L1: The cytoskeleton as a dynamic system
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
51 Terms
What is the cytoskeleton
system of protein filaments found in all eukaryotic cells
What fundamental cellular functions does it perform
maintenance of cell shape
locomotion
intracellular trafficking that organises the cells’ contents
Rearrange components of cell in space and time
How do the compoentns of the cytoskeleton perform these functions
organised into higher order strucutures
What processes can these higher order structures support examples
cell migration in developing embryo or adult
spread of cancer cells
swimming of sperm
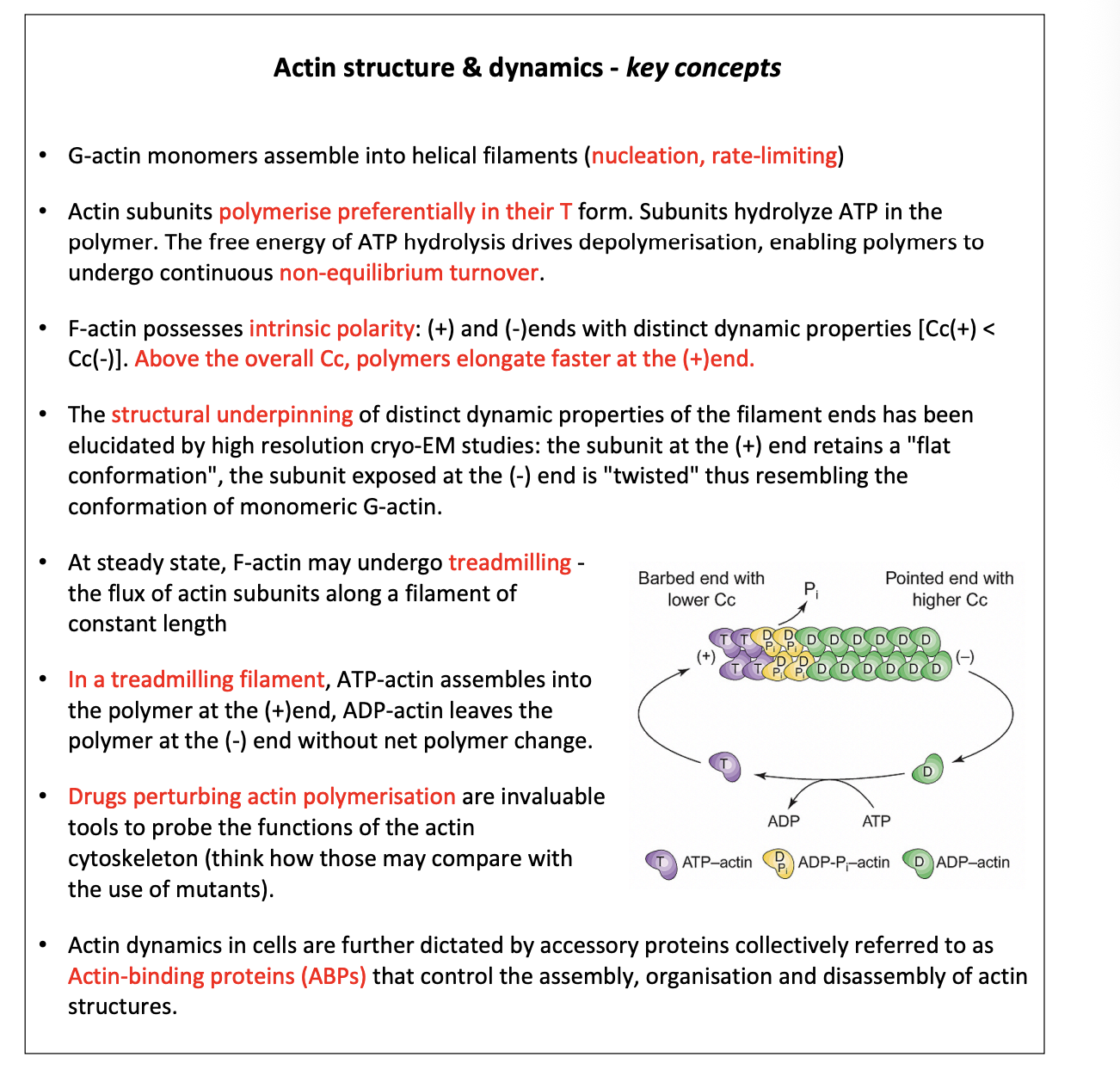
muscle contraction
meiotic and mitotic divisions
cell cycle divisions every ten minutes
segregating chromosomes
contractile ring for cell division
Organelle partition
establishment of polarity and asymmetrical positioning of cell determinants for alternative developmental fates
i.e not just in muscle cells→ also in all cells
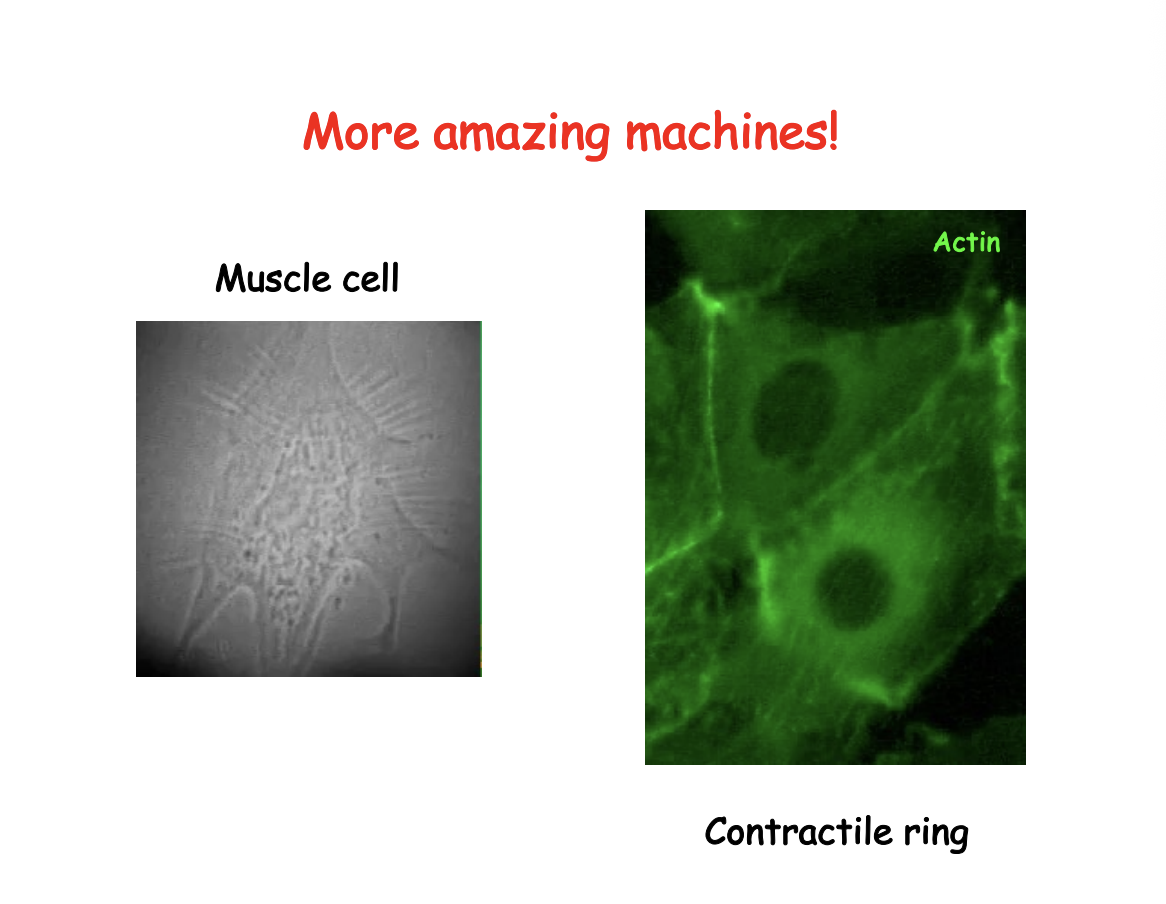
Example of where it is used for transport and motility
Intracellular→ axonal transport
Change in cell shape movement → granulocyte in blood vessel for healing wound
Dorsal closure in development→ actin creates a zipper that closes the gap
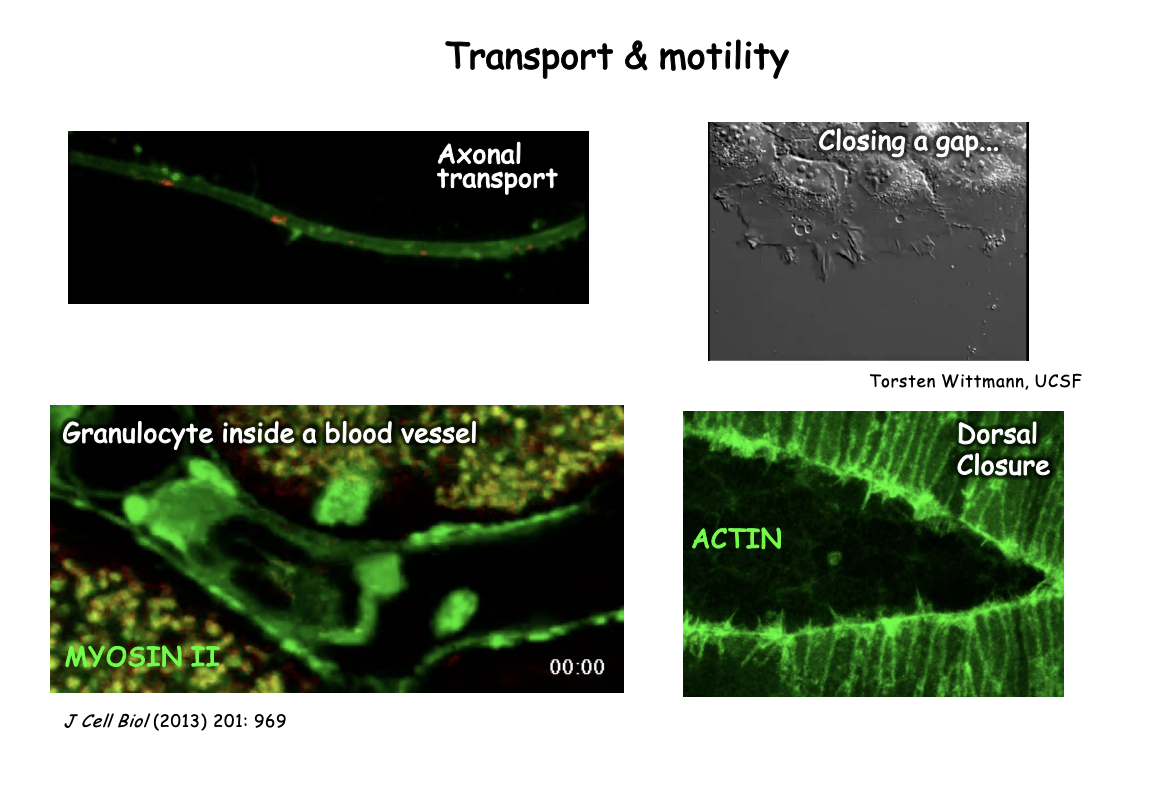
