Strengthening Mechanisms and Annealing
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What is the yield point?
The point at which dislocation motion initiates (i.e. plastic deformation begins)
How do you increase the yield strength of a material?
Stop the dislocations in the material from moving
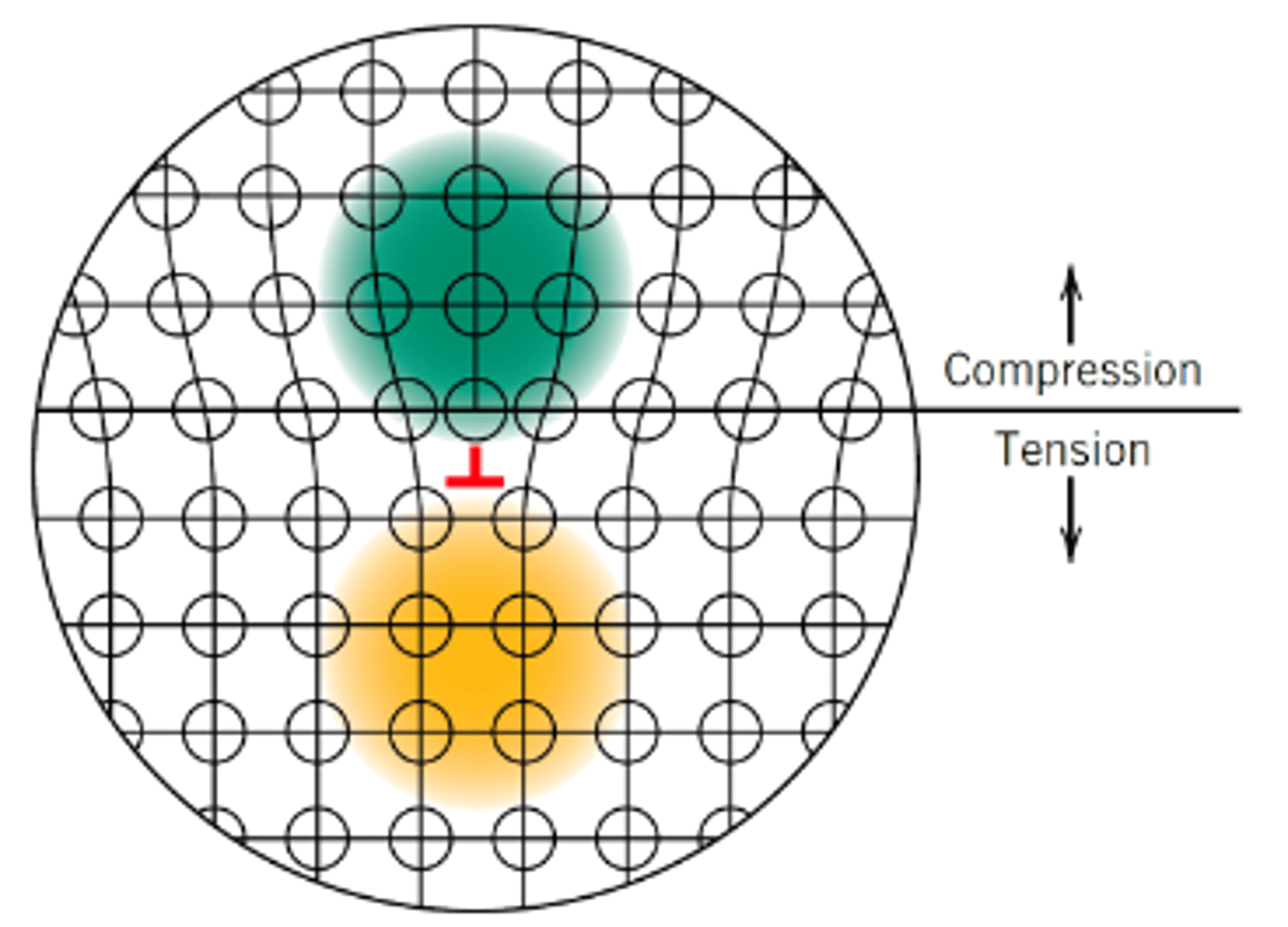
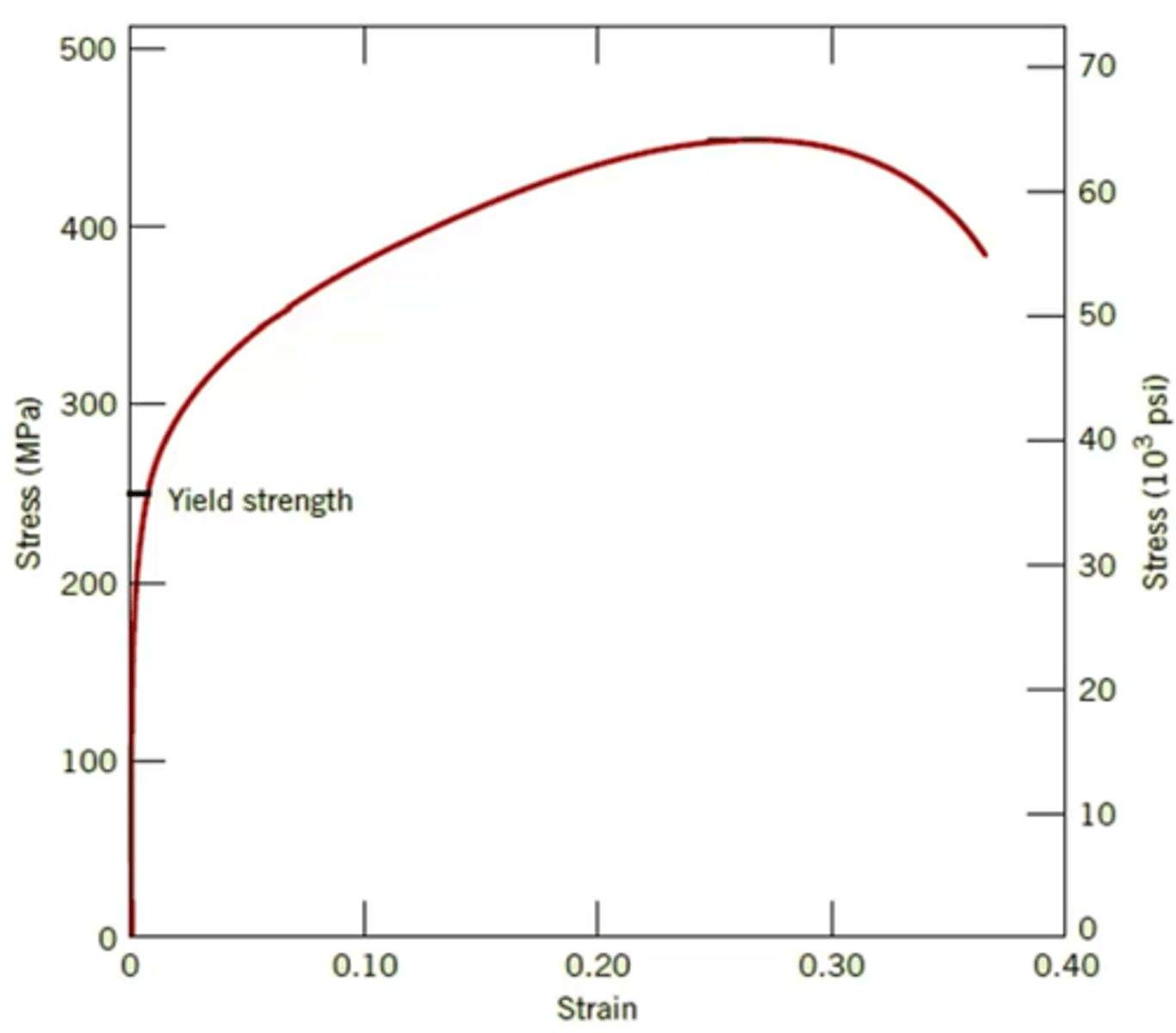
Why do we have to increase the stress (σ) to keep dislocations moving?
