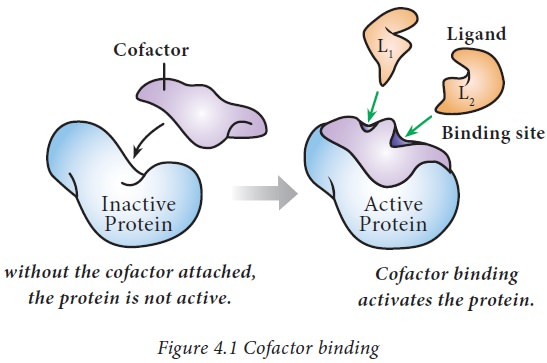
What is a non protein organic molecule typically a vitamin B that commonly acts as a Cofactor?
Coenzyme
These are organic molecules that assist enzymes in their catalytic activity. In coagulation, calcium ions (Ca2+) act as essential cofactors helping clotting enzymes bind phospholipid membranes, thus indirectly acting as coenzymes
What is a molecule that binds to another molecule called a receptor to send signals within or between cells?
Ligand
Molecules that bind specifically to proteins. For example, tissue factor acts as a ligand for factor VIIa, forming a complex essential for clot initiation.
The action that causes or accelerate (a reaction) by acting as a catalyst
Catalyze
What are compounds whose reaction an enzyme catalyzes?
Substrate
These are molecules upon which enzymes act. In the cascade, clotting factors in inactive forms (zymogens) such as factor X or prothrombin are substrates for activated enzymes that convert them into their active forms (e.g., factor Xa, thrombin).
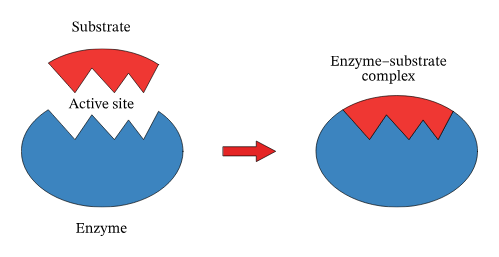
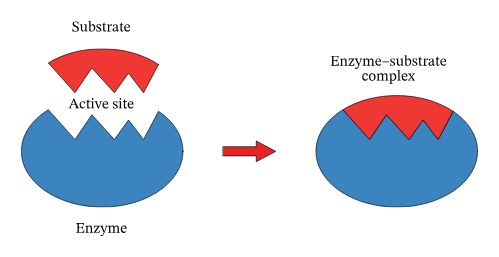
What is the specific site of the enzyme where a substrate binds during a reaction?
Active Site
A process that initiates or increase the activity of an enzyme?
Activation
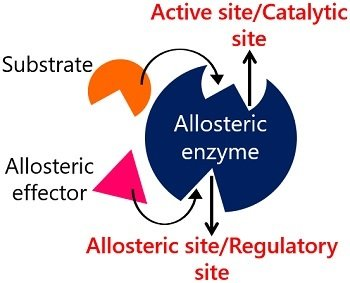
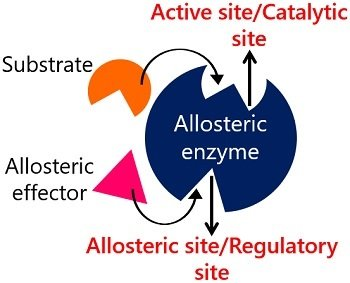
A portion of an enzyme where activators/inhibitors bind to regulate catalytic reactions?
Allosteric Site
The term "allosteric site" is called so because it comes from the Greek words "allos," meaning "other," and "stereos," meaning "solid" or "space." This implies that the allosteric site is a distinct or "other" site on an enzyme or receptor, separate from the enzyme's active site where the substrate normally bind
What is a thing that slows down the rate of reaction?
Inhibitor
Molecules that decrease enzyme activity. Examples in coagulation include activated protein C that inhibits factors Va and VIIIa, thus downregulating clotting.
In the Light Blue Tube, Citrate inhibits the chelation of Ca2+ (a coenzyme) but is reversible in comparison to EDTA
What is the term for an inactive form of enzyme?
zymogen or proenzyme
Formed upon the basis of structural complementarity of Substrate and Active site?
enzyme and substrate complex
What is a molecular entity formed when a central metal atom or ion is surrounded by one or more molecules or ions called ligands?
Complex
What is the specific enzyme that catalyzes the reaction of urea with water?
Urease
What reactions to Enzymes Catalyze? How do they Catalyze it? What is its implication in regards to Cells?
Enzymes are agents of metabolic function that catalyse thermodynamically favourable reactions at rapid rates, enabling cells to exert kinetic control over biochemical processes essential for life.
What does “Thermodynamically Favorable mean”?
if it naturally tends to happen on its own, without you having to constantly add energy to make it go.
Ex: Coagulation Cascade
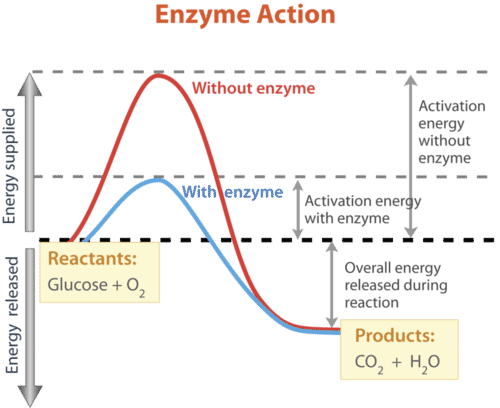
How does a catalyst work in increasing the reaction rate of a substance?