Examples of other motile strucutures
Swimming→ flagellum
Beating→ cilia
Listeria→ invading cell must hijack the cytoskeleton system to propel themselves
What features of the cytoskeleton allow it to have so many functions
Built from small diffusible subunits
Subunits held together mainly by non-covalent interactions→ makes them really strong
Accessory proteins modulate the spatial distribution and dynamic behaviour of cytoskeletal systems and provide an interface with diverse signalling pathways
Cytoskeletal structures ca be highly dynamic and may undergo rapid remodelling
How are cytoskeltal strucutres highly dynamic
under the control of accessory proteins
undergo continuous turnover in cells
small subunits can then diffuse and become reorganised into strucutre to match requirments of the cell
When do these rearrangements occur
Abruptly in response to
intracellular cues
or
Extracellular signals
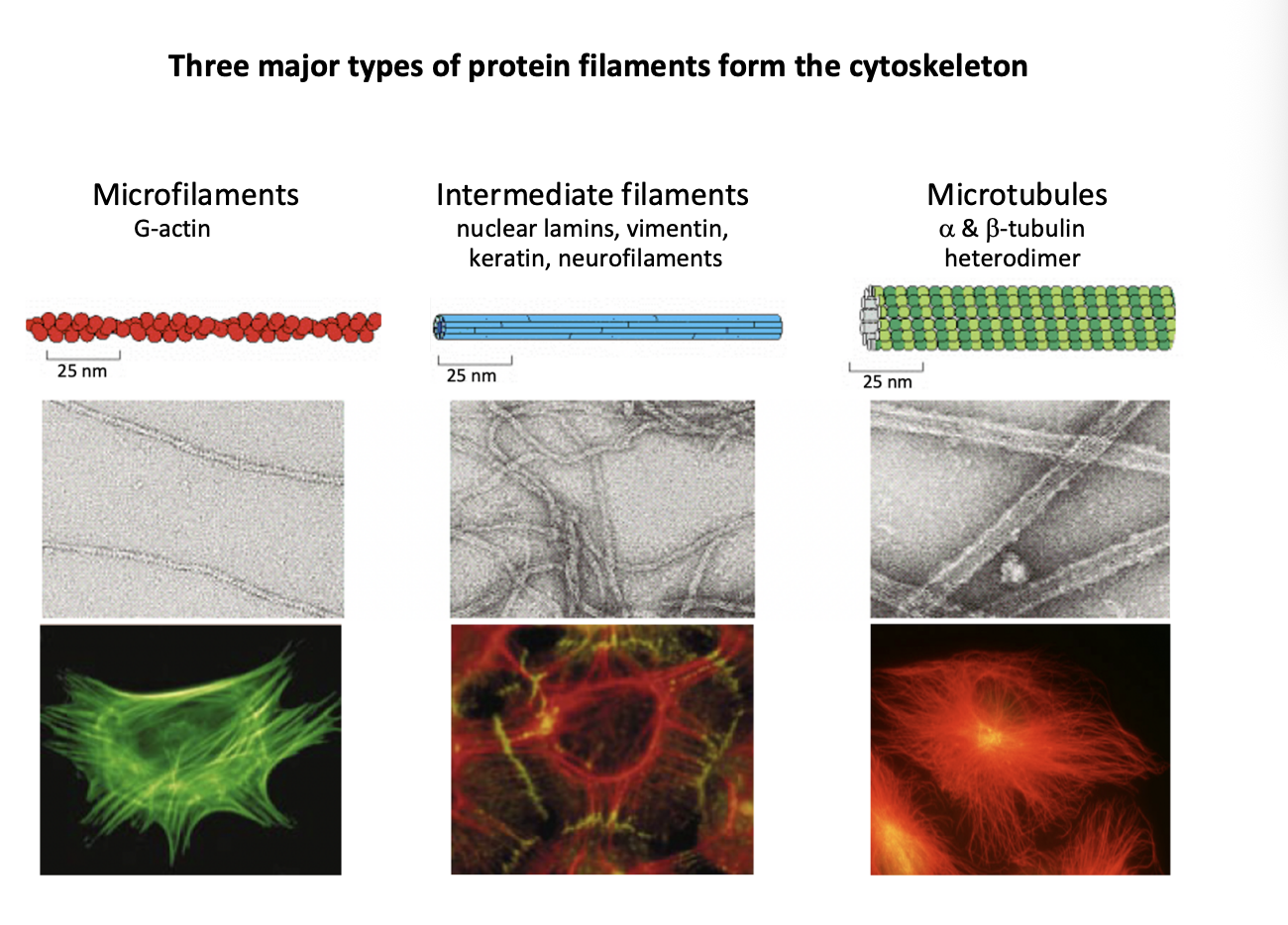
Three major types of protein filaments from the cytoskeleton and rough diameters
Microfilaments (MFs)
G actin
7nm
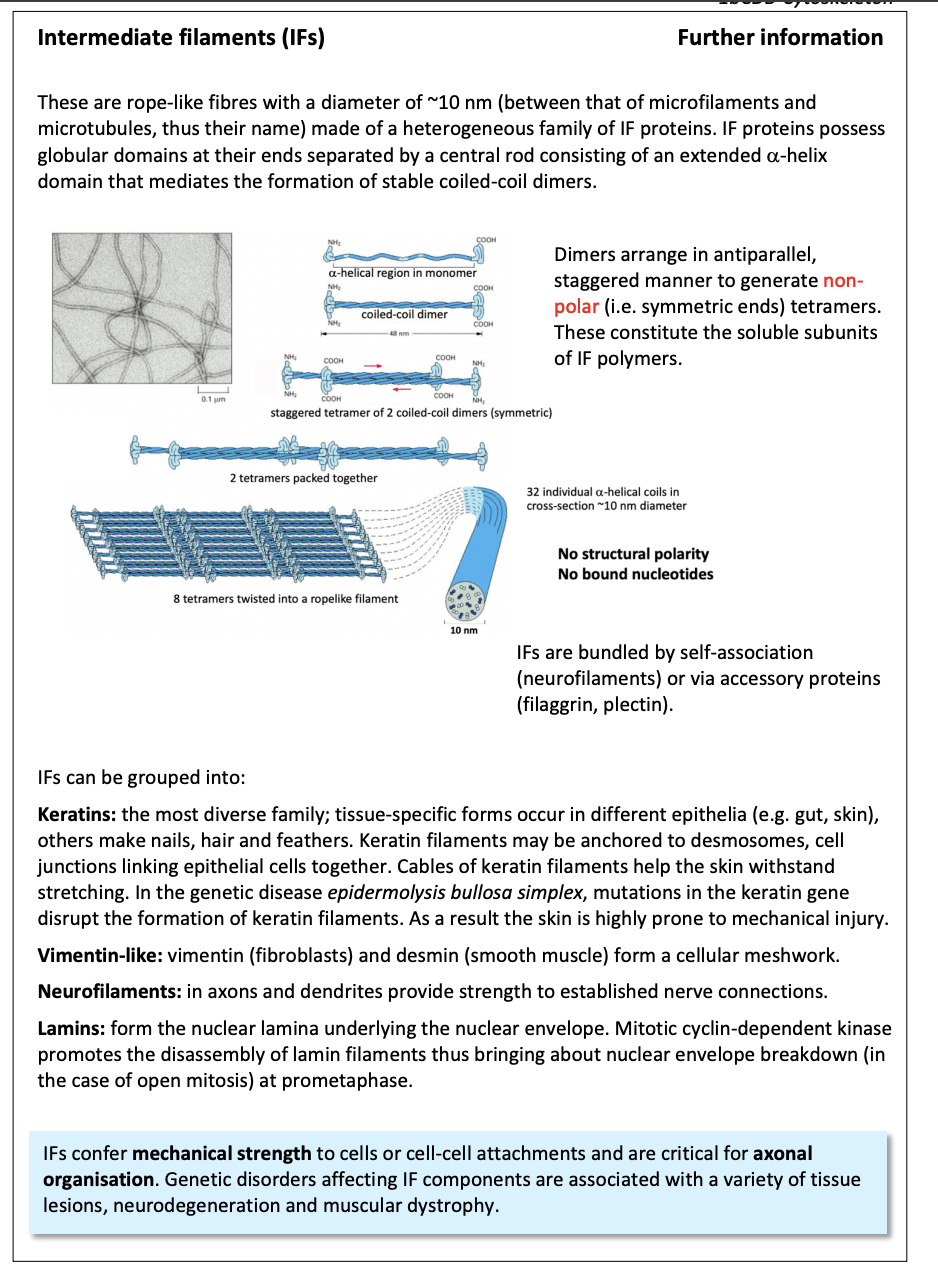
Intermediate filaments (IFs)
Repetitive subunits of different proteins
neulcear lamins, vimentin, keratin, neurofilaments
10nm
Microtubules (MTs)
Alpha and beta tubulin heterodimer
makes walls and hollow cylinder