Dislocations multiply and tangle during plastic deformation, making further dislocation motion more and more difficult (this is the concept of work hardening)
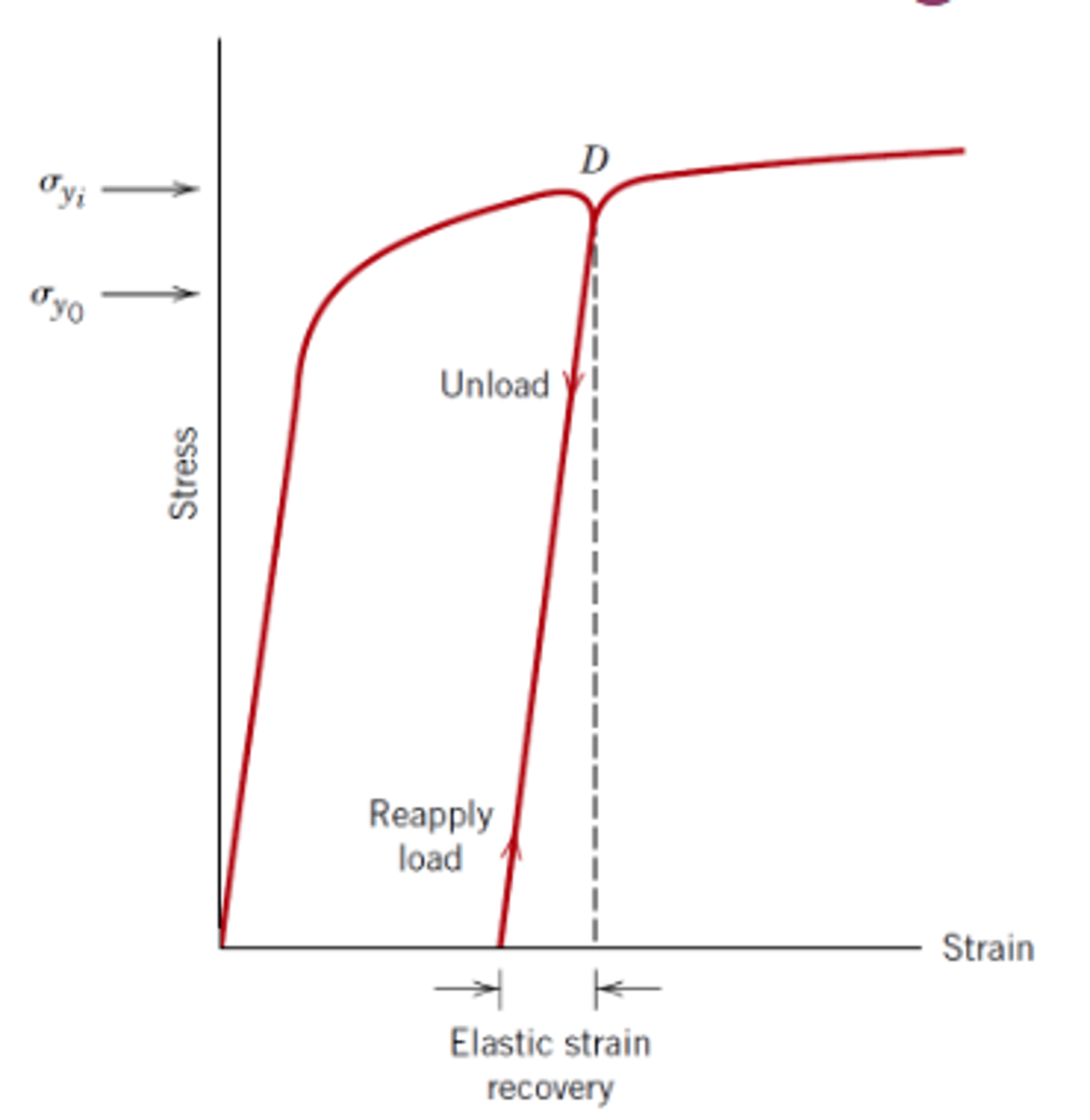
How do you increase the yield stress of a material through work hardening?
Load material beyond original yield stress (but not enough to cause failure) then unload at the desired new yield stress (the material will also unload at exactly the same gradient it loaded at)
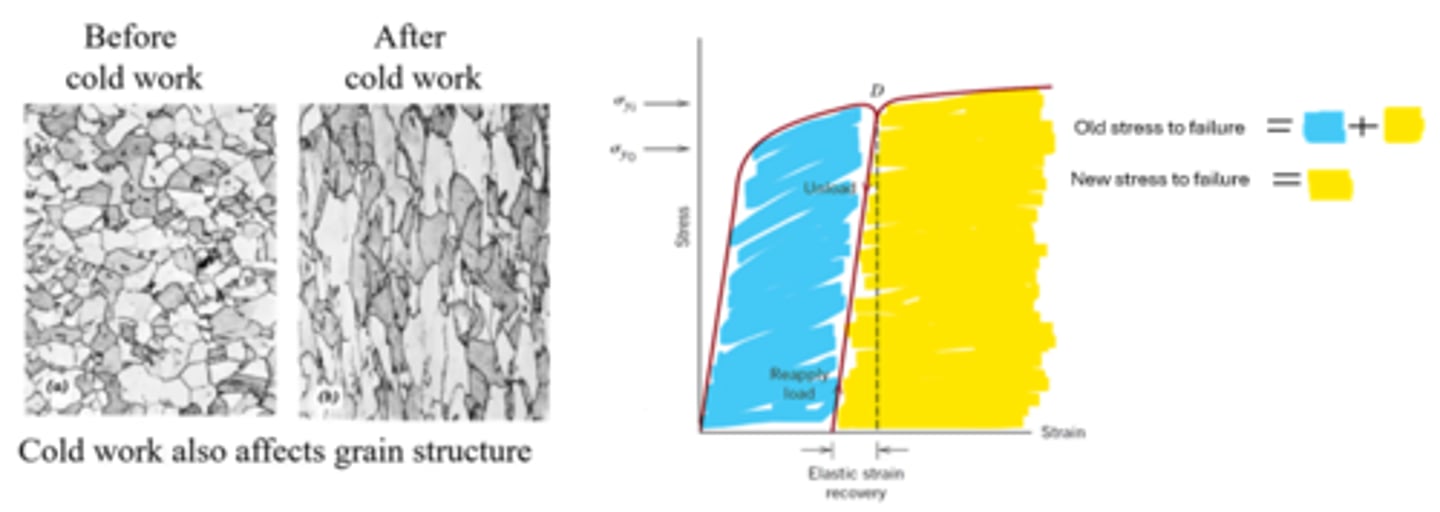
What are some of the side effects of work hardening?