It lowers the activation energy of the reaction by binding and stabilizing to transition state
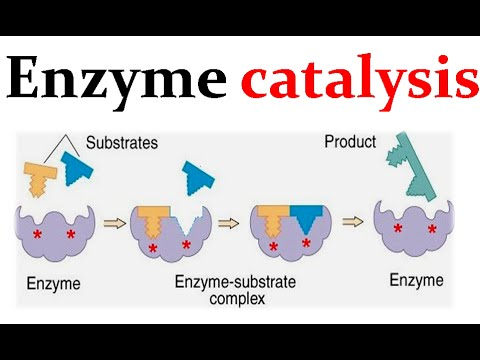
What is the lock and key model?
The lock and key model depicts the formation of the Substrate Enzyme Complex. The activation site (acting as the lock) contains amino acids that bind to the attracted substrate (key) via hydrogen bonding if Polar or Hydrophobic Interactions if Non-Polar. These reasons are secondary to Structural Complementarity. This also explains the high specificity of the reaction
What is the Induced Fit Model?
The active site becomes modified to accommodate the substrate. Shape of Active Site and Substrate should be closely similar
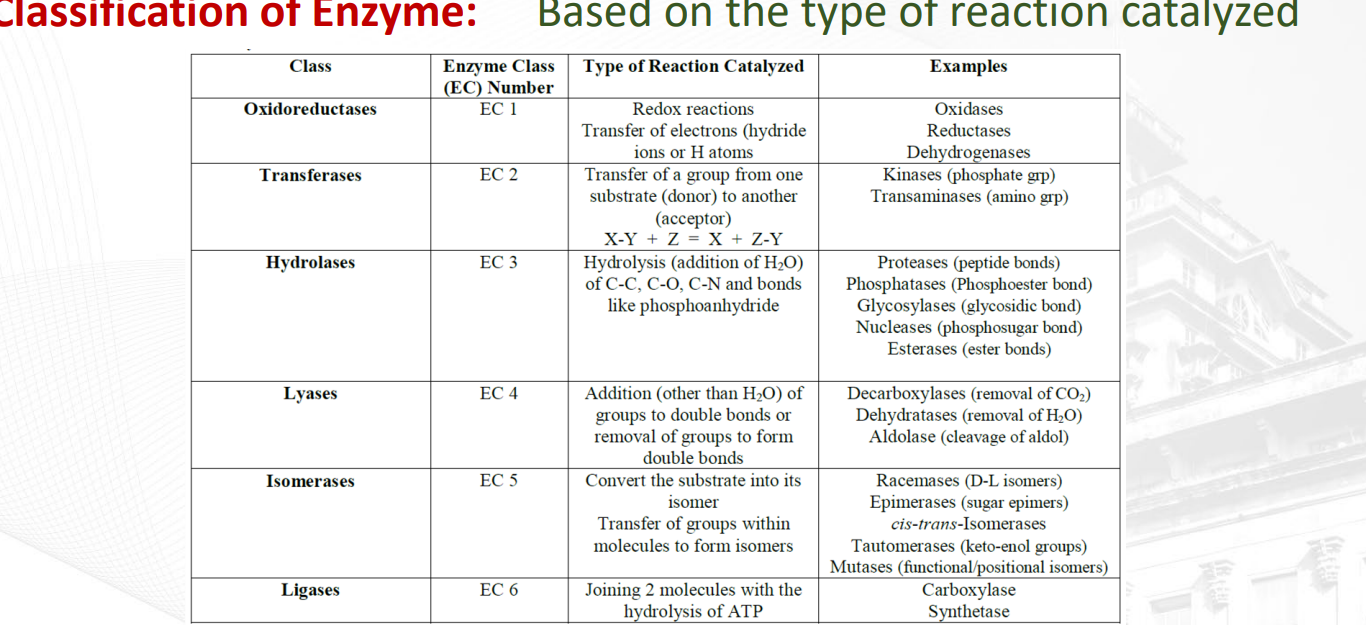
What are the six classes of enzymes? OTHLIL
Oxidoreductase
Transferase
Hydrolase
Ligase
Isomerase
Ligase
Oxidoreductase
Transferase
Type of inhibitor that resembles the structure of the substrate competing for the active site? How can it be reversed or counteracted?
Competitive Inhibitor, addition,
Types of inhibitor that binds to the allosteric site of the enzyme, can bind to both free enzyme and enzyme substrate complex, increase Vmax, and does not structurally resemble the ?
non-competitive/mixed inhibitor
How does a mix inhibitor “inhibit” the reaction?
Decrease the amount of enzymes
Km Increases, Vmax is Same,
competitive inhibitor
Km is Same, Vmax decreases
non-competitive/mixed inhibitor
Both Km and Vmax decreases
uncompetitive inhibitor
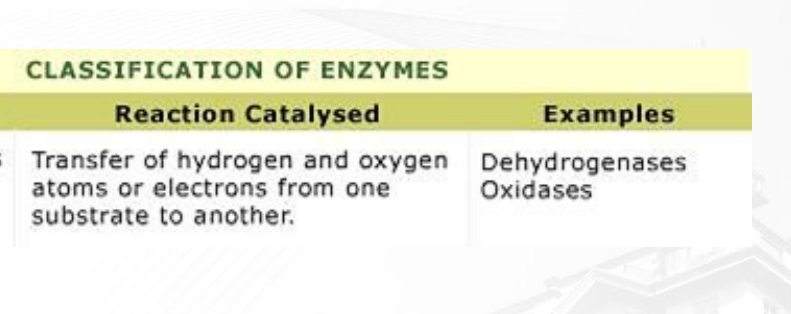
What oxidoreductase?