25nm
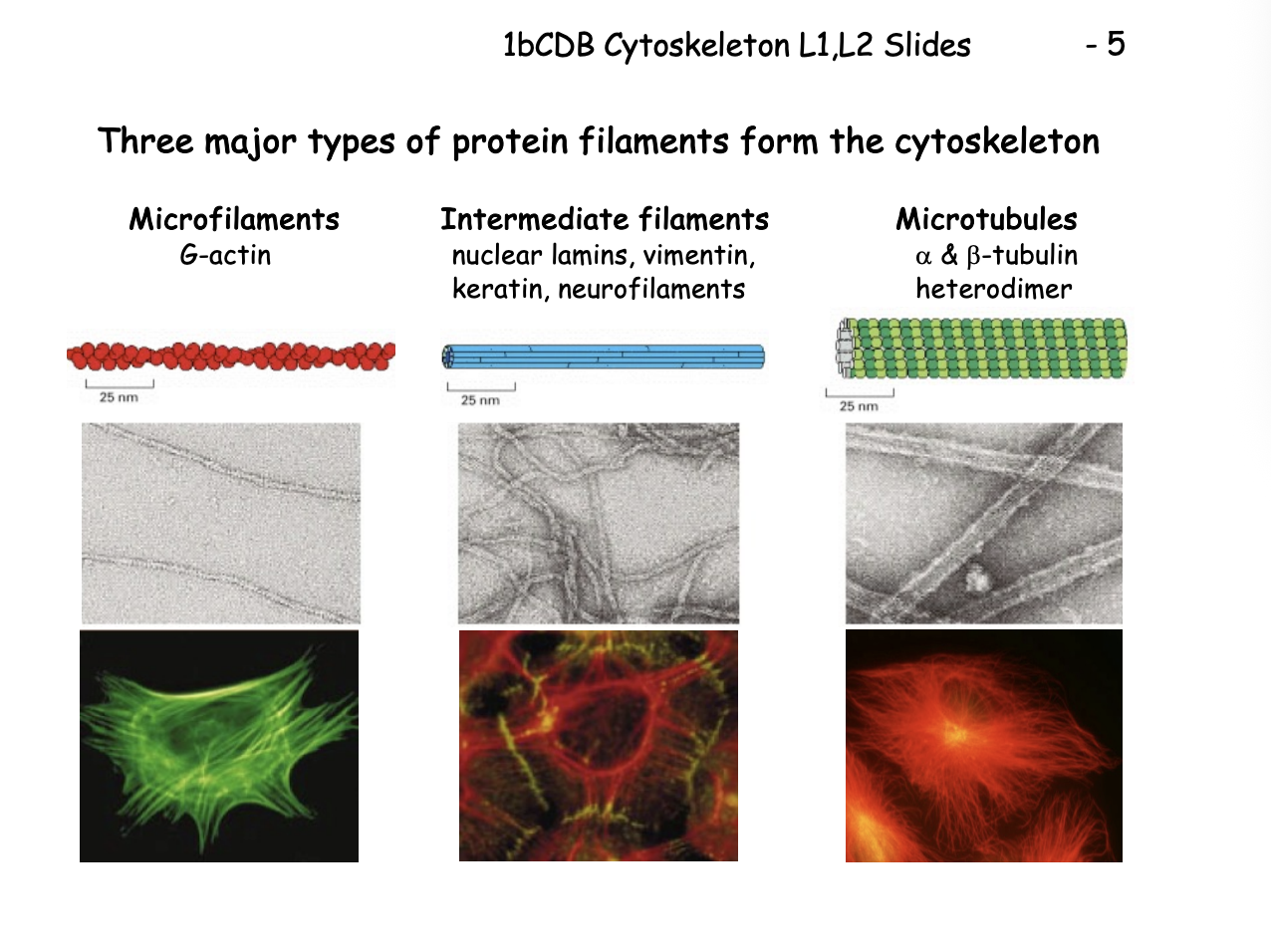
Microfilaments
actin monomers
in all eukaroytic cells
Functions:
form cell cortex→ underneath cytoplasmic membrane network
Filaments bundle to form chracteristic cell protrusions
Microvilli→ e.g intestine
Sterocilia→ e.g inner ear
filopodia or lamellipodia in crawling cells
Intracellular transport
provide tracks
Contractile strucutres
stress fibres
myofibrils in muscle cells
Cytokinesis
actomyosin ring
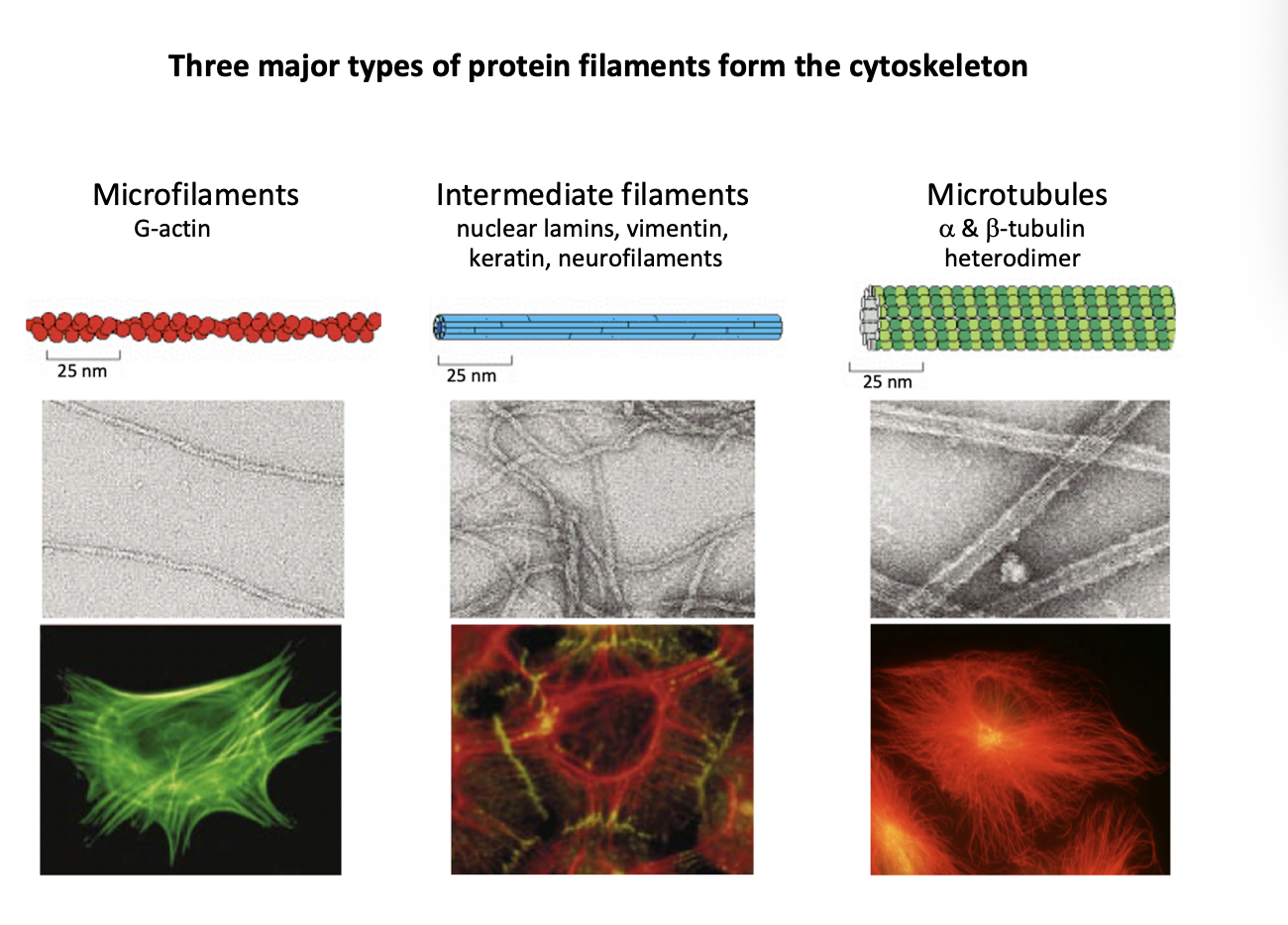
Role of nuclear actin?
remain more mysterious
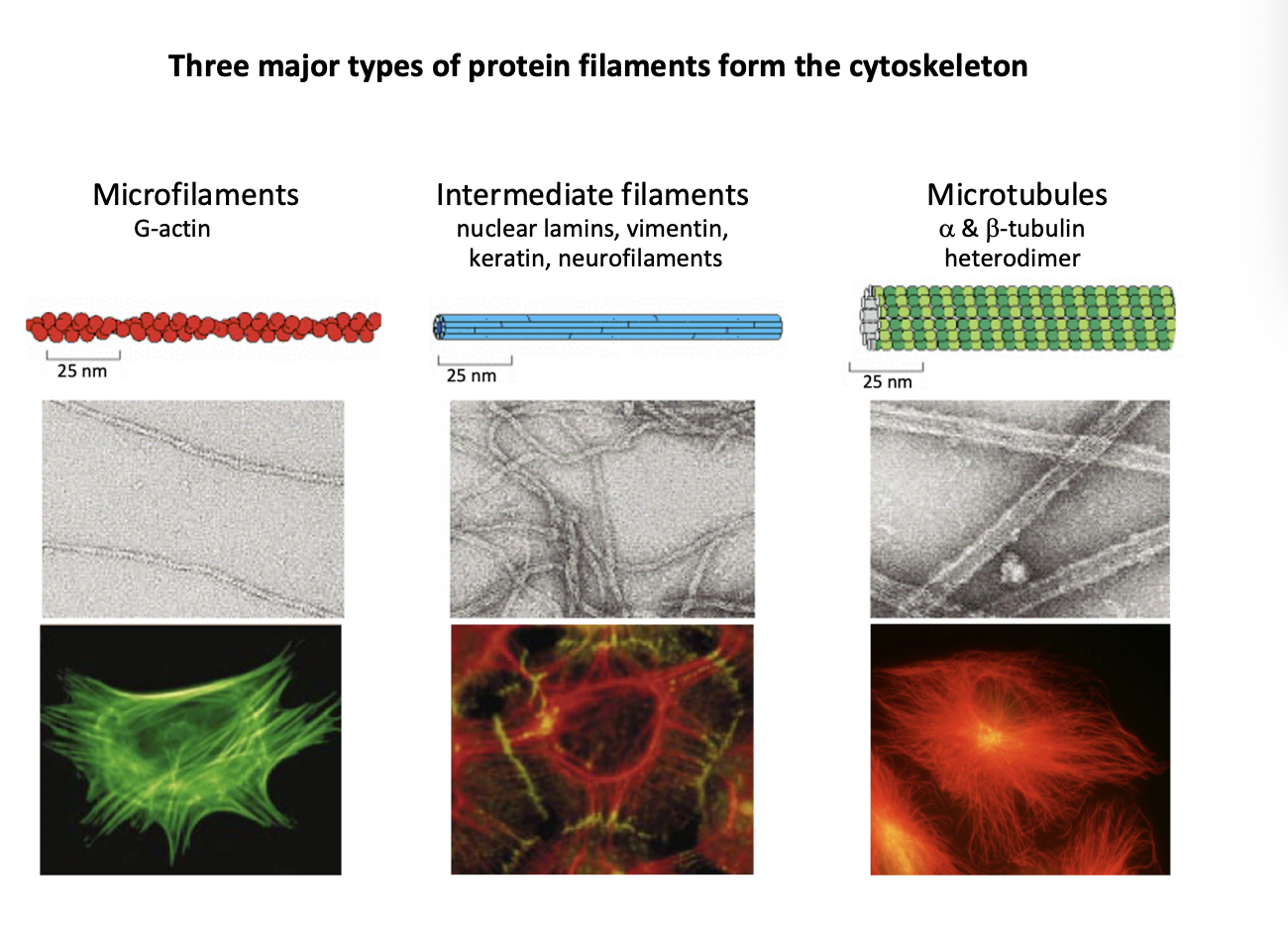
2 . Intermediate filaments
more restricted distribution
strong polymers with common overall ‘rope-like’ strucutre
contribute to the remarkable strength of tissues
e.g skin and muscle
withstand stretching
Full development of neurons
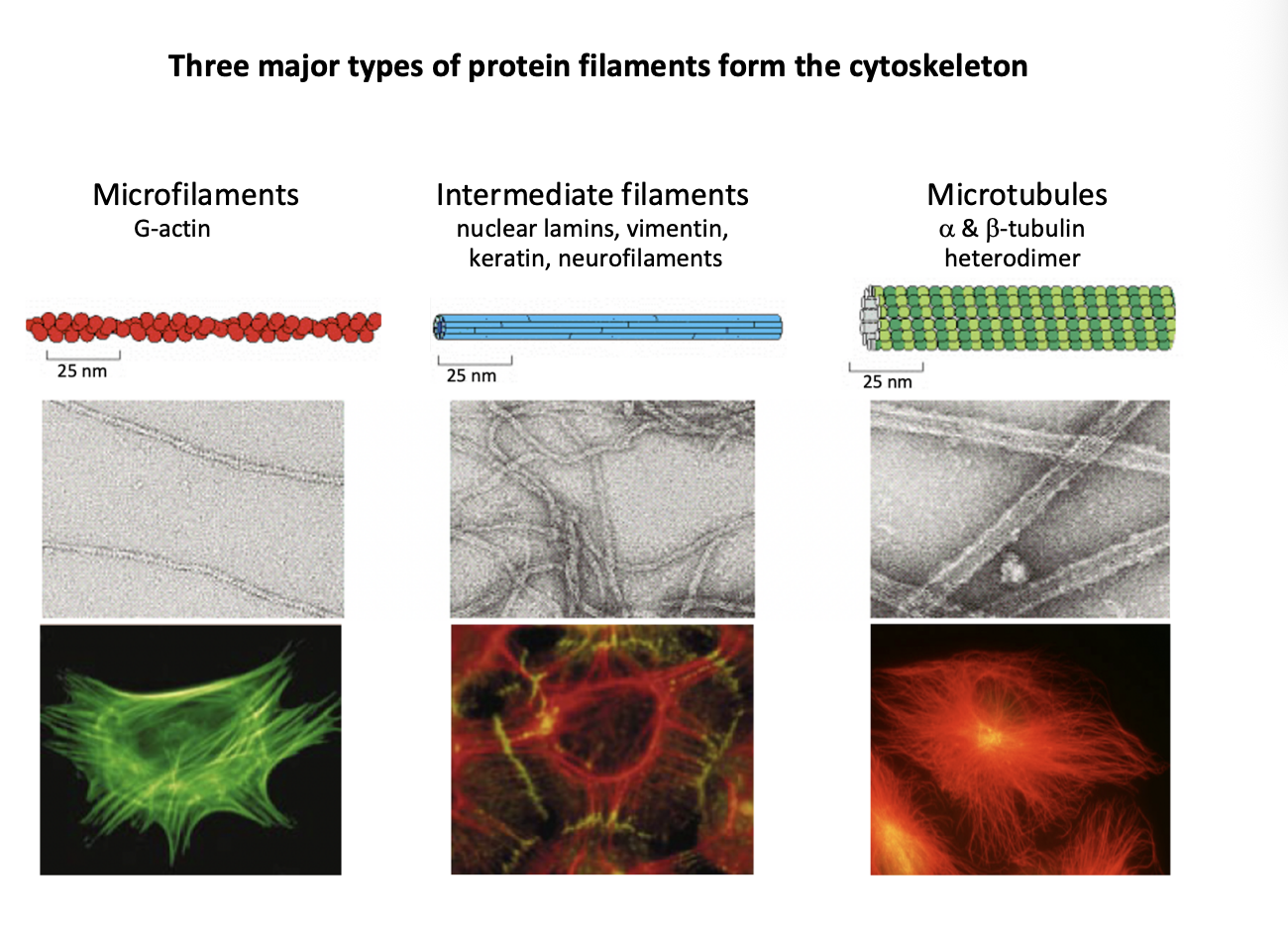
intermediate filaments
further info
Microtubules (MTs)
hollow cylinder of heterodimeric subunits
a/b-tubulin
Functions:
Form tracks for vesicular and organelle traport
e.g axonal transport
Compartmentalisation of Golgi and ER within the cell
Mitotsis> rearrange mitotic apparatus
In order to understand MFs and MTs→ must explore
Structure
Filament nucleation
Organisation
Dynamic beahviour
Functional integration→ mitosis and cell division (case study)
Microfilaments: Actin