• Could change the microstructure of the material (i.e. change the way the crystals are in the material)
• Material will become more brittle (i.e. σy ↑, %el. ↓)
Can dislocations move through a grain boundary?
No
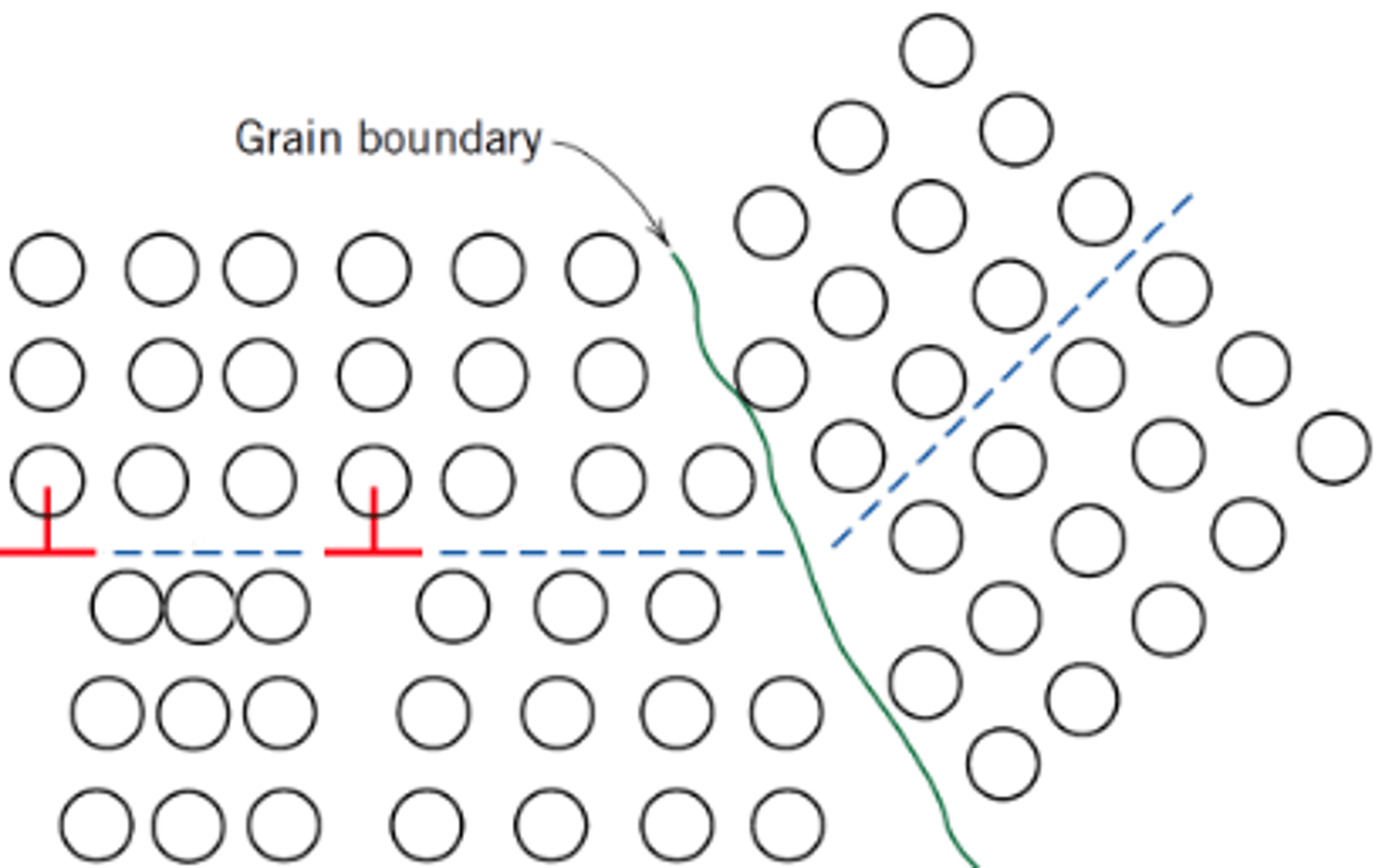
What is grain boundary strengthening?