Involves transfer of Hydrogen or Oxygen atoms from one atom to another
example: Dehydrogenase (tanggal hydrogen), Oxidase (tanggal oxygen)
in the picture, two hydrogen atoms are transferred from NADH2 to Pyruvate forming Lactate + NAD+
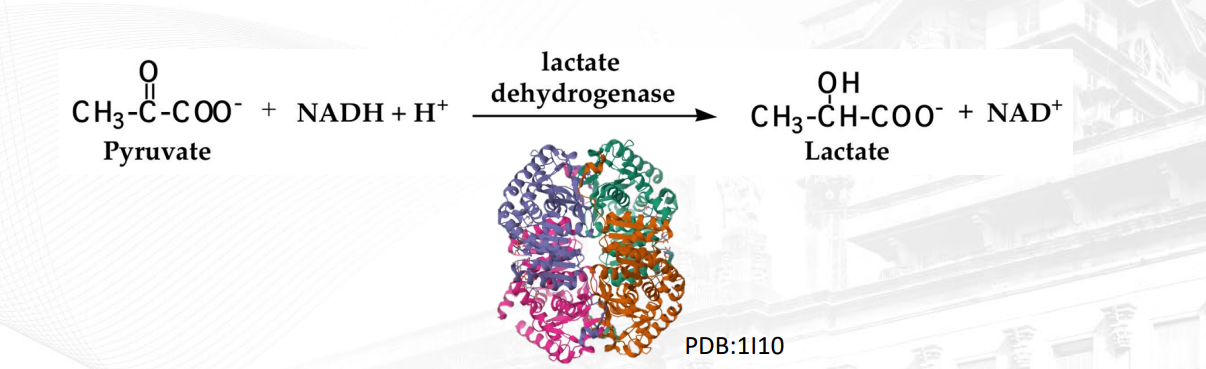
What does oxidation mean? What does oxidant mean?
Gain of Oxygen Gas, loss of hydrogen, loss of an electron.
is the substance that gains electrons and becomes reduced. By gaining electrons, it causes another substance to be oxidized. In this case it is the reactant FAD
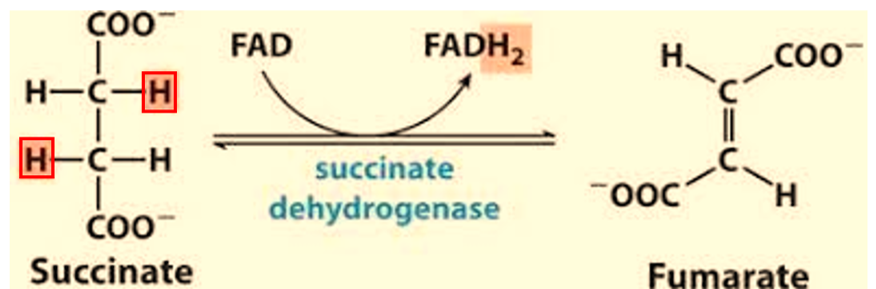
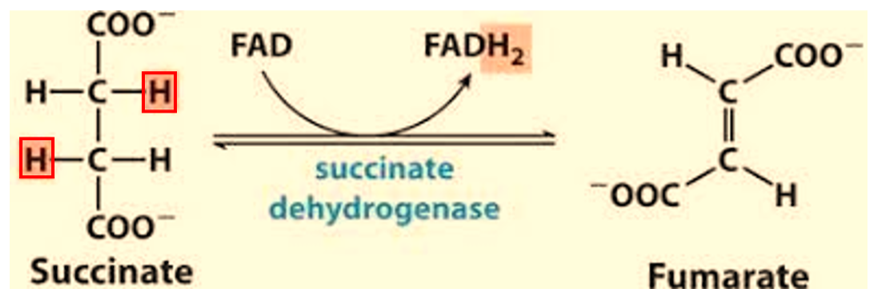
What does reduction mean? What does reductant mean?
Loss of Oxygen, Gain of Hydrogen, Gain of Electron.