in all euakroytic cells
most abundant cellular protein
10% muscle cells
1-5% non-muslce cells
Main roles in cell
cell motility
Cell polarity
Cell shape
Great range of roles it has
Endocytosis and intracellular trafficking
Contractility
Surface protrustion and adhesion
Mitotic spindle orientation
Cytokinesis
Cell division patterning
Embryonic development
Whole cell motility
Elongation of nerve axons
Defence against infection
Wound healing
Metastasis
How is it able to do these roles
structrual and dynamic properties
cycles of polymerization and disassembly
between globular and filamentous forms
constantly remodelling
used for force-generating system
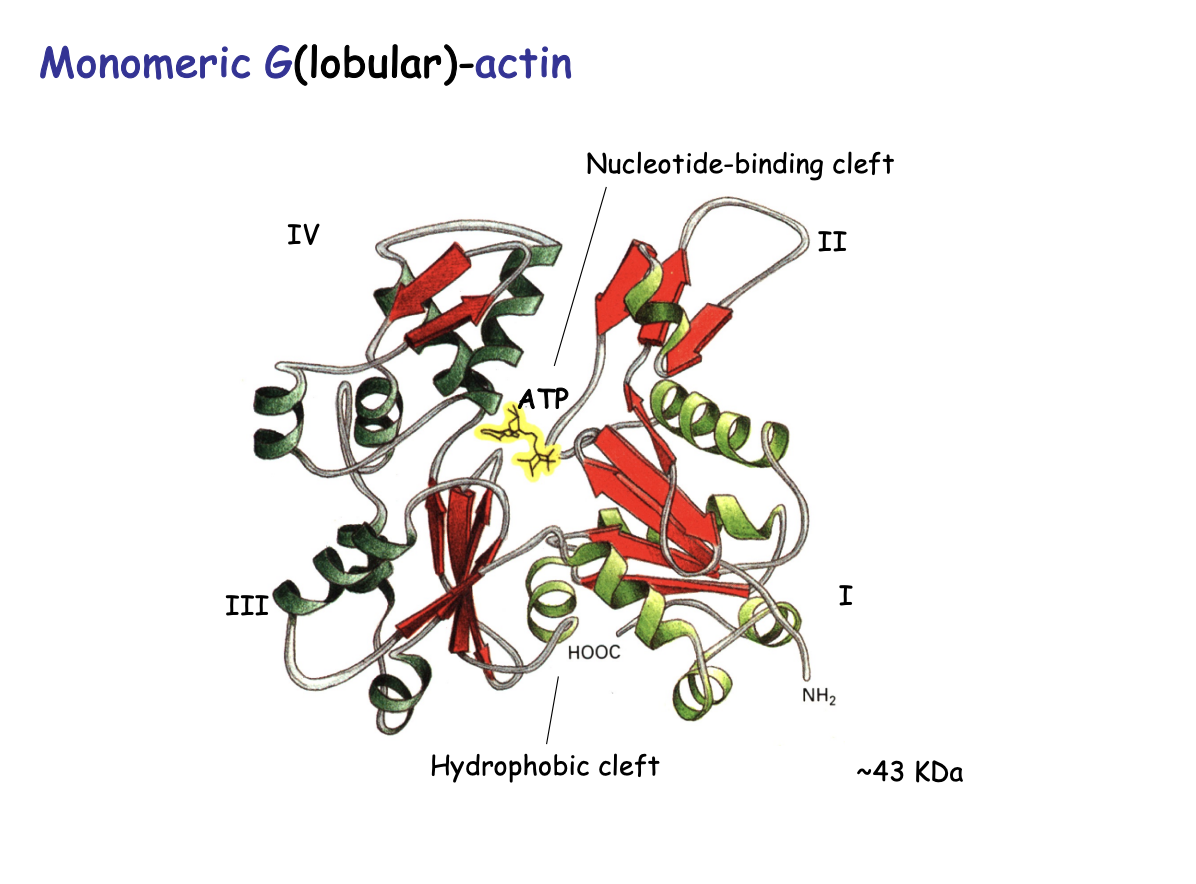
Actin monomers strucutre
Globular (G) Actin:
bi-lobe (two distinct lobes)
separated by a deep hydrohobic cleft
binds ATP or ADP
ATPase activity
43kDa
Polymerised actin
Filament (F) actin
Flexible
7nm diameter
double helix
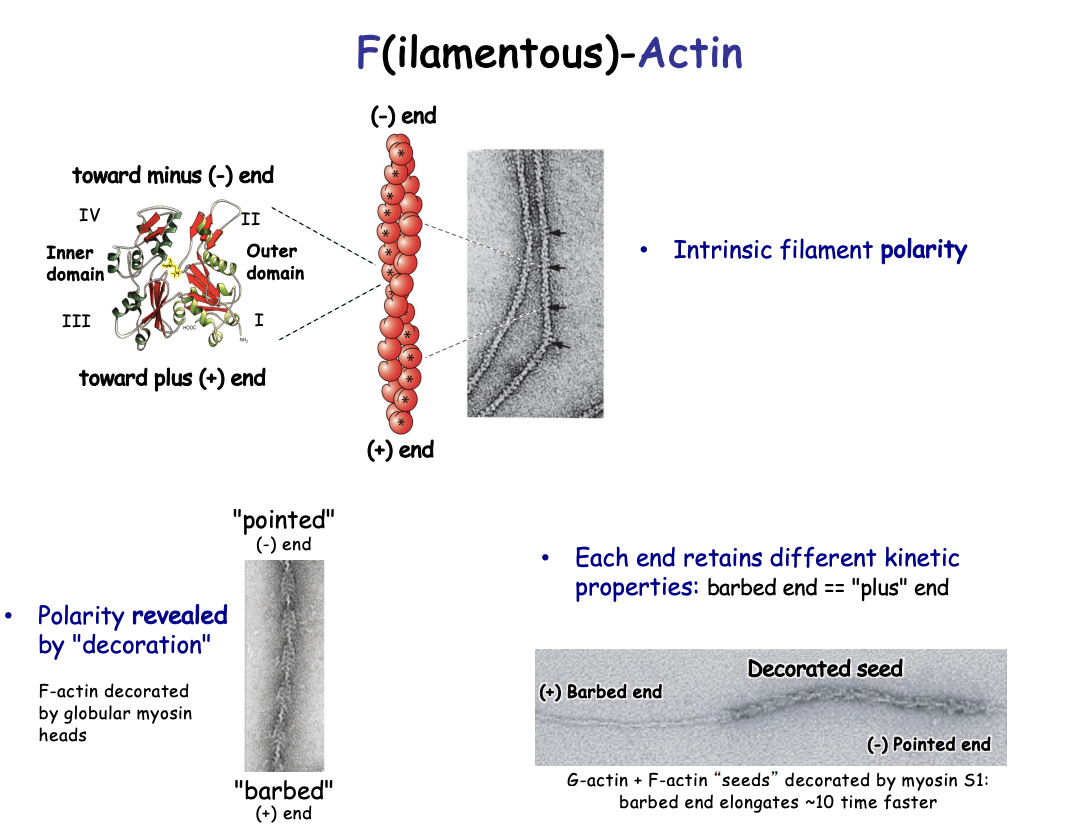
non covalent bonds→ strong
Same orientation/polarity→subunits pointed in the same direction
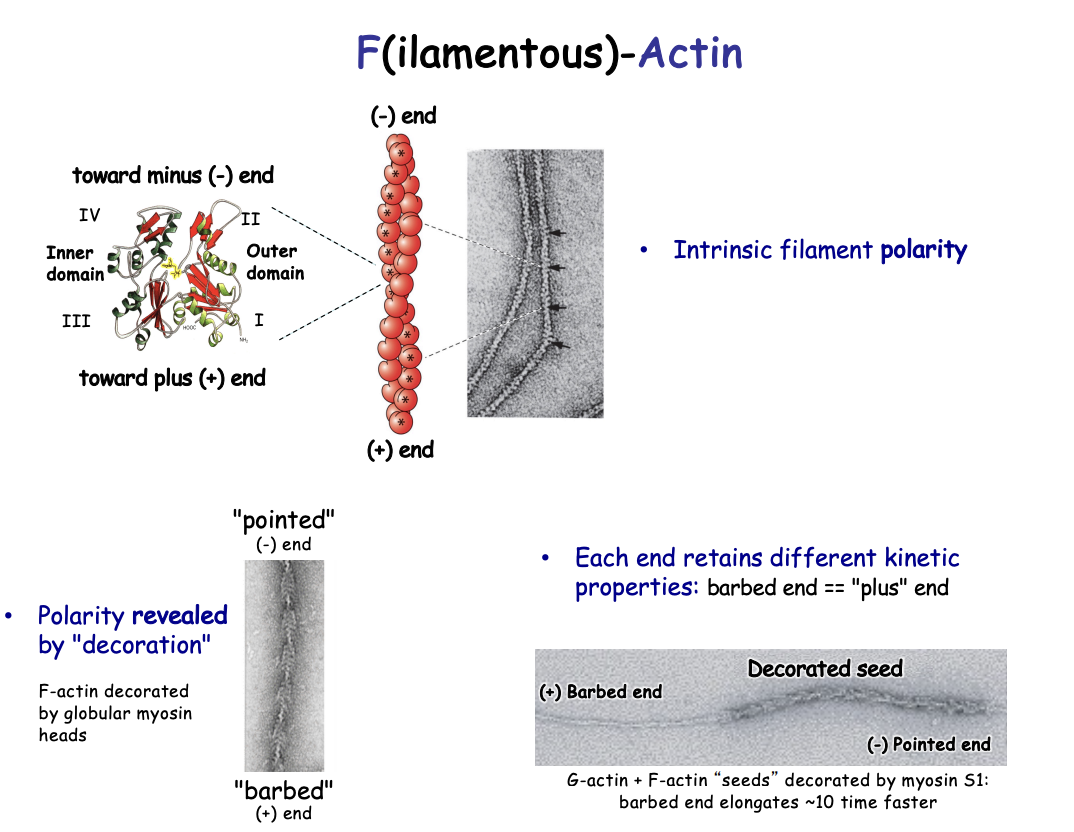
How was polarity investigated/shown
Decoration Experiments
add proteolytic S1 fragment to microfilaments in vitro (myosin globular heads)
i.e F actin decorated with globular muosin heads
results:
revered a ‘barbed’ or ‘pointed’ ends