Dislocations pile up at the grain boundary, causing a local stress concentration, until they initiate slip (dislocation motion) in the next grain
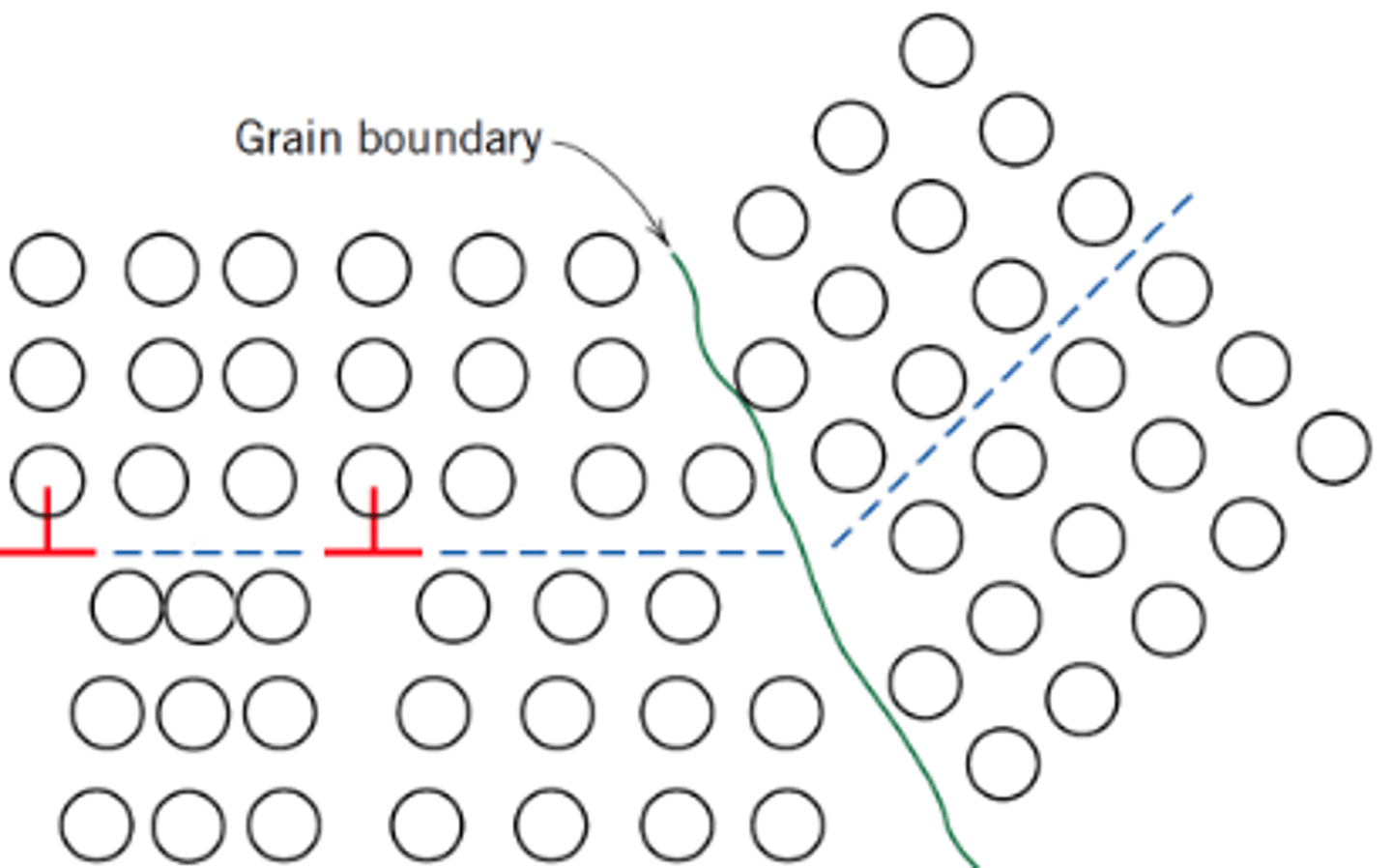
Which is stronger, fine grained materials or large grained materials?
Fine grained materials, as the bigger the grain, the longer the pile-up, the bigger the stress concentration and the easier it is for slip to initiate in the next grain
What is the Hall-Petch equation?
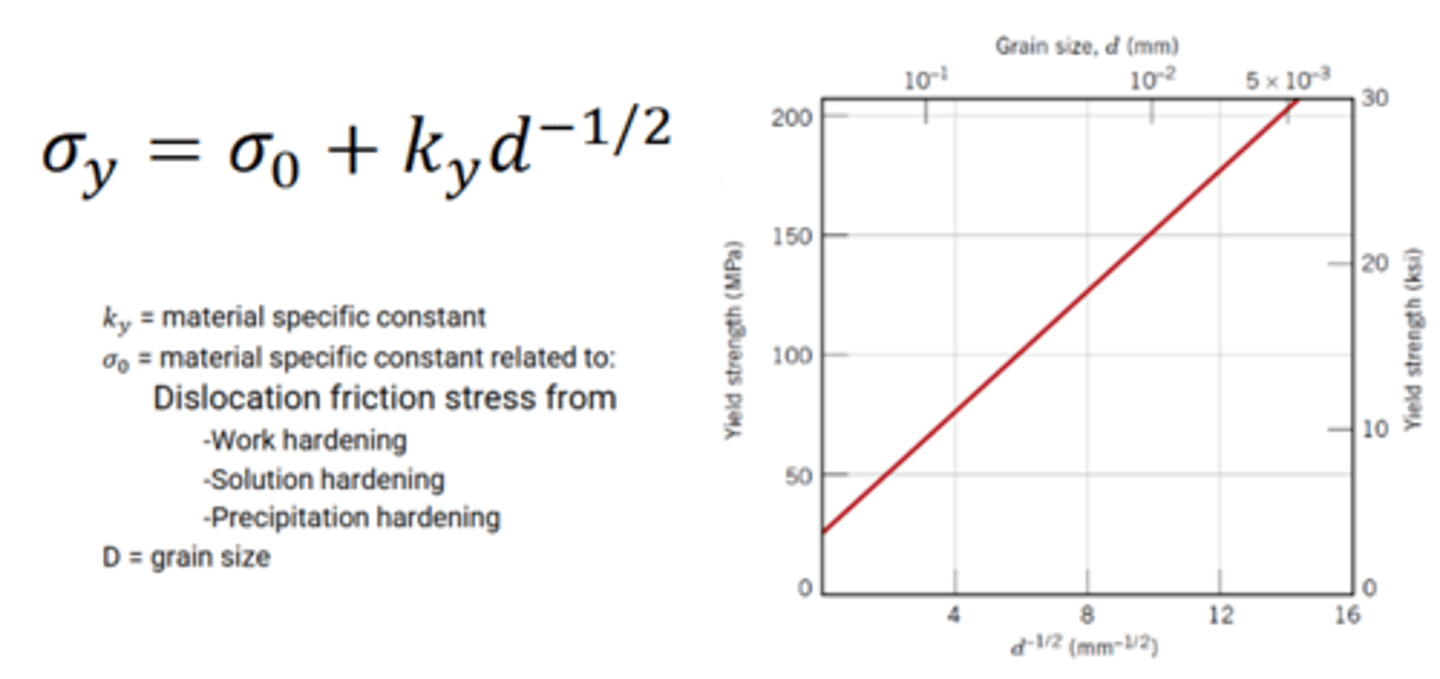
Why is grain boundary strengthening not great in high temperature environments?
Lots of diffusion around grain boundaries when using fine grains, however using coarse grains can help reduce this
What are precipitates?
Regions within a crystal with a different structural arrangement
What is precipitation hardening?
Precipitates are formed within a crystal to act as obstacles to dislocations. This strengthens the material by impeding plastic deformation
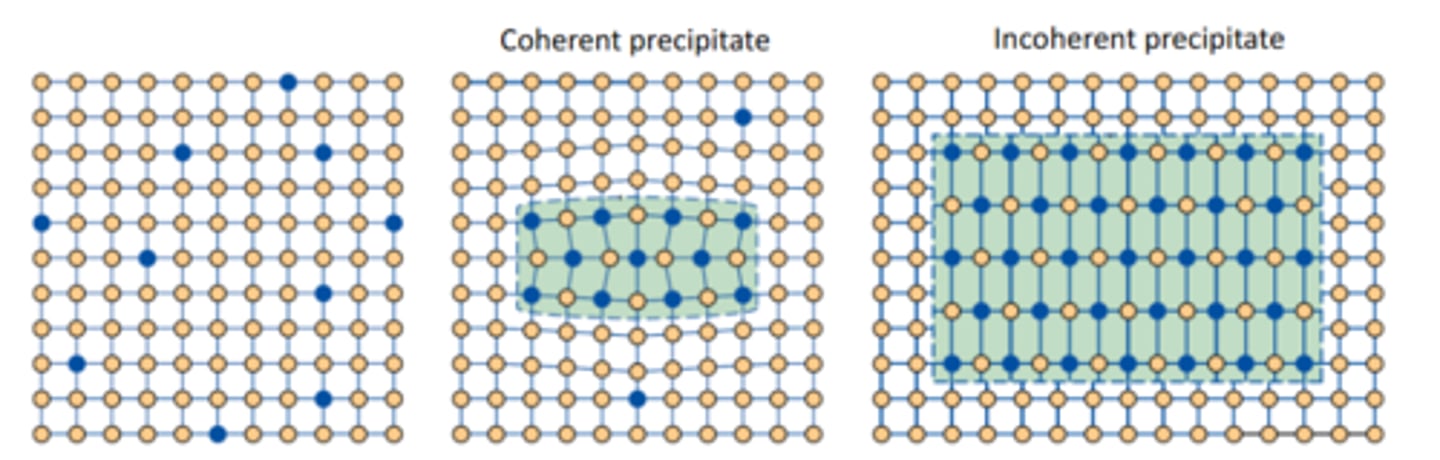
What are the 3 heat treatment steps for precipitation hardening?