is the substance that loses electrons and becomes oxidized. By losing electrons, it causes another substance to be reduced. In this case it is the reactant Succinate
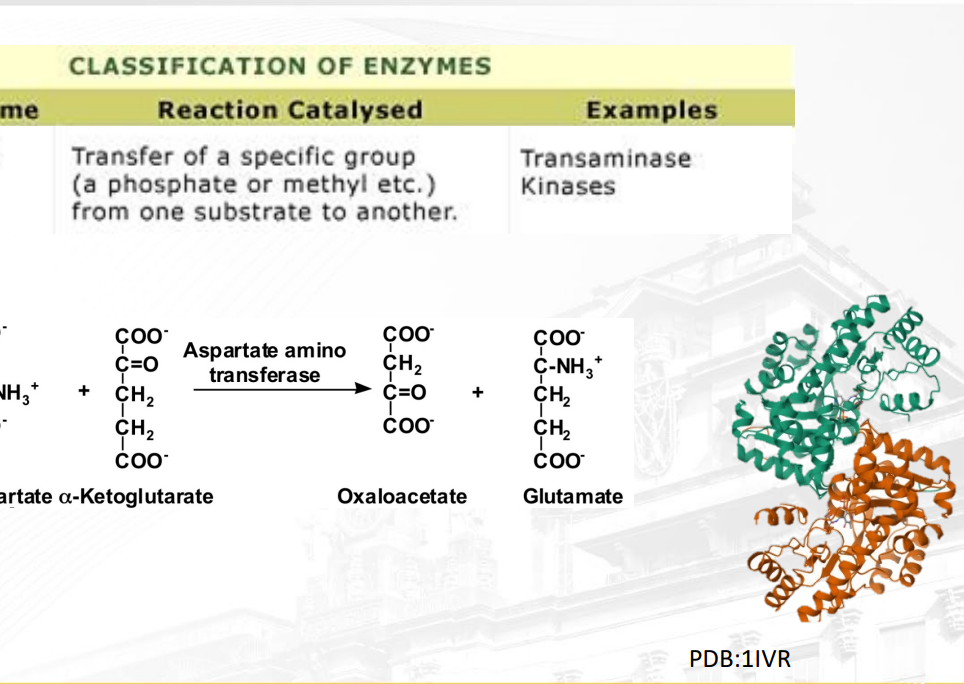
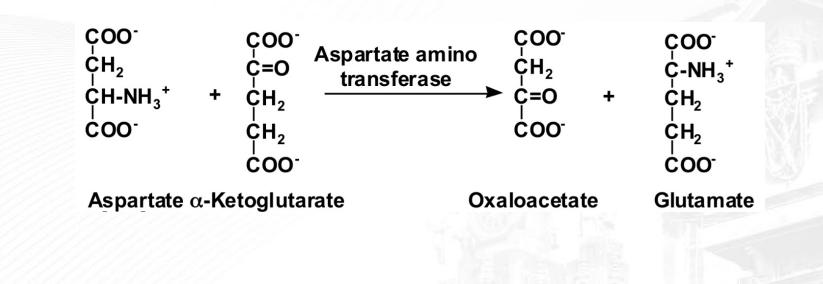
What is a transferase?
Transfer of a specific group from one substrate to another (displacement rxn). The picture shows double displacement of NH3 and O
ex: Transaminase (transfers amino groups to other amino acids or keto acids)
Kinase (addition of phosphate to substrate)
What is a Hydrolase?
Enzyme that initiates breakdown of a substrate in the presence of water
Ex: Lipase (lipid breakdown), Peptidase (peptide chain breakdown), Nuclease (Nucleic acid breakdown), Phosphatase (breakdown of phosphate), esterase (smaller lipids)

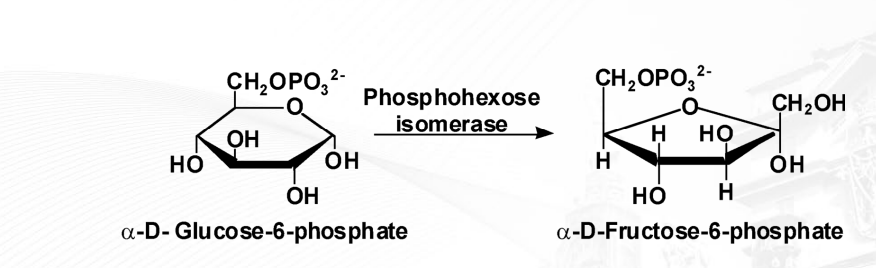
What are isomerases?
enzymes that change a substrate into one of its isomers, which means they rearrange the atoms within a molecule without adding or removing any atoms.
ex: phosphohexoisomerase (is an enzyme that catalyzes the reversible conversion of glucose-6-phosphate to fructose-6-phosphate)
Fumarase (function is to catalyze the reversible hydration of fumarate to L-malate)
What are lyases?
Enzyme that either breaks down or recombines stuff into the substrate without the presence of water (non-hydrolytic)
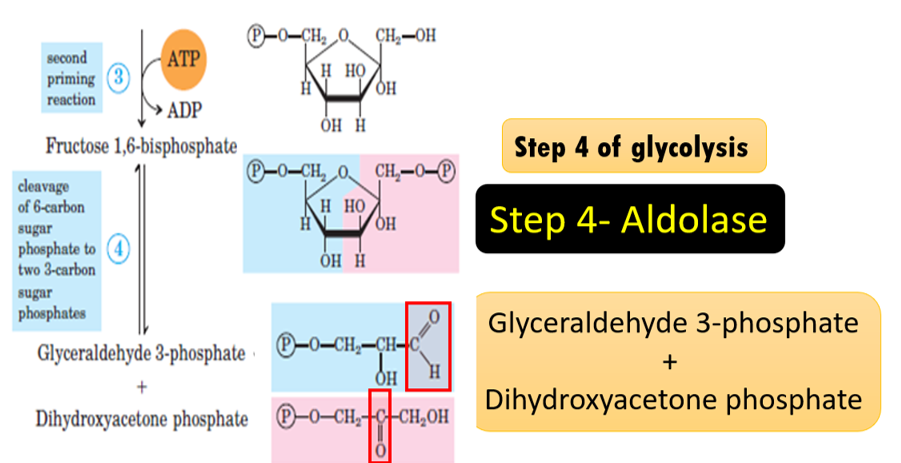
ex: Synthase, Aldolase a six-carbon sugar is split into two three-carbon molecules), Decarboxylase (removal of carboxyl group)
Types of isomerism
What are ligases?