with polarity→ all point in same direction
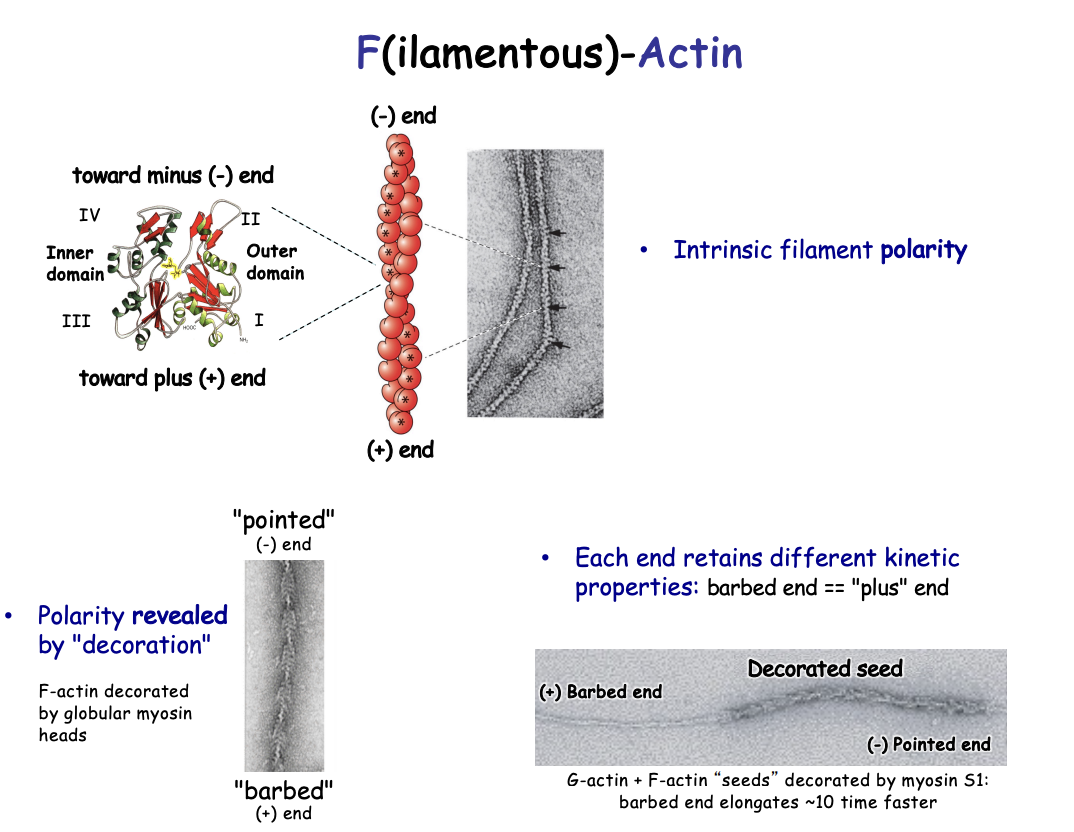
Investigating the different dynamics of each head
Procedure:
G actin and F actin seeds decorated by myosin S1
shows the barbed end elongates 10 x faster
Different end retains different kinetics:
Plus end (+)→ fast-growing barbed end
Minus end (-)→ less dynamic pointed end
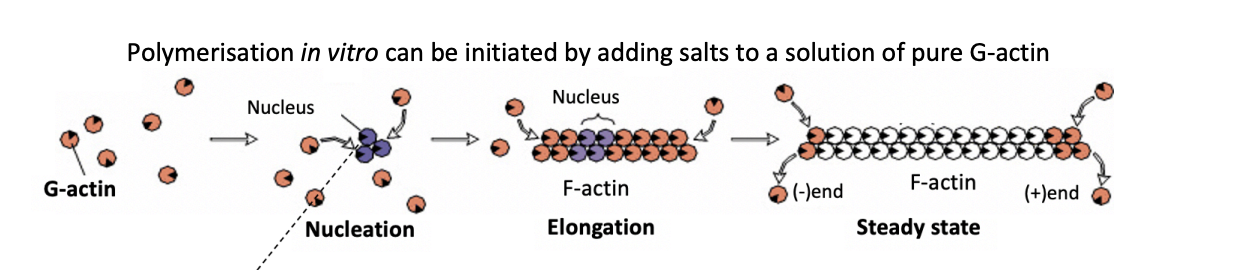
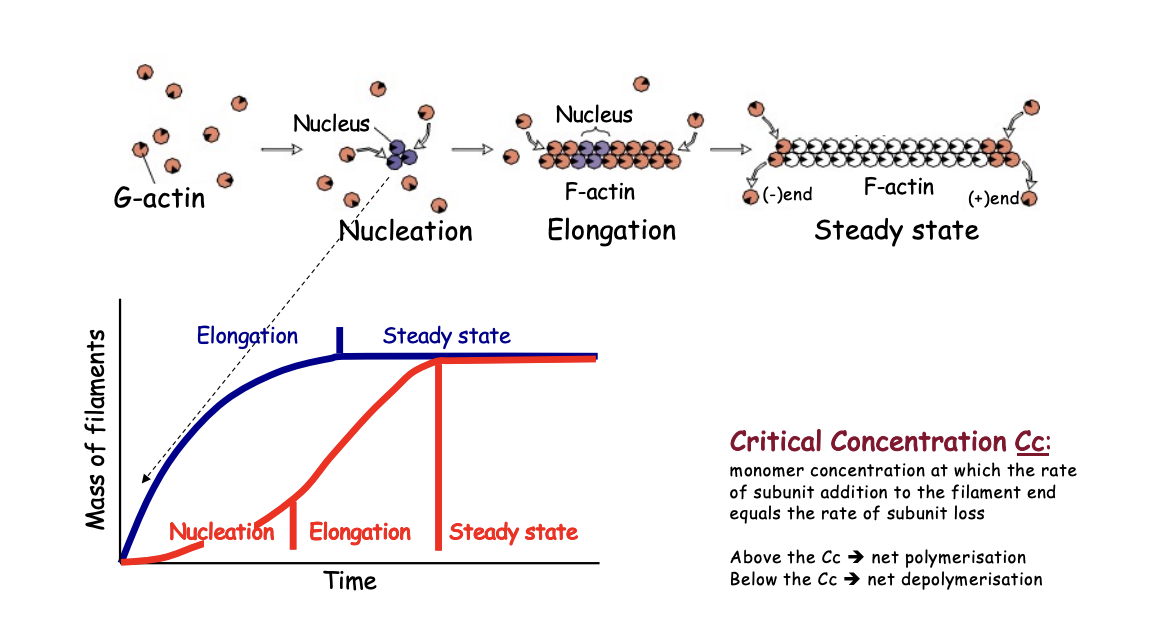
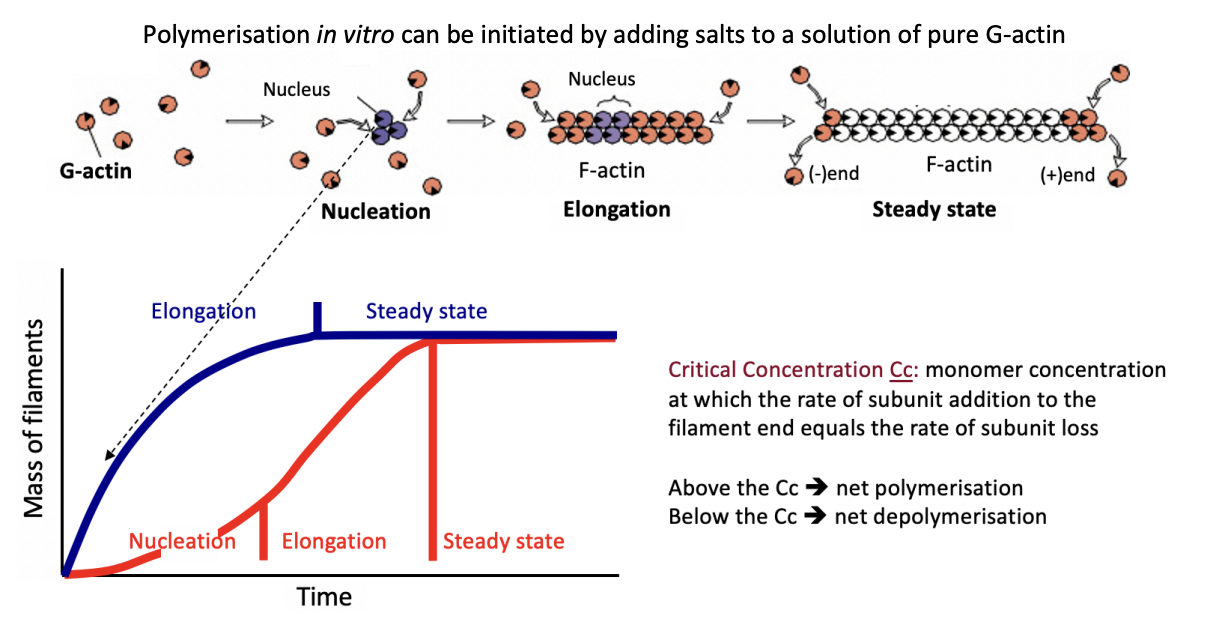
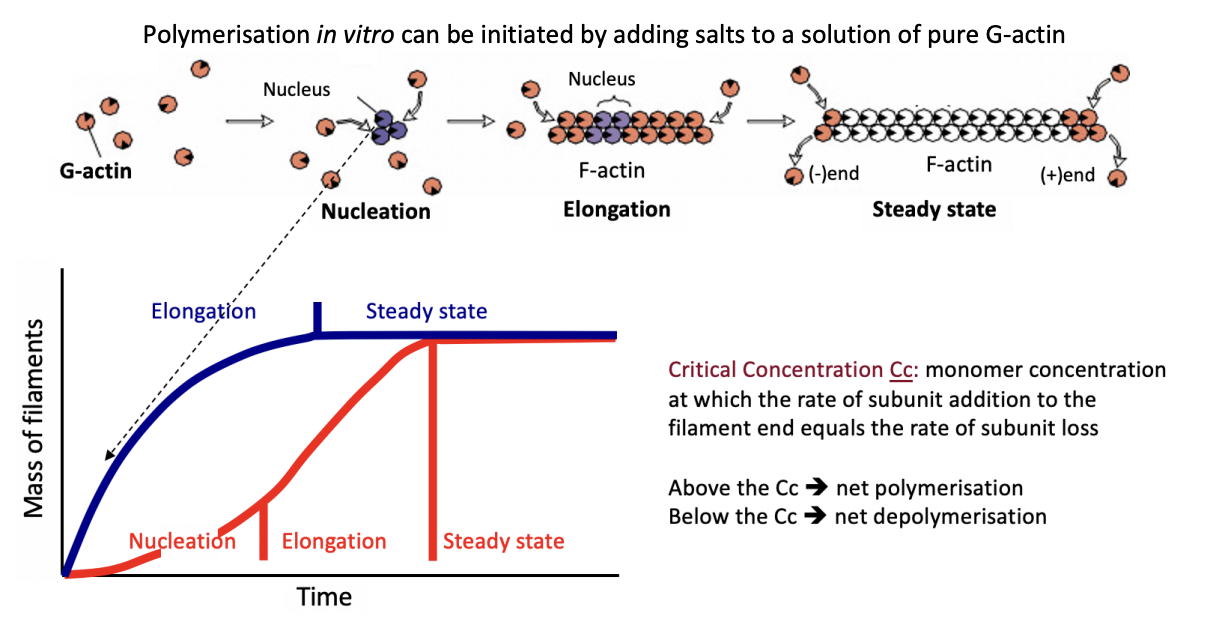
Polymerisation of actin
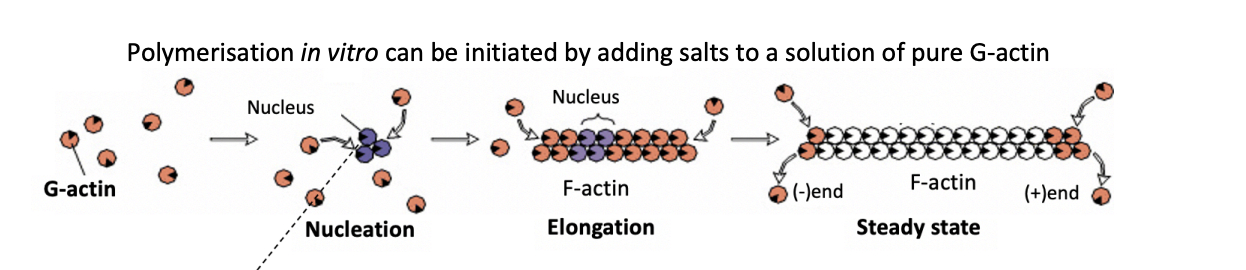
How can polymerisation in vitro be initiated
adding salts to a solution of pure G-actin
What is the rate limiting step in actin polymerisation
Nucleation
initial formation of oligomers with few subunits
energetically unfavourable
Evidence that the RLS is nucleation→ Actin polymerisation kinetics if add stablilised oligomers to the reacation→ blue line
suppresses the lag
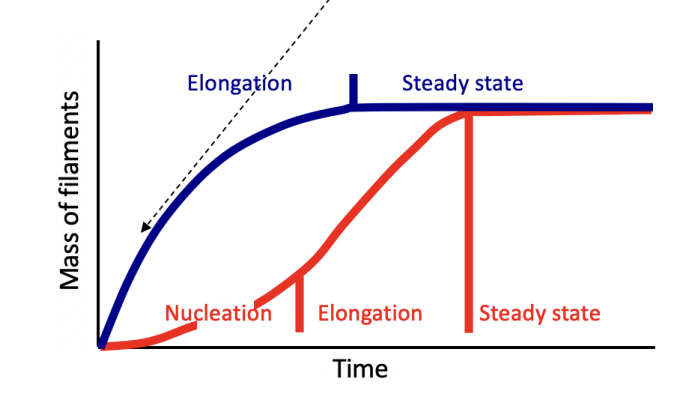
Actin polymerisation kinetics (red line)
Nucleation
Elongation
Steady state
As filaments elongate, the concentration of free monomers of free monomers falls until critical concentration (Cc)
What happens at the critical concentration (Cc)
actin subunits add to or leave the filament at the same rate