• Solution Treatment
• Quenching
• Aging
What happens during Solution Treatment? (Precipitation Hardening)
Alloy heated until all components dissolve into a single solid solution
What happens during Quenching? (Precipitation Hardening)
• Metal is rapidly cooled (quenched) at room temperature.
• The atoms don’t have time to diffuse and form precipitates, so the high-temperature solid solution becomes “frozen in.”
• This creates a supersaturated solid solution
What does it mean if a solid solution is supersaturated?
The solid contains more of the dissolved substance (solute) than it could normally hold under equilibrium conditions at that temperature
What happens during Aging? (Precipitation Hardening)
Alloy reheated to a moderate temperature allowing precipitates to form and spread throughout the metal
How does precipitation hardening strengthen the material?
Hard precipitates of a different structure within the metal matrix are difficult to shear, and thus act as barriers to dislocation motion
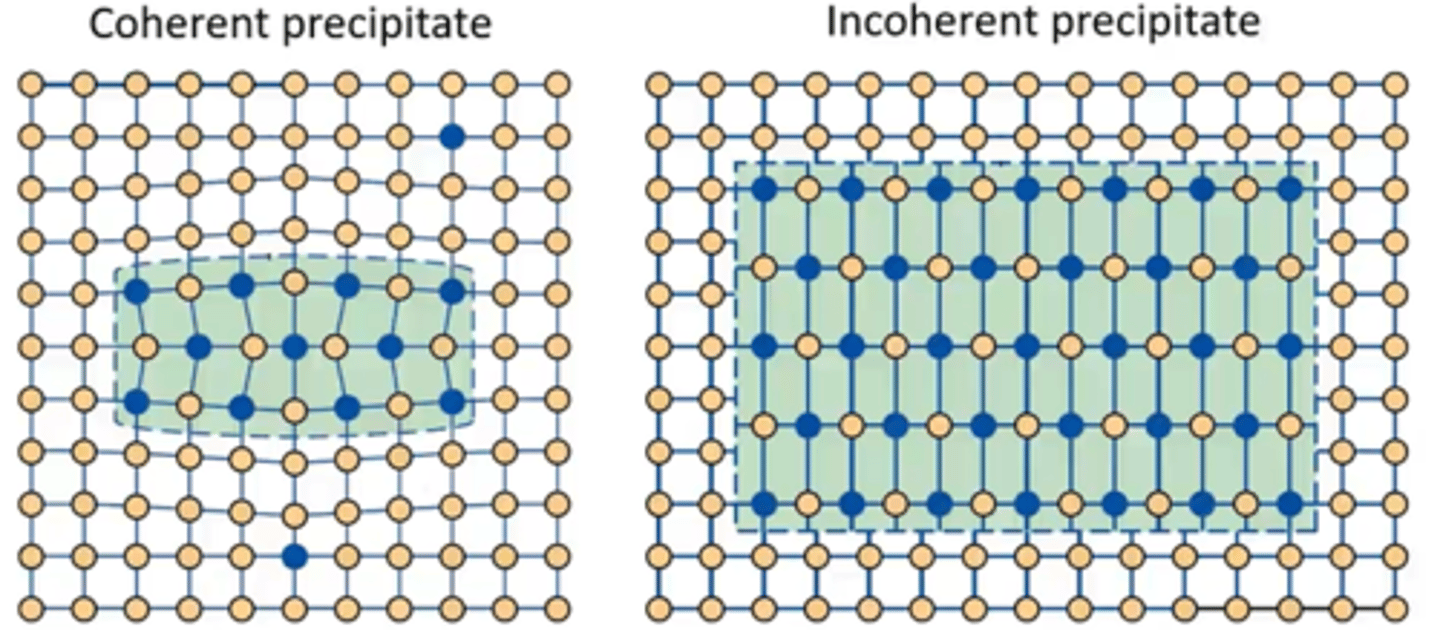
What must the precipitates be for dislocations to cut through them?