Enzymes that bind two molecules together with energy expenditure

EC number meanings

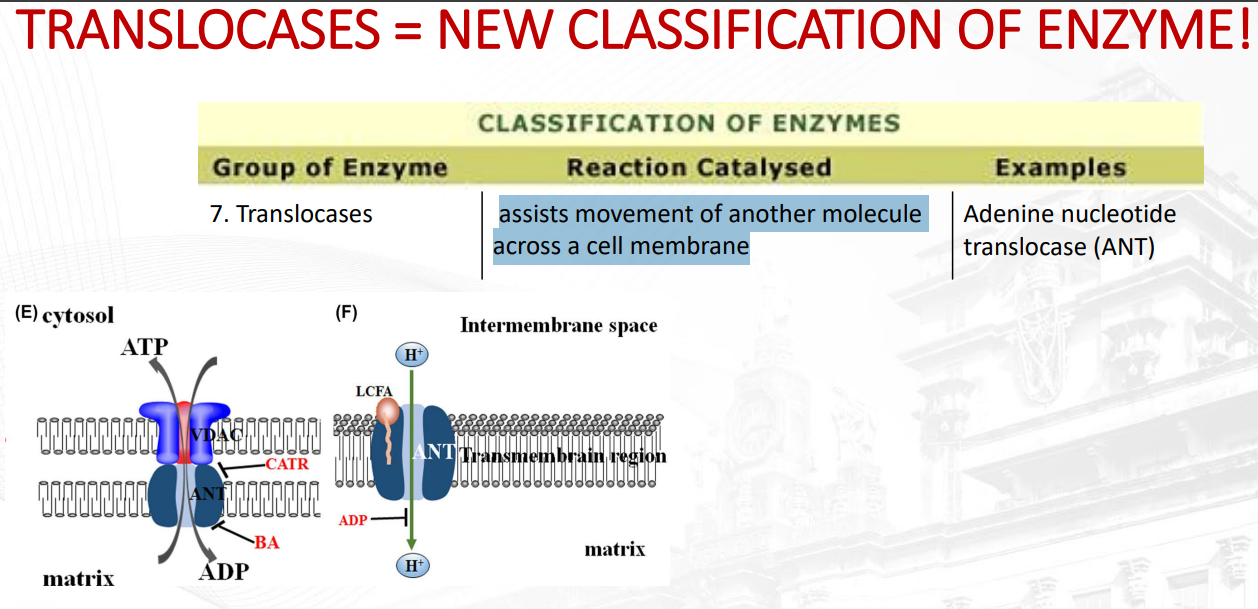
What is a translocase?
assists movement of another molecule across a cell membrane
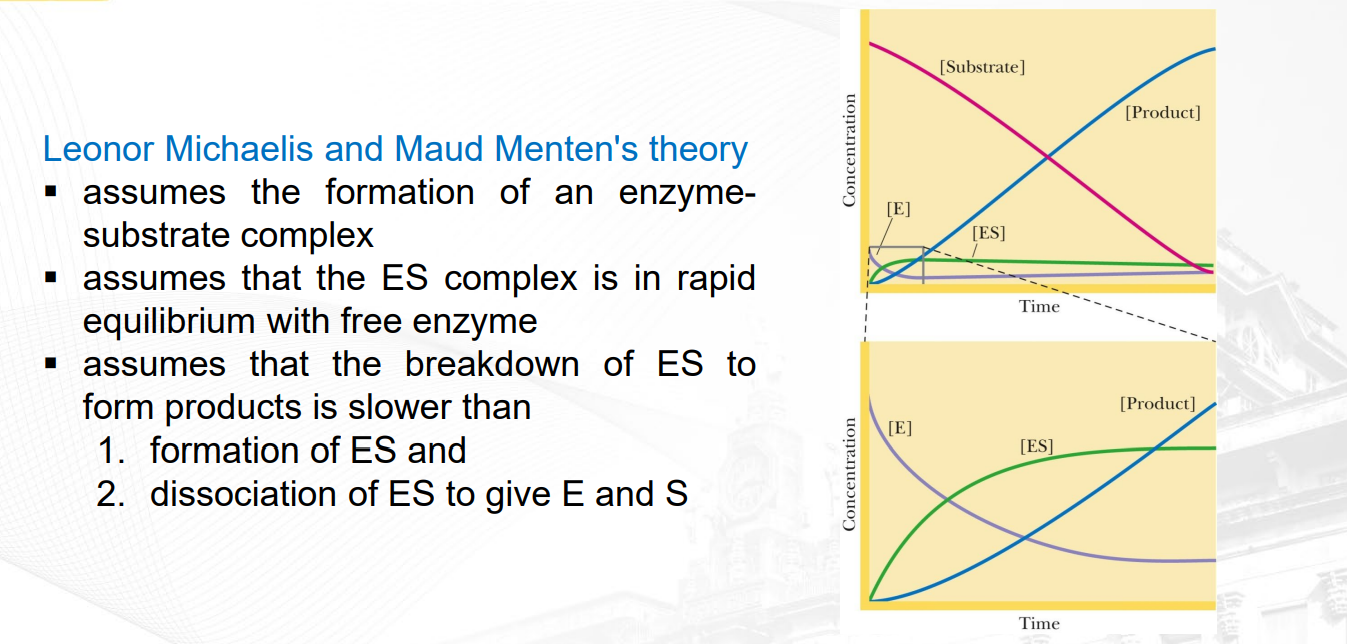
What is the assumption in the steady of state theory?
during an enzyme-catalyzed reaction, the concentration of the enzyme-substrate complex (ES) remains constant over the time course of the reaction. Which mean that the rate of breakdown of the ES complex whether to E + L or E to P is the same or constant
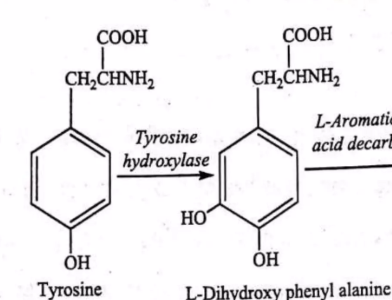
Tips on identifying whether an enzyme if an oxidoreductase (kasi mahirap mag bilang ng oxidation state) like here in Catecholamine synthesis
Key Clues: Involvement of NAD+/NADH, FAD/FADH2, O2, or H2O2 (pagdag ng Hydrogen = reduction; or Oxygen = Oxidation; Which is electron transfer)
Names like Dehydrogenase, oxidase, reductase, peroxidase.