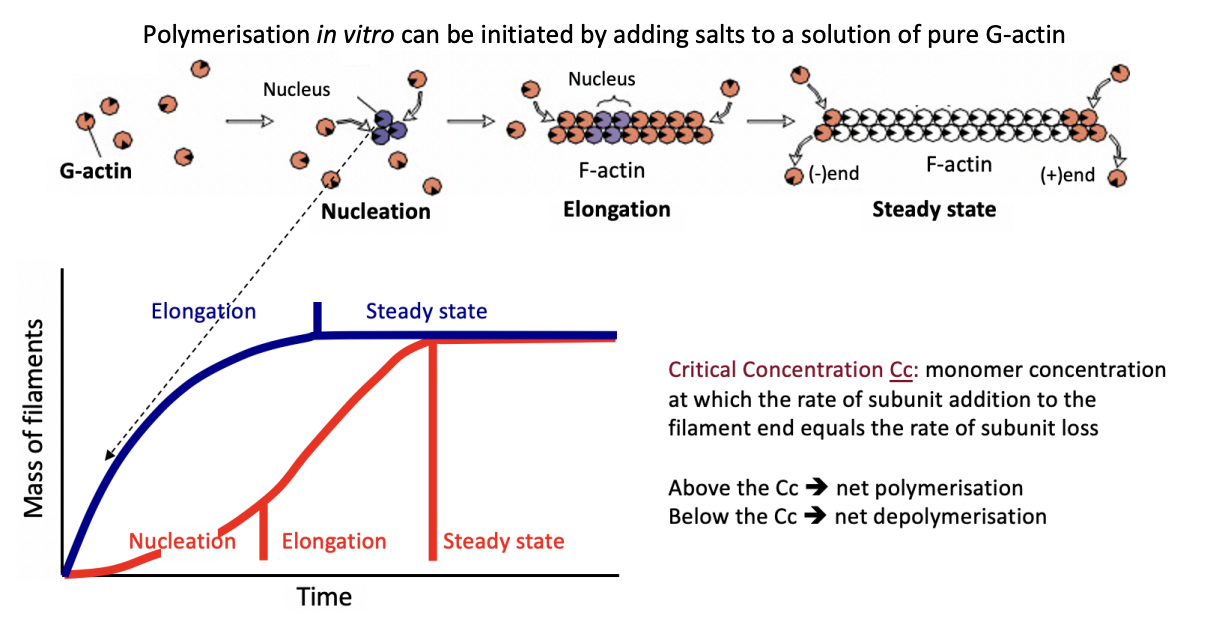
What happens below Cc
no new filaments form
any present→ depolymerise
net depolymisation
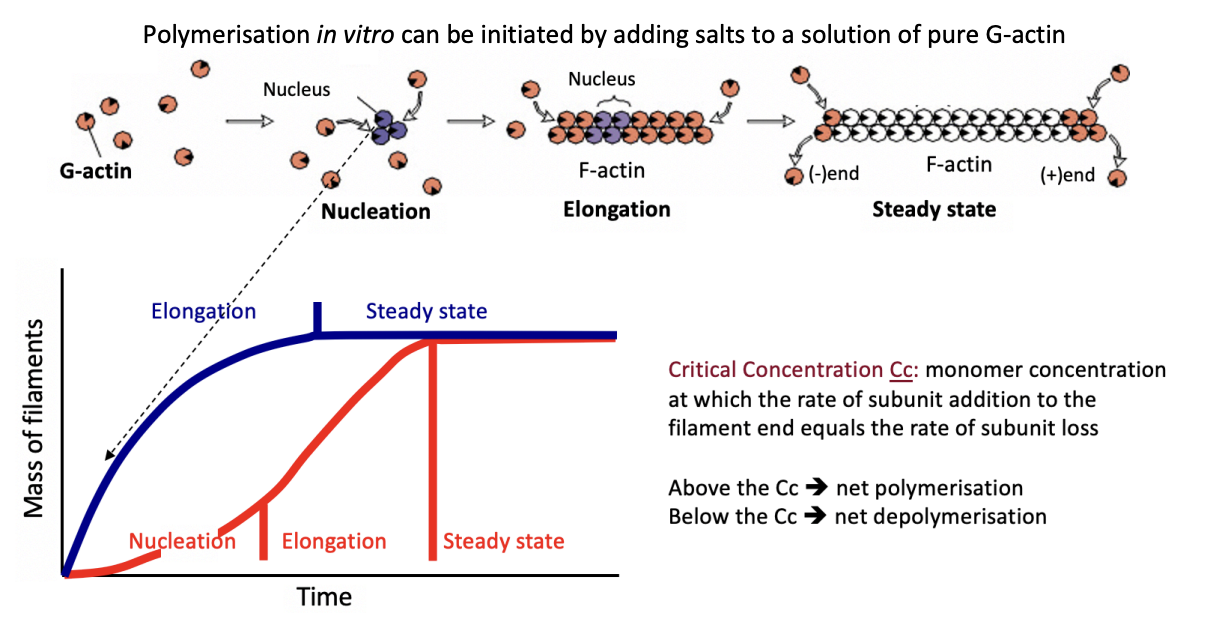
What happens above
Net polymerisation
Filament end dynamics and ATP cycle
incoming monomer→ ATP-boud actin monomers (T form)
Preferentially incorporated
Domains are twisted
Newly polymerised→ 20 degree scissor like rotation
Flat conformation on outer domain
(the one facing outwards in the helical filament)
Rotation enhances ATP hydrolysis to ADP-Pi-ACtin
Slow release of Pi
Yields ADP-actin→ D form
This process explains why there are two different properties of the two ends
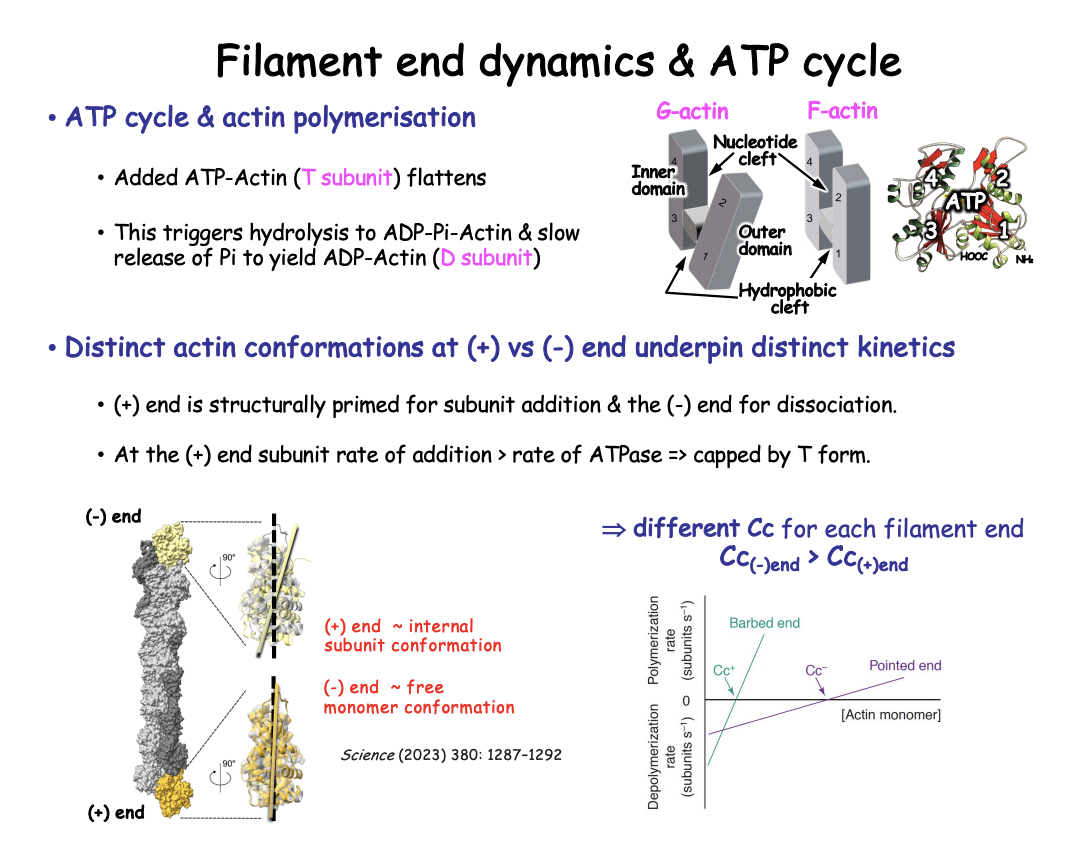
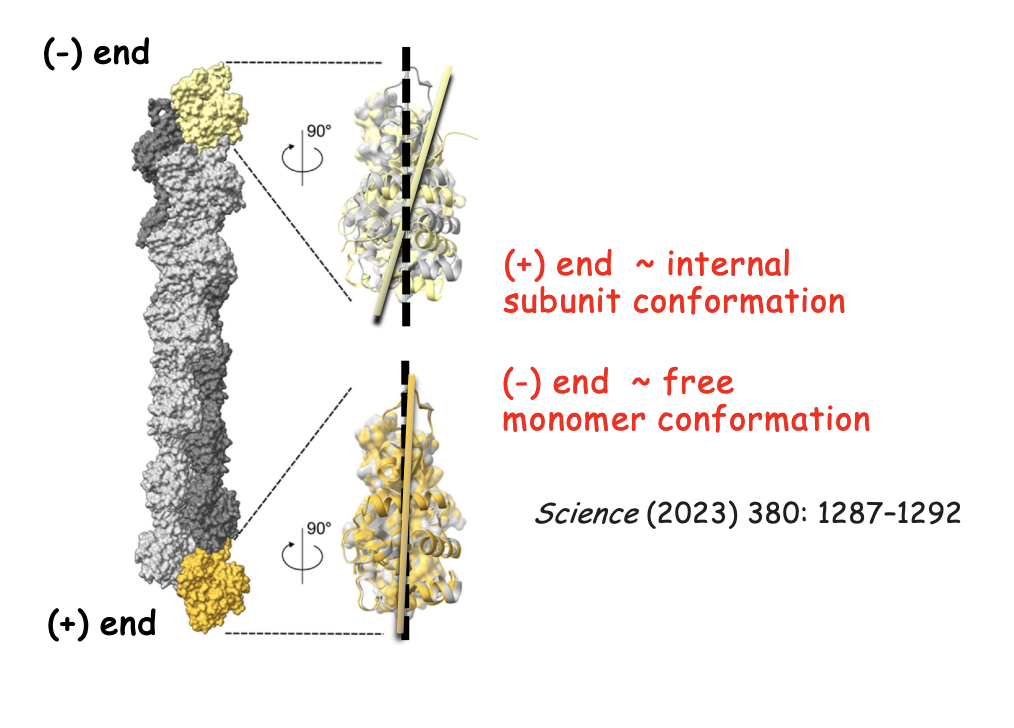
Distinct actin conformations at (+) vs (-) end
Plus (+)
retains flat conformation
typical of internal subunits
→ therefore: Favours subunit addition
Rate of addition of ATP-actin is > Rate of conversion to D form
so + end retains T subunit form
Minus (-)
twisted
monomer-like conformation
→ Therefore: primed for subunit dissociation
disfavours incorporation of new subunits
contains the D form
This has been revealed with what
Cryo-electron microscopy (Cryo-EM) high resolution
Right side→ + and - ends with internal F-actin subunit
structures aligned by their inner domains (surface representation)
