Acid base reactions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Define acid
Something that produces excess H+ ions in an aqueous solution
Define alkali.
something that produces excess OH- ions in an aqueous solution
What does acid do in a chemical reaction?
donates a proton
What do bases do during a chemical reaction?
accepts a proton, base is called an alkali if it dissolves in water
What do compounds that can act as an acid and base called?
amphoteric
How do we find the strength of acids?
greater the dissociation, greater the strength
Why is HCL a monobasic acid?
only release one proton per molecule
What is the name of acids that can release 1,2 or 3 protons?
mono-
di -
tri-
What do acids reactions with alkalis make?
salt + water
What do acids reactions with carbonates make?
salt + water + carbon dioxide
What do acids reactions with metals make in hydrochloric acid?
Salt + hydrogen
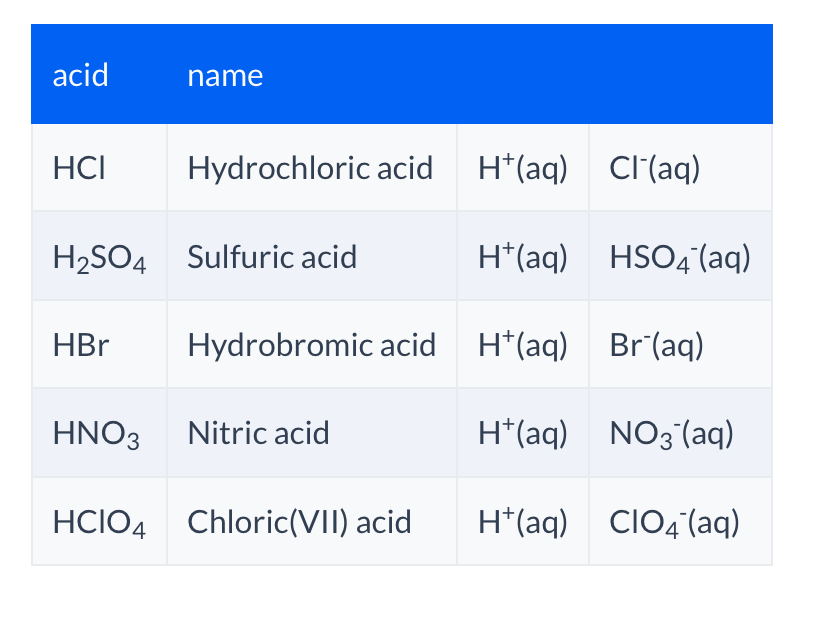
Acids names and chemical equation?
equation for pH ?
pH = -log10 [H]
How to find hydrogen ions if you have a pH?
H = 10^-ph