Tip: If an OH group or O atom is added/removed, it's almost always an oxidoreductase.
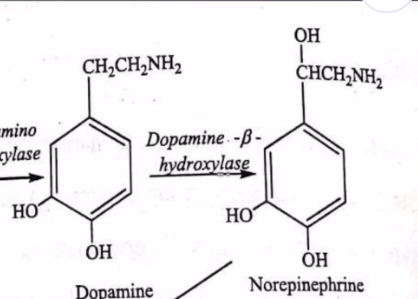
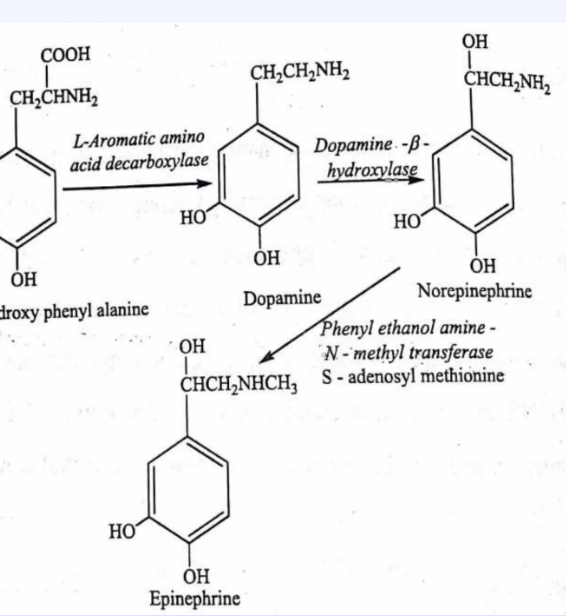
Tips for transferase detection ex here is addition of methyl group to the norepinephrine
What to look for: Transfer of a functional group (C, N, or P group) from one molecule to another.
Tip: If a small group (like phosphate, amino, or methyl) moves from one molecule to another (or appears as a "tag" on the product), it's a transferase.
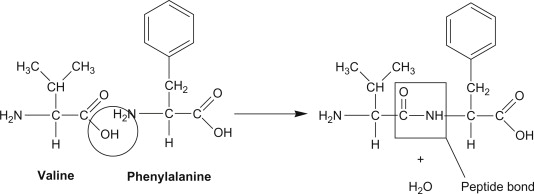
Tips for Hydrolase identification (sepration of primary acid structure into residues)
What to look for: Bond cleavage (hydrolysis) using water (H2O) as a reactant.
Key Clues: Protease, lipase, phosphatase, nuclease, ATPase (when breaking ATP into ADP+Pi).
Tip: If H2O is consumed to break a bond (C–O, C–N, ester, or peptide), it’s a hydrolase.
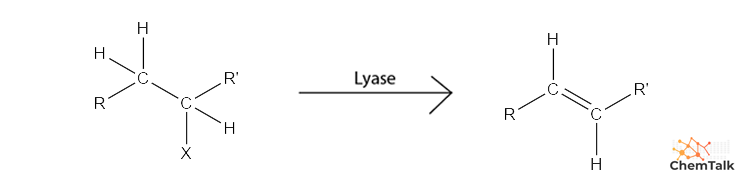
Tips for Lyase identification
What to look for: resulting in the formation of double bonds or the release of small molecules.
Key Clues: Synthase, decarboxylase, aldolase, lyase.
Tip: If something "just pops off" (like CO2 or NH3) or a double bond appears/disappears on the main substrate, think lyase.
Tips for isomerase identification
What to look for: Intramolecular rearrangements (changes to structure, but molecular formula stays the same).
Key Clues: Isomerase, mutase, racemase, epimerase.
Tip: If the substrate and product have the same atoms but are rearranged internally, it’s an isomerase.
Tips for Ligase identification
What to look for: Bond formation (C–C, C–O, C–N, etc.) that is coupled with ATP hydrolysis (ATP is required and ADP+Pi are products).
Key Clues: Synthetase, carboxylase, ligase.
Tip: If ATP is used to join two separate molecules into a larger one, it’s a ligase.
General Tips for enzyme class identification
Track the atoms:
OH added/removed → Oxidoreductase.
Small group (CH3,PO4,NH2) added → Transferase.
CO2 or NH3 lost → Lyase.
Only arrangement changed → Isomerase.
Look for bond changes:
One molecule split into two pieces → Hydrolase or Lyase.
Two molecules joined into one → Ligase (assume ATP was used).
Check for movement: If the enzyme name implies transport, pump, or unwinding (like helicase), it's a Translocase.
What is the effect of Optimal, slight, and extreme pH conditions in enzymes? (kaya kung uminom ka ng snake wine hindi ka magkakaroon ng immunity dahil na denature ang protein)
Optimal= V max is achieved
Extreme= loss of catalytic activity or denaturation
Slight= change of charges in the active sites (yung sa pKa determination ang mangyayari)

What are the Effects of optimal, low, or beyond optimal temp (basis is collision theory)
Optimal= more energy, more collisions, Vmax is achieved
below= less energy, less collision, slower reaction rate
Beyond= alteration in 3d structure (ayaw na ni substrate) disrupting the bonds or reformation of new ones
What is the Reaction order of Enzyme-catalyzed reactions at low substrate concentration?
rate is proportional to rate, first-order reaction
What is the Reaction order of Enzyme-catalyzed reactions at high substrate concentration?
all enzymes are filled, no more rxn processor, Zero order kinetics
called as saturation effect
What are the 3 assumptions of MME?
What is the effect of ES complex formation on entropy (rate or disorder)?
results in entropy loss. The ES complex is a more highly ordered, low-entropy state for the substrate
What is solvation?
the process where individual solute molecules or ions in a solution become surrounded and stabilized by solvent molecules
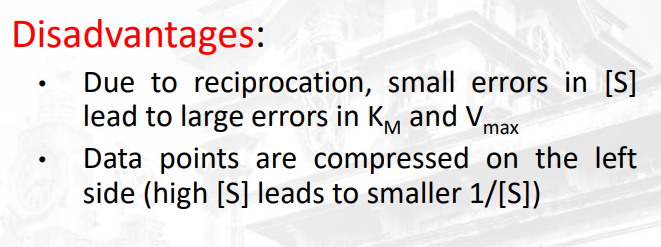
Describe the disadvantages in using the lineweaver burk plot
yung number 4 hahahahahhahahahhahahahahhahahahhah
What is the type of Inhibitor that form strong covalent bonds with the side chains or prosthetic groups at an enzyme’s active site and permanently block the enzyme’s activity?
irreversible inhibitor.
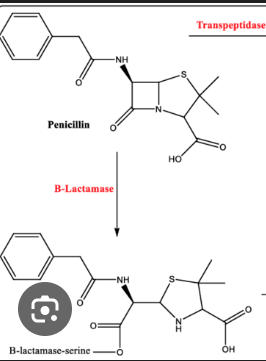
ex is Penicilin: The Attack and the Break: When the enzyme tries to act on penicillin, the highly reactive, four-membered β-lactam ring in the antibiotic breaks open.
The Irreversible Bond: The broken penicillin snaps onto a vital part of the enzyme's active site, forming a permanent, extremely stable covalent bond. (The enzyme is a hydrolase da make the sira to the square shit
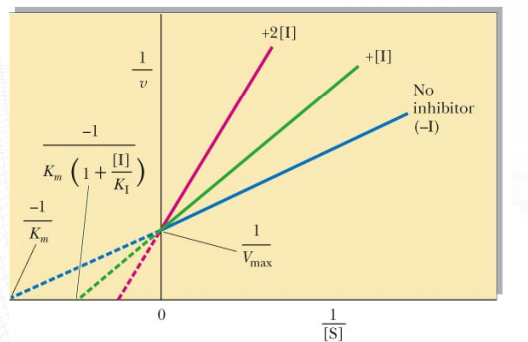
What inhibition is shown? What makes you say that (distinct characteristic in this graph which is lineweaver burk, which is Lineweaver-Burk)?
Competitive inhibition, lines intersect at y intercept, x-intecept is changed
in a Lineweaver-Burk plot, the x-intercept has a direct relationship with the Michaelis constant (Km)

Why is Km decreased in competitive inhibition and Vmax is the same? (parang GSW lang)
because they make agawan to the active site, to make this agawan in the favor of the substrate, the substrate must be many
with an increase in substrate, the Km increases as it describes the concentration of substrate to reach ½ vmax

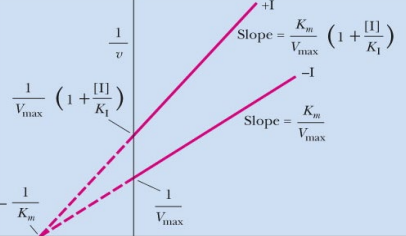
What inhibition is shown? What makes you say that (distinct characteristic in this graph which is lineweaver burk)?
pure-non competitive, they differ in the y-axis which is the reciprocal of Km
Km in line whatever bert graph is inverse relation with y intercept.
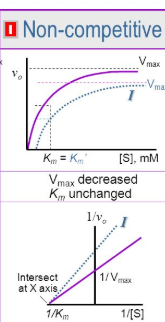

Why is Vmax decreased in pure non competitive (basically Michael Jordan)
The max velocity is reduced by stopping the process altogether as Vmax is the max velocity at which catalysis occurs. In order for this velocity or rate to increase catalysis must occur which in this inhibition, halted by either binding to the allosteric site of E at equal rates.
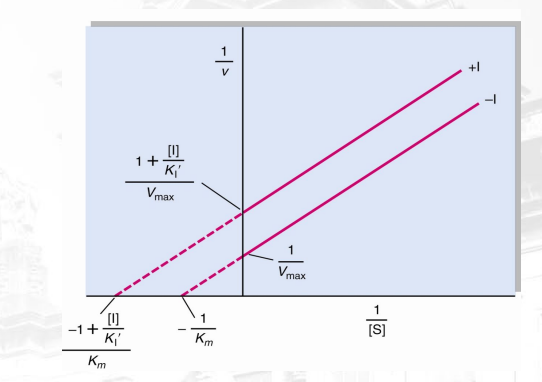
What inhibition is shown? What makes you say that (distinct characteristic in this graph which is lineweaver burk)?
Uncompetitive, the lines are parallel and backward
X-inter of the inhibitor is farther from origin (which means lower km) and the y-inter is higher which means that Vmax is lesser
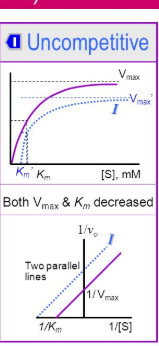
Why does uncompetitive inhibition cause Vmax and Km lowering
It targets the allosteric site of the ES complex locking in said complex. Which means that it shuts down the change of structure to release the products, process the substrate, or dissociate.
it decreases Vmax by shutting down catalysis which velocity is dependent upon
It decreases Km because a locked ES means less enzyme. Less enzyme means less substrate needed to reach Vmax 1/2
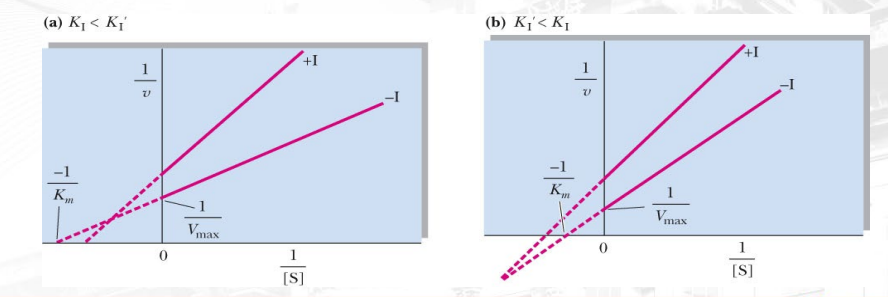
What inhibition is shown? What makes you say that (distinct characteristic in this graph which is lineweaver burk)?
Mixed inhibition as they intersect not on the intercepts (both x and y). Effects vary depending on the location of intercepts
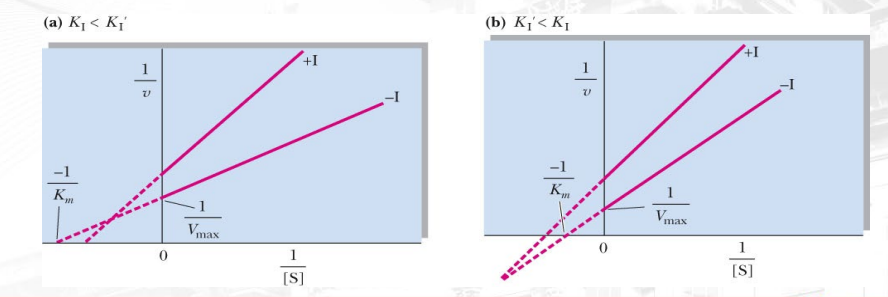
Explain Vmax and Km for this graph of mixed inhibition? Why does the change vary (based on non-comp and uncomp)? Explain the change in Km and Vmax.
They are both meaning the inhibitor can bind at both the allosteric site of ES or E
left one: x-inter is more negative meaning Km is decreased, Vmax is increased due to y-inter being lower.
Right one: km is increased, but vmax is low
I cant fking explain this shit cuz it don’t make sense (yet) mali kung titignan ang intercepts
How many amino acids typically participate in the active site of most enzymes studied? Which five amino acids are most common in enzyme active sites? What are the side chains present?
Five amino acids participate in more than 65% of enzymes, Histidine (His) > Cysteine (Cys) > Aspartate (Asp) > Arginine (Arg) > Glutamate (Glu), Four have acidic or basic side chains; one (Cys) has a sulfhydryl (-SH) group
What is feedback control in enzyme regulation? What types of inhibition can occur in feedback control? What types of inhibition can occur in feedback control?
It’s when the product of a series of enzyme-catalyzed reactions inhibits an earlier step in the sequence. Comp and Uncomp
What is a proenzyme (zymogen)? What structural changes occur
An inactive enzyme that requires part of its polypeptide chain to be hydrolyzed and removed to become active.
Both the primary and tertiary structures change, enabling the enzyme to adopt its active form.
What is allosterism in enzyme regulation? What is an allosteric enzyme?
Regulation at a site other than the active site that causes a change in the active site. An enzyme regulated by allosterism, often with multiple polypeptide chains.
Define negative modulation and positive modulation.
A: Negative modulation: inhibition of an allosteric enzyme.
Positive modulation: stimulation of an allosteric enzyme.
What is a regulator in allosteric enzyme regulation?
A substance that binds to an allosteric enzyme and changes the shape of the active site.
How does protein modification regulate enzyme activity? Give an example of enzyme regulation by protein modification.
covalently modifying the enzyme, often through phosphorylation/dephosphorylation, Pyruvate kinase (PK) is inactivated by phosphorylation to pyruvate kinase phosphate (PKP). $
What are isoenzymes (isozymes)?example
Enzymes that occur in multiple forms but catalyze the same reaction. Lactate dehydrogenase (LDH): H4 in heart muscle, M4 in liver and skeletal muscle.
What is a transition-state analog? Give an example of a transition-state analog.
A molecule whose shape mimics the transition state of a substrate. Pyrrole-2-carboxylate mimics the planar transition state of a substrate.
What happens when COX-2 is activated? How does COX-2 inhibition help patients?
It reduces prostaglandin synthesis, relieving pain and inflammation
Name some drugs that inhibit COX enzymes.
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, and acetaminophen.
What is the role of angiotensin-converting enzyme (ACE) in the body? How do ACE inhibitors help patients with high blood pressure?
ACE narrows blood vessels, increasing blood pressure prevent the formation of active ACE from its zymogen, lowering blood pressure.
How do HIV protease inhibitors work?
They block the activity of HIV protease, decreasing virus replication in the patient
Basta yung table AAAHHHHHH