showing relative rotation of outer domains (ribbon representation)
Top→ - end→ Black dotted line→ axis of outerdomain in internal subunit→ Shows twisted (monomer like)
Bottom→ + end→ Flat conformation→ (polymerised conformation)
Each end has a different Critical concentration (Cc)
Cc pointed (-) end > Cc barbed (+) end
So for there to be a steady state→ needs to be more monomers around for the - end because this end is prone to depolymerisation
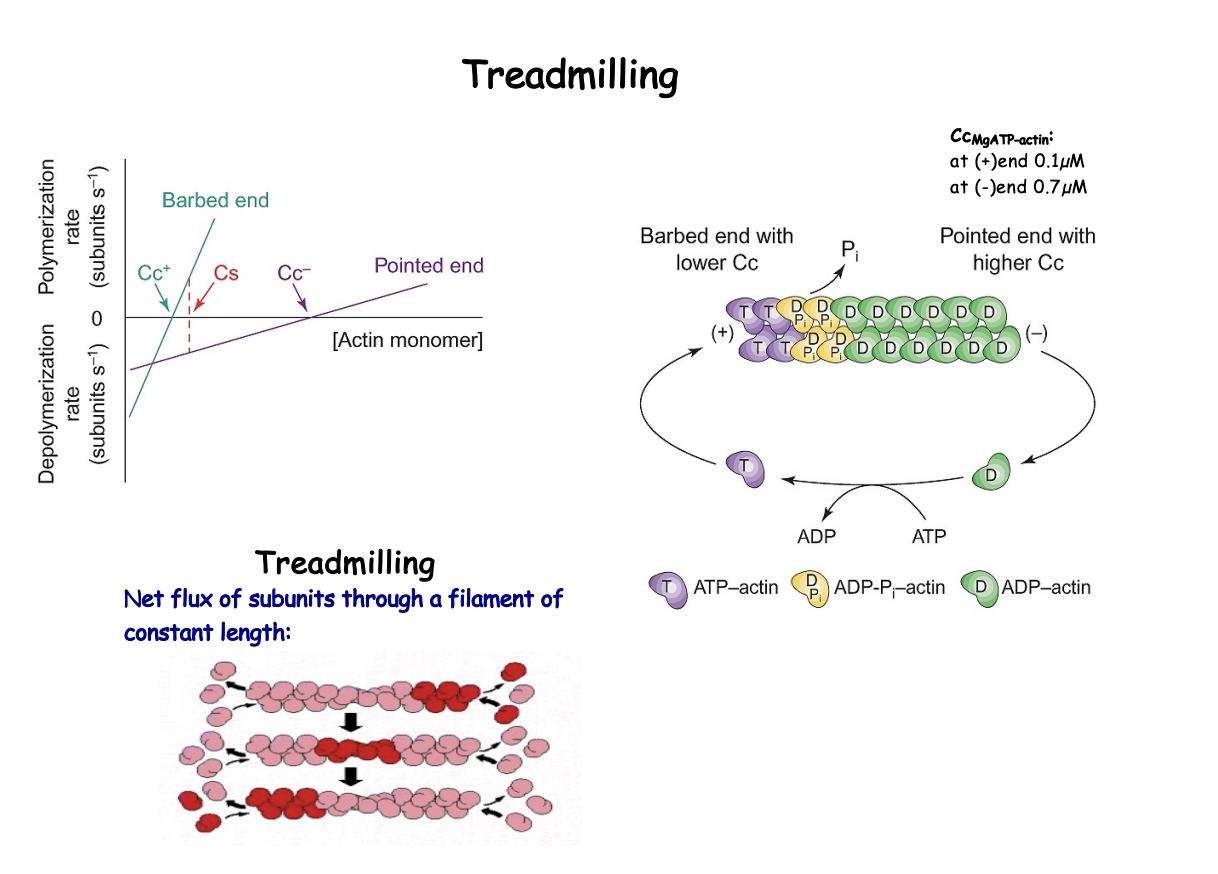
What is treadmilling
net flow of actin subunits through a filament of constant length
seems static but is continuously breaking down and building up
Contains:
ATP-bound subunits at the + end
ADP bound subunits at the - end
When does treadmilling happen
At a set concentration of G actin intermediate bewteen the Cc + and Cc-
F and G actin are at steady state (see graph)
filament length and the concentration of monomeric actin (Cs) will not change over time
Rate of loss of molecules at pointed end BALANCES addition at the barbed end
Treadmilling is done due to
ATP hydrloysis and distinct dynamics of the two ends
Helped by existence multiple polymer conformation
Actin-binding proteins
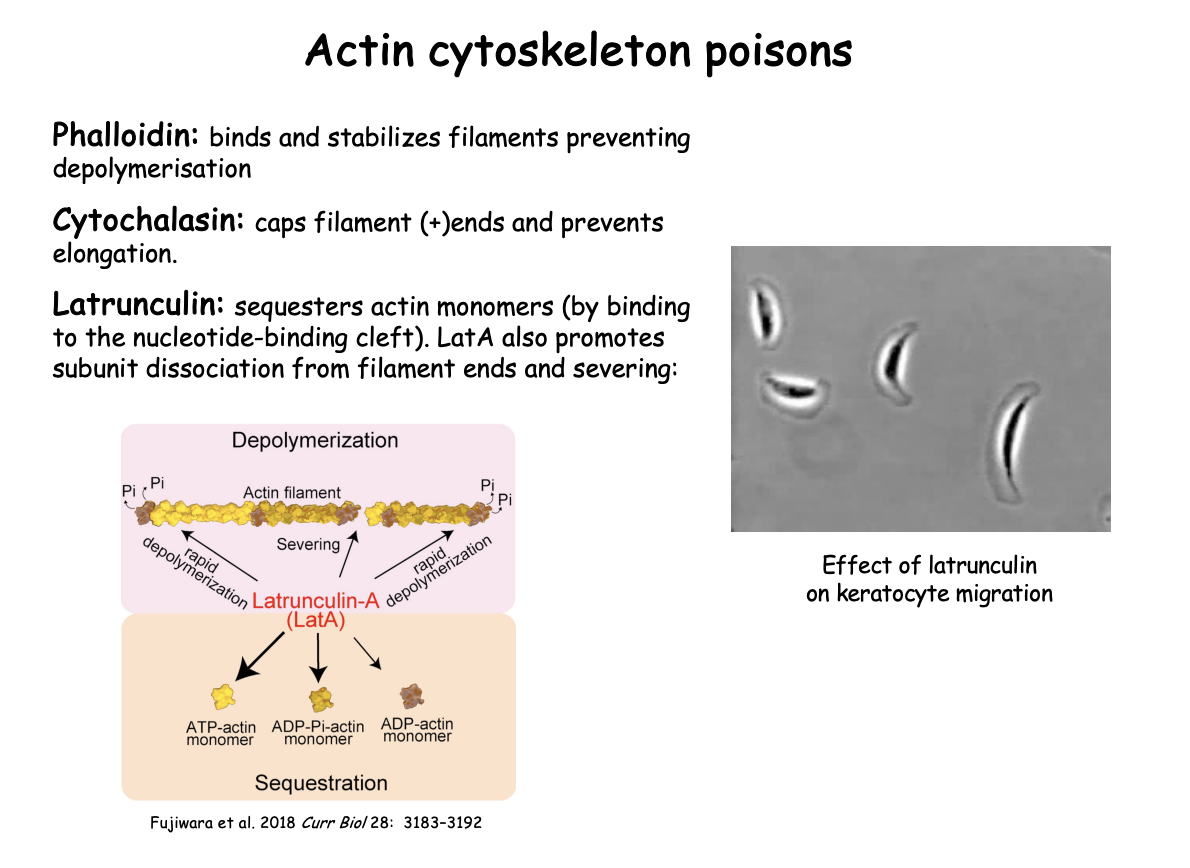
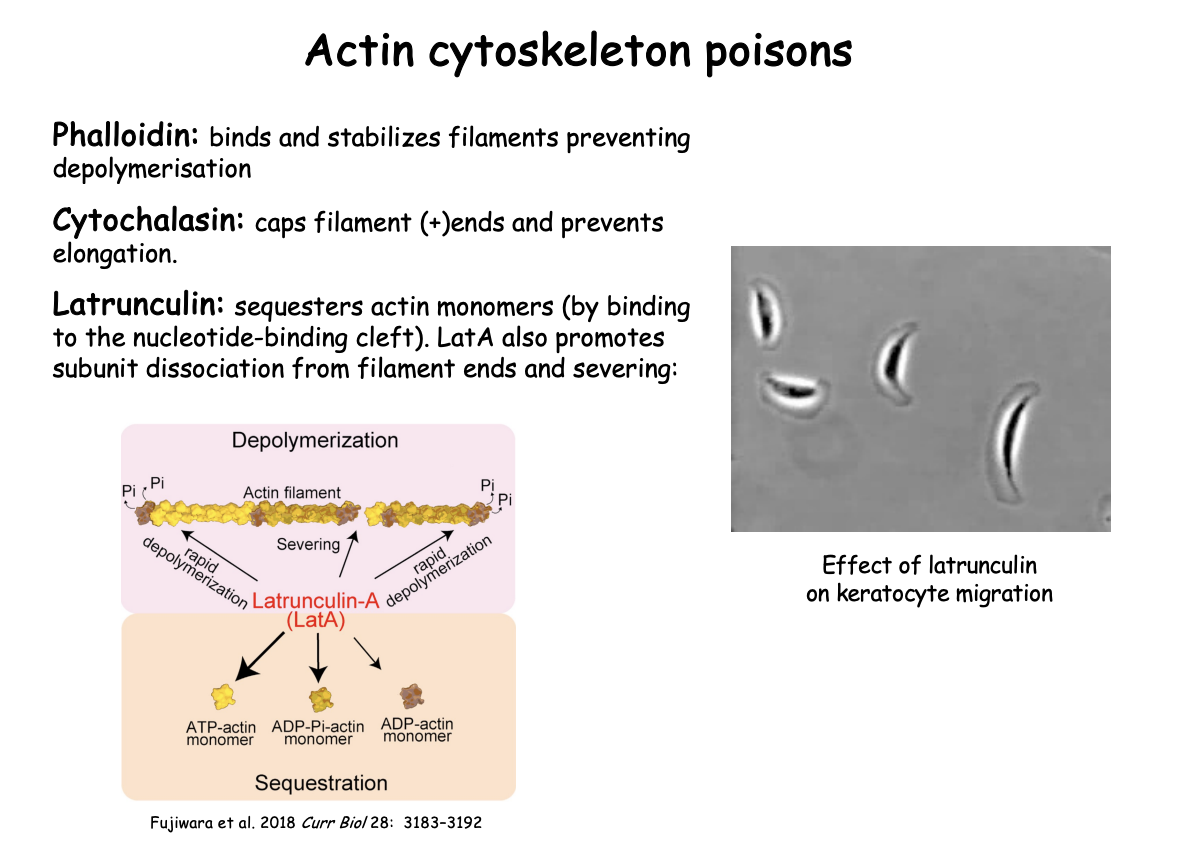
What types of actin poisons have been used to study dynamics?