Small and Coherent
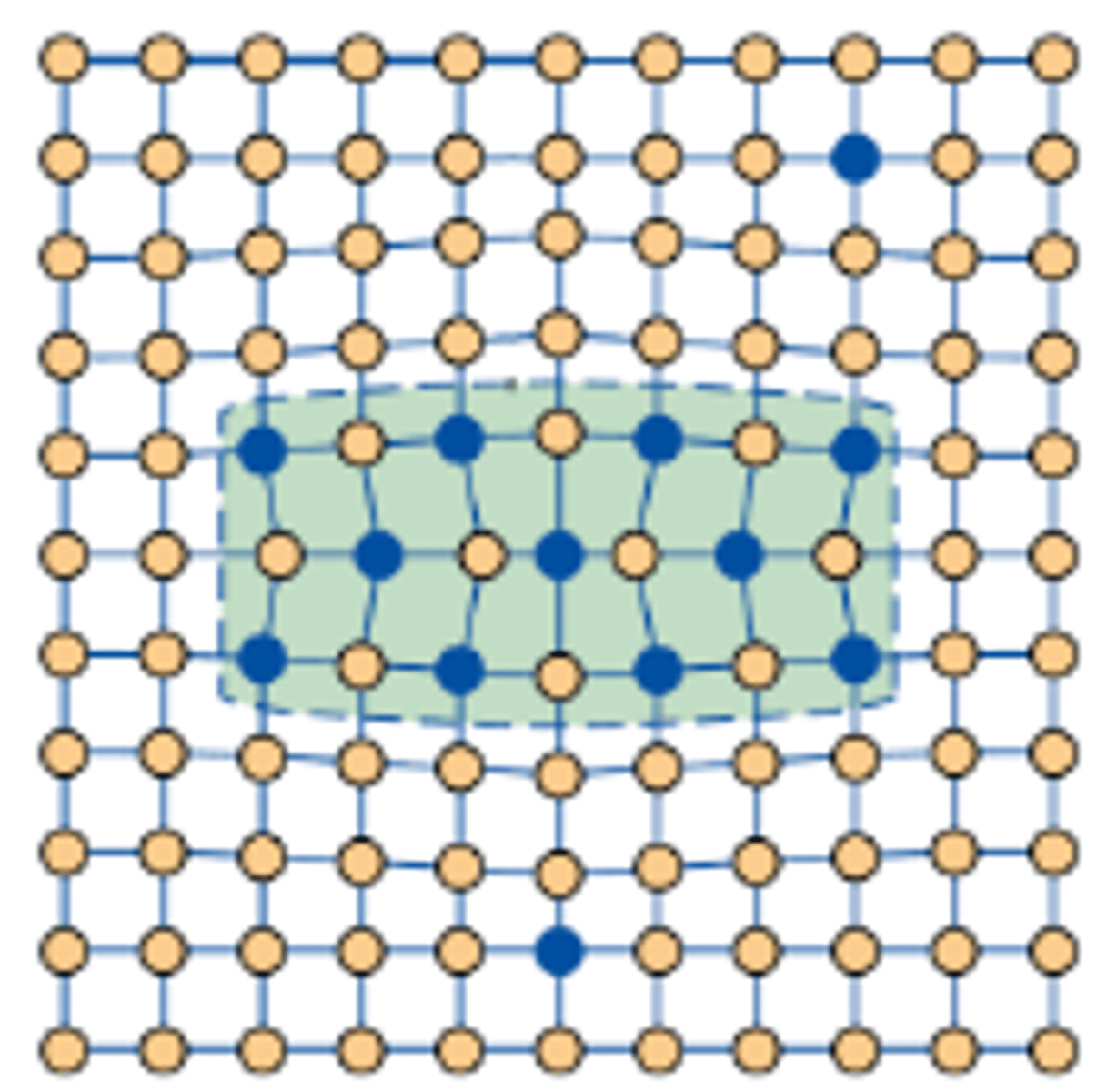
How do dislocations cut through precipitates?
The dislocation enters and shears precipitates, which requires extra energy due to lattice distortion in the precipitate’s structure, thus increasing alloy's strength
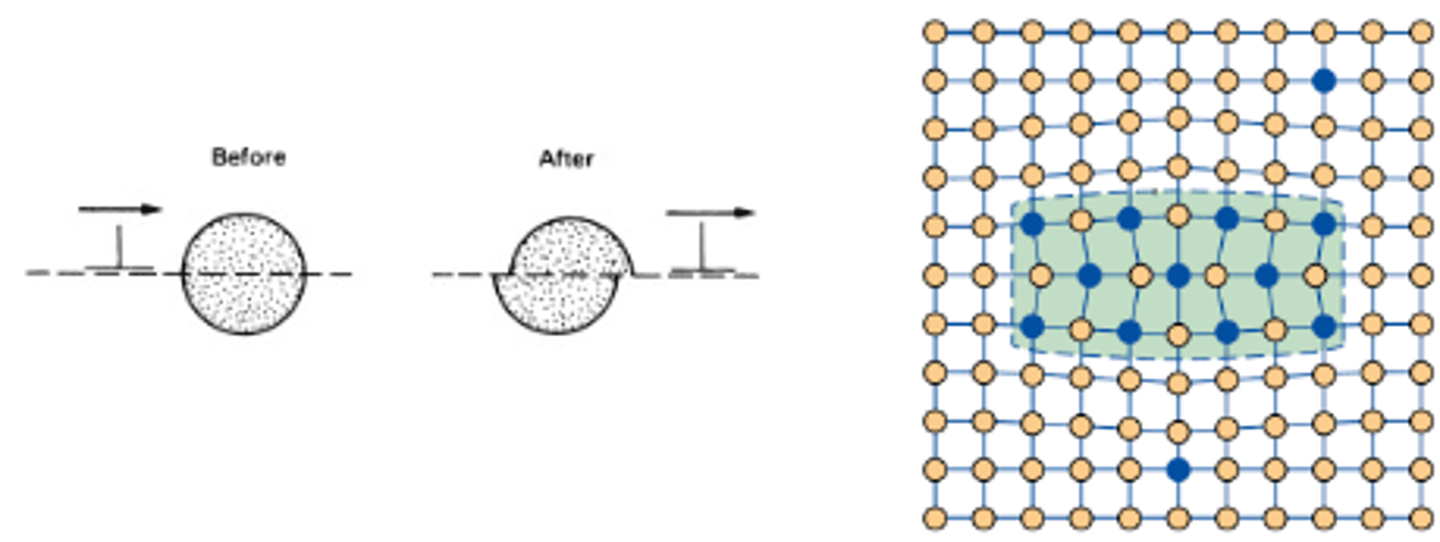
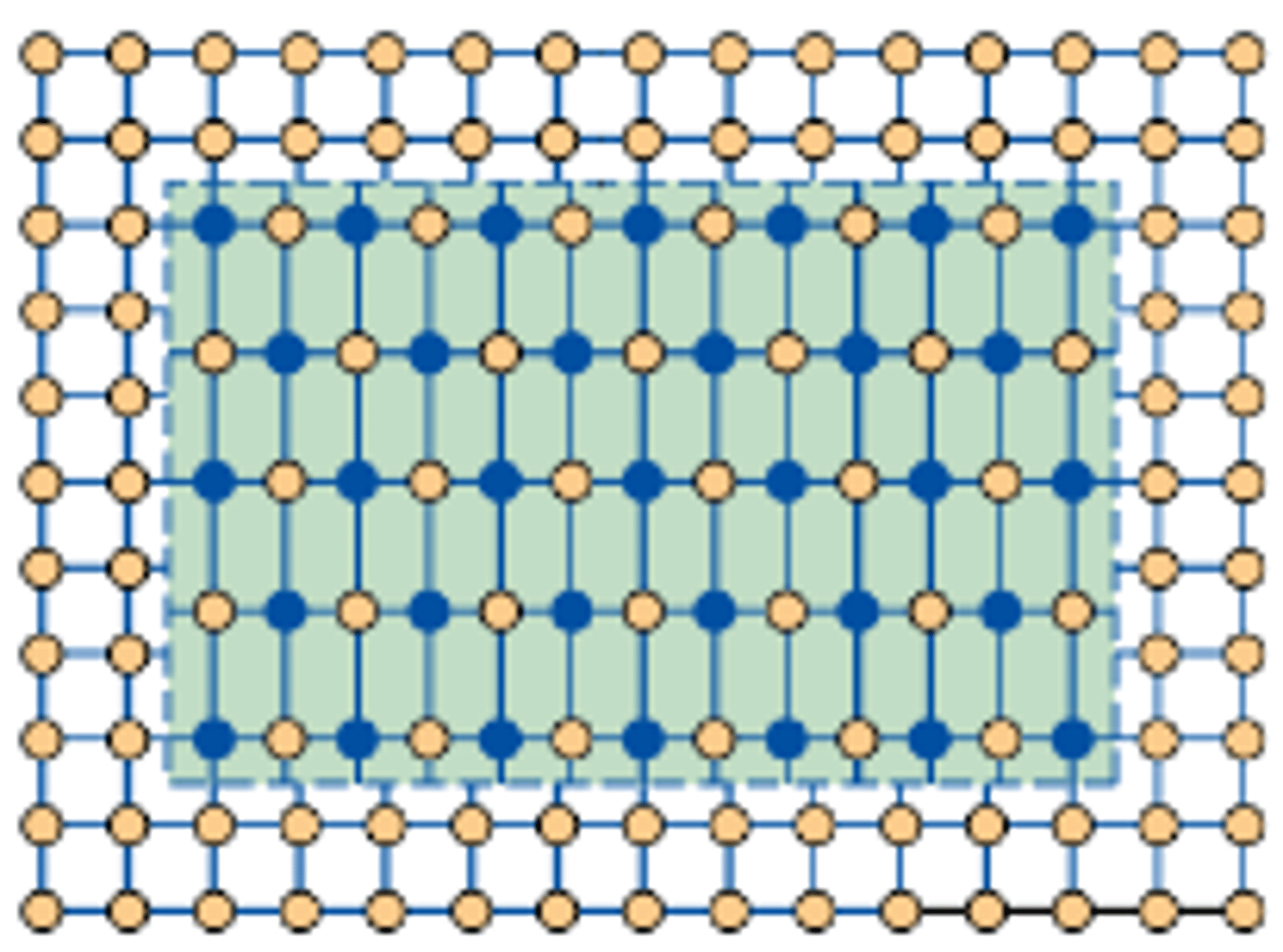
What must the precipitates be for bowing to occur?
Large and Incoherent
What is bowing?
When a dislocation gets pinned between precipitates and stress begins to increase, it can bow out between them. This keeps the dislocation moving however this process requires extra stress
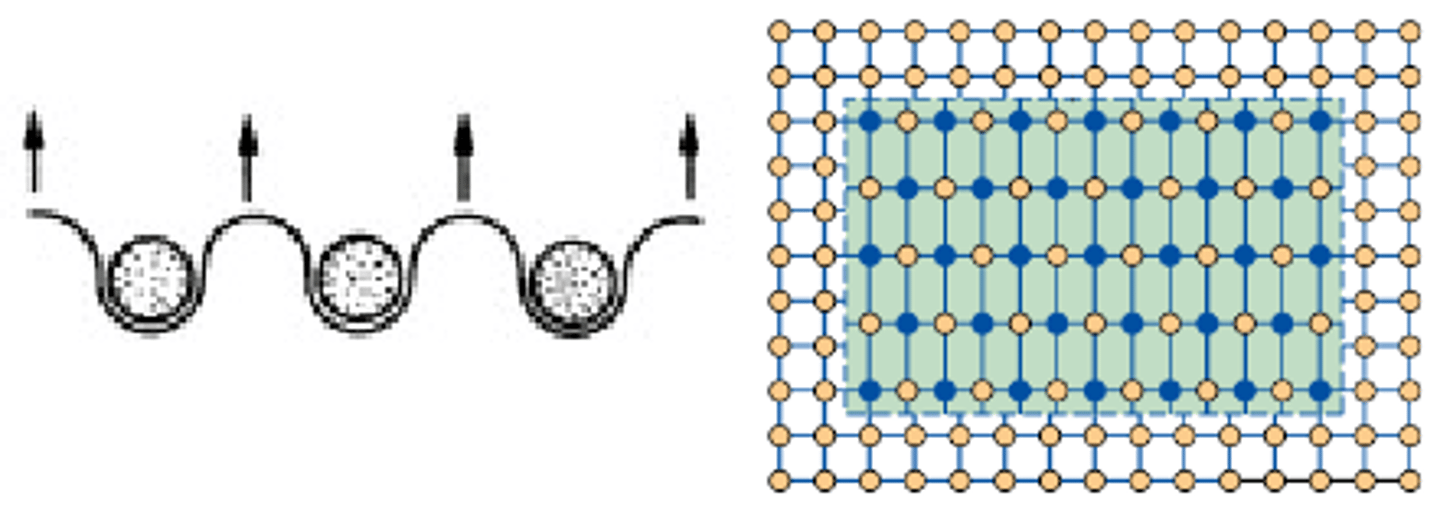
What are the 3 stages of annealing?
• Recovery
• Recrystallisation
• Further Grain Growth
What happens during Recovery? (Annealing)
Dislocations rearrange and thus some stresses from cold work are relieved. Metal is slightly softer, but still relatively hard, and there is no change in grain structure
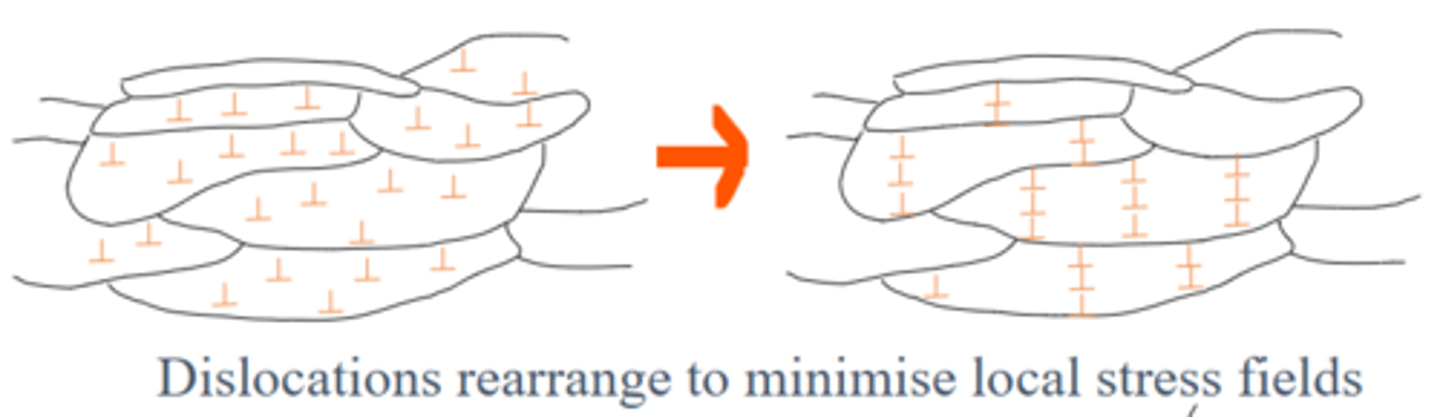
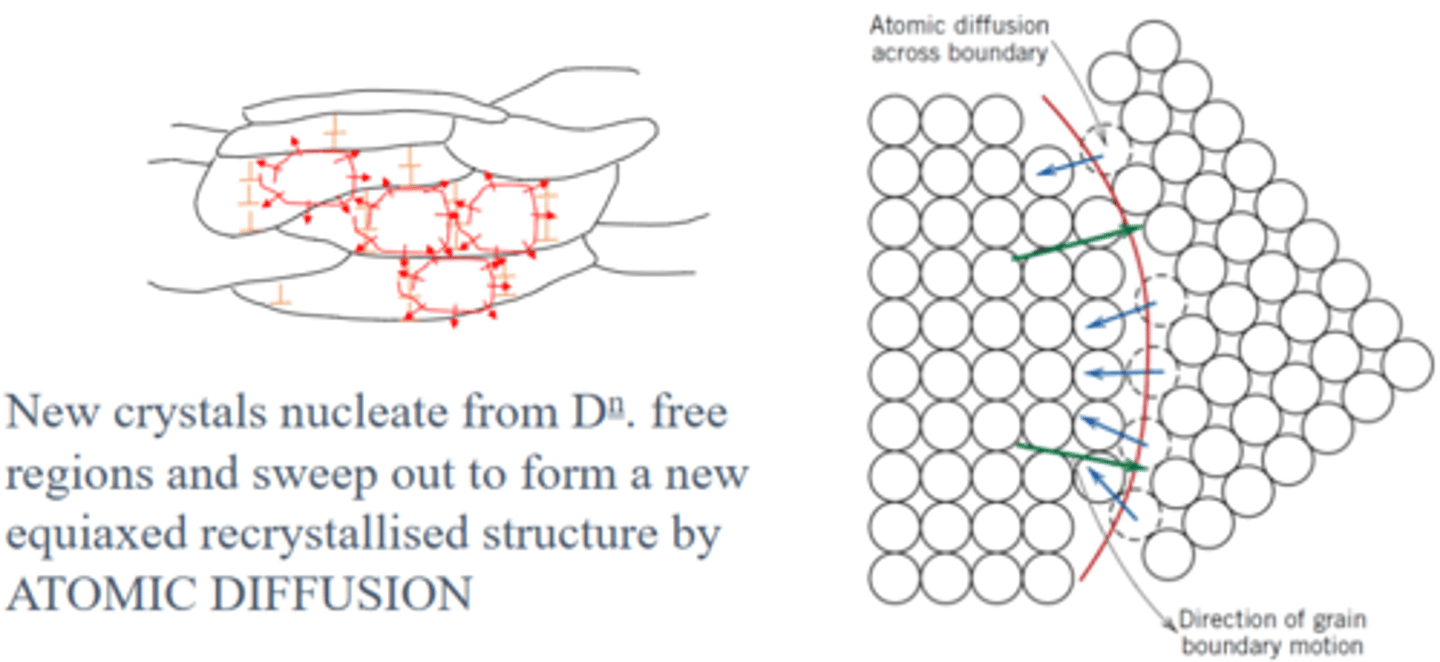
What happens during Recrystallisation? (Annealing)
New, strain-free grains form in dislocation free regions and replace the deformed, worked-hardened ones. Atoms diffuse across grain boundaries so that the new strain-free grains expand. Hardness and strength drops but ductility is restored
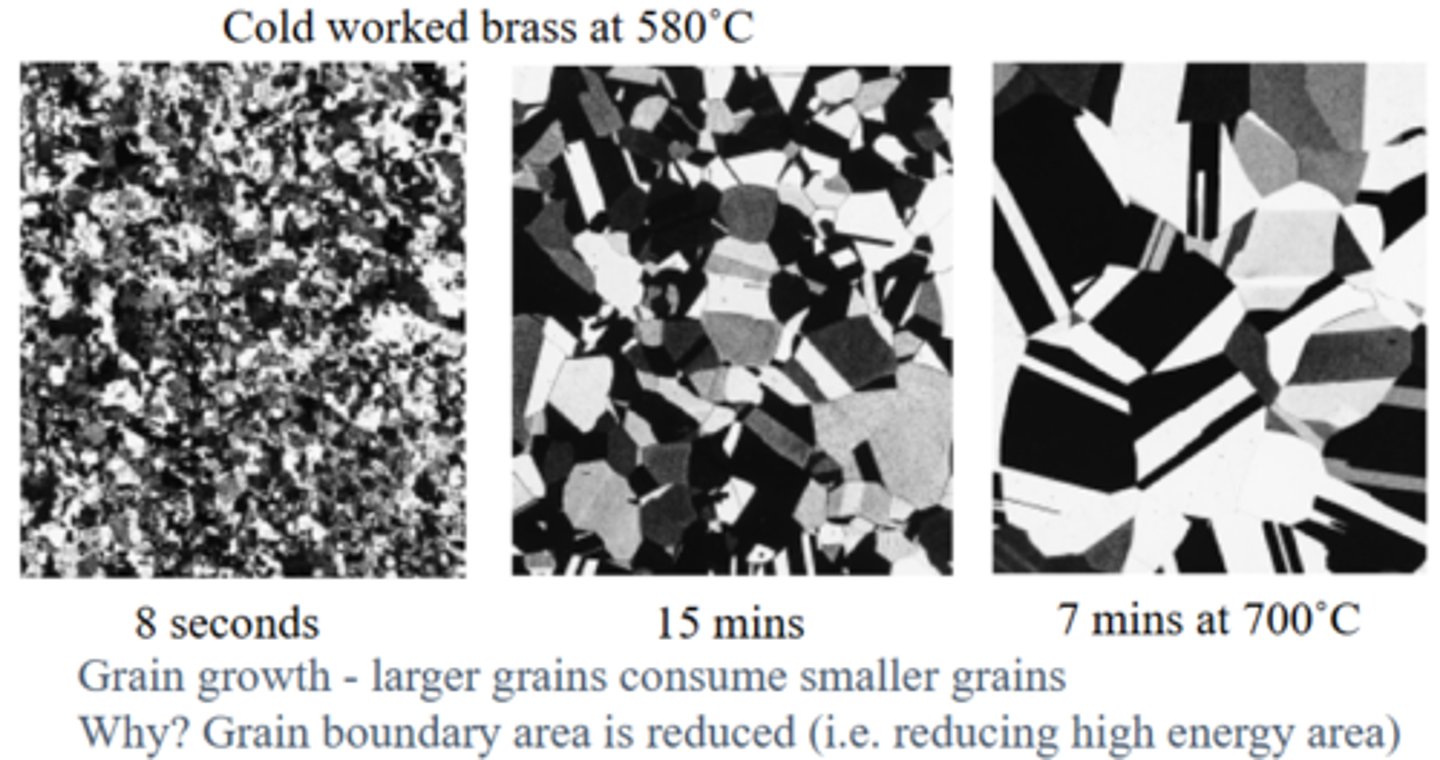
What happens during Further Grain Growth? (Annealing)
New grains grow larger if the metal is kept hot for too long, consuming smaller ones to reduce total grain boundary area and lower the system’s energy. Strength decreases further but ductility increases
What does the size of recrystallised grain structures depend on?
• Amount