Phalloidin→ binds and stabilizes preventing depolymerisation
Cytochalasin→ caps filament (+) ends and prevents elongation
Latrunculin→
Binds to nucleotide binding cleft
sequesters actin monomers
Also→ LatA promotes subunit dissociation from filament ends and severing
Phalloidin
Bicyclic heptapeptide from mushroom→ Amanita phalloids
What is does
Stabilizes F-actin
prevents depolymerisation
shows that if stabilised→ there will not be enough MFs to continue to live
Use
Rhodamine-conjugated phalloidin
high selectivity
Used for reagent to speciffically stain and visualise F-actin
for fluoresence microscopy
Cytochalasin
fungal alkaloid
What it does
caps filament + ends
prevents elongation
But as Cc- is higher than Cc+→ blocking + end leads to depolymerisation of the filament
Latrunculin and what it demonstrates
from certain sponges
what it does
sequesters actin monomers and prevents polymerisation into filaments
LatA→promotes subunit dissociation from filament ends and severing
Effect on keratocyte migration demonstrates:
requirement for actin polymerisation in generating protrusion at the leading edge and associated movement
i.e shows essential for movement
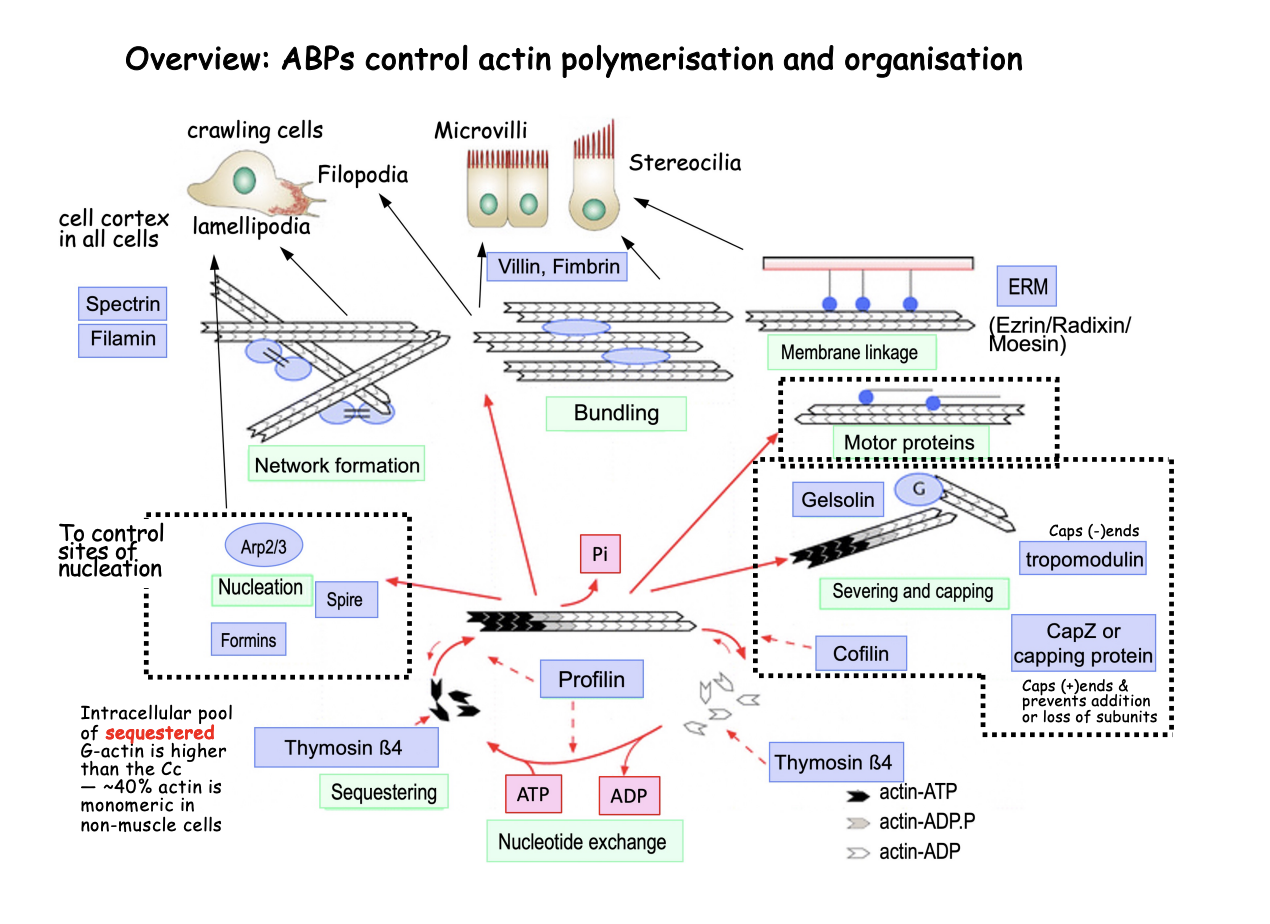
Overview of how Actin Binding proteins (ABP) control actin polymerisation and organisation
Help general actin polymerisation cycle
But also help organise into different structures for different functions
Organisation of the cell cortex
Network formation
Bundling
Membrane linkage
Motor proteins
Help in general actin polymerisation cycle
Sequestering→ e.g thymosin beta4
40% of all monomeric actin is in a pool
but this is higher than the critical concentration→ so why doesn’t it cause polymerisation?
ABP sequester the G actin into a pool
Nucleation→ e.g Formins, Spire and Arp2/3
used to control the generation of new actin filaments spatially and temporally
Nucleotide exchange→ e.g profilin
Promodes ADP/ATP exchange and delivers ATP-actin for polymerisation
Severing and capping→ e.g Gelsolin, CapZ or capping protines, Topomodulin
limits elongation as the cap of the + end
or causes filament fragmentsation
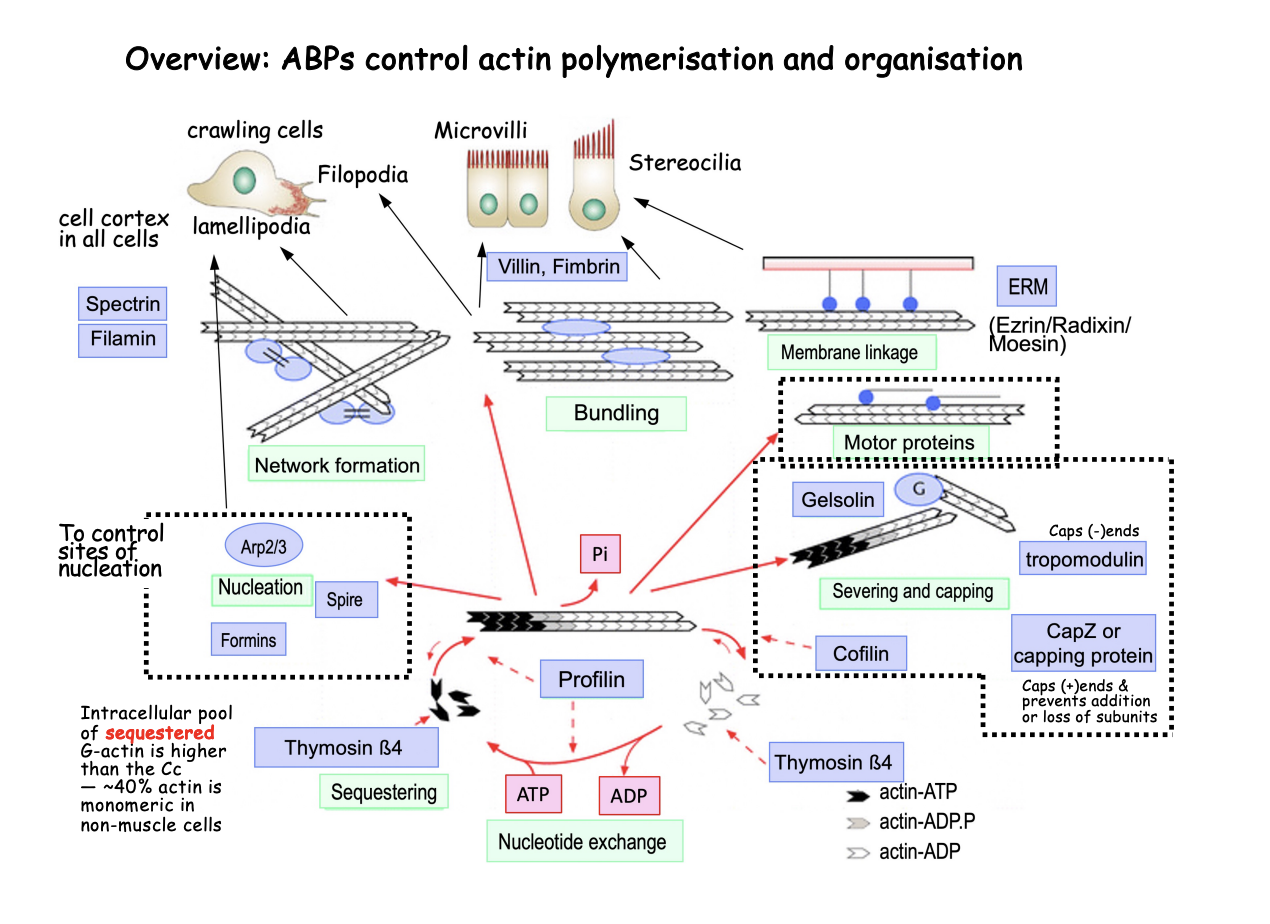
Why need sequestering
ensures that it causes rapidly changes in polymerisation
no need to wait for it to be produced
stops under signals?
How ABP are used for more specialised functions
Motor proteins→ e.g myosins
Actin-dependent motor proteins
powered by ATP hydrolysis
move along actin tracks and transport cargo or mediate contractility by sliding antiparallel filaments with respect to each other
Organisation of the cell cortex
Way the cell cortex can be organised
Network formation→ spectrin, filamin
Crosslinking proteins→ loose network
Bundling→ Villin, Fimbrin
bundling proteins→ tight bundle
Membrane linkage→ ERM (Ezrin/Radixin/ Moesin)
anchor filaments to membranes
Combinations of these ABPs can form higher order actin strucutres, imparting overall shape and function
Lamellipodia and filopodia→ drive cell crawling
dynamic membrane protrusions at the leading edge
Tight actin bundles support persistent strucures:
Microvilli→ brush boarder to maximise SA
Sterocilia→ transudce sound by mechanical displacement in hair cells
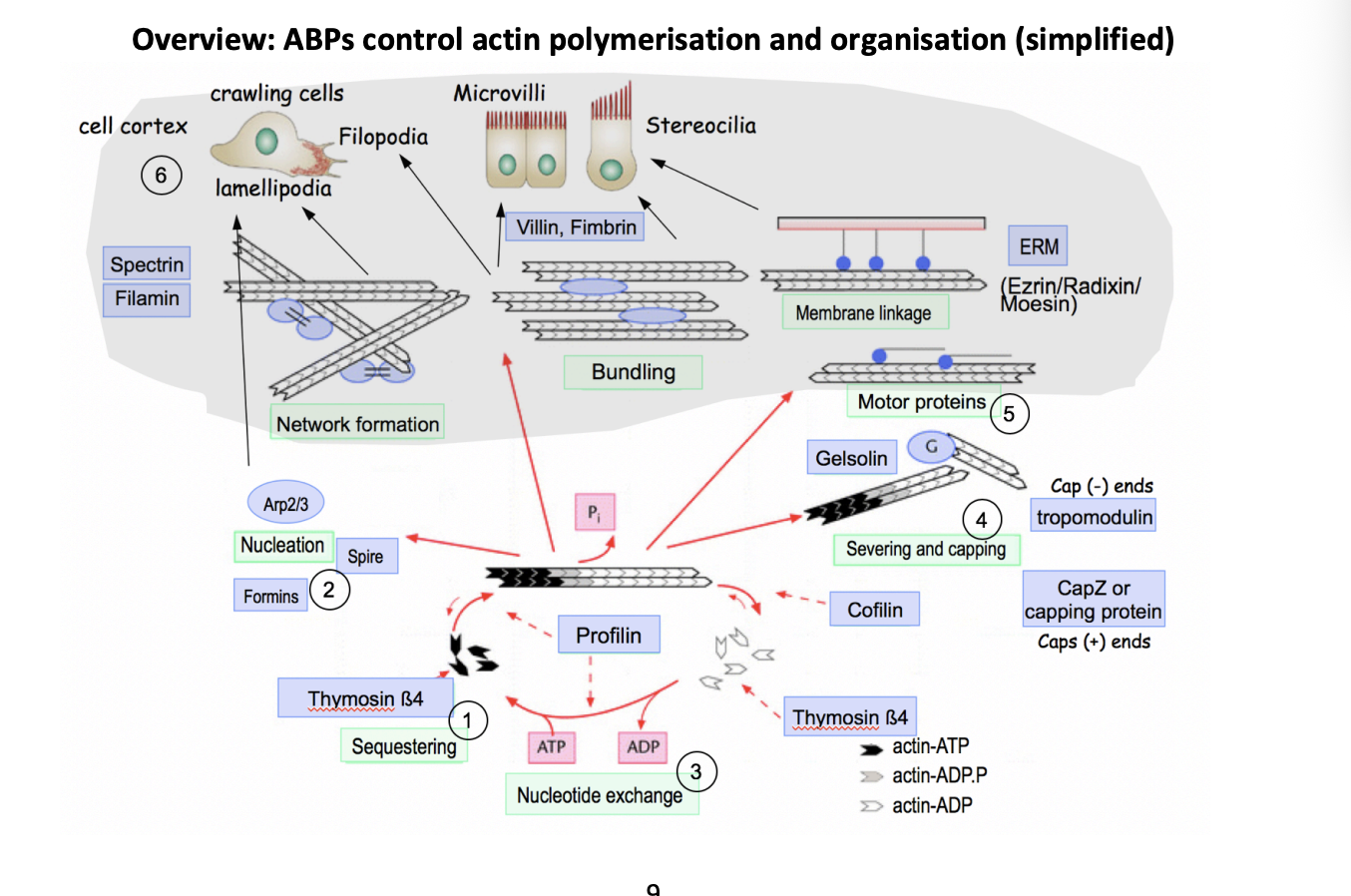
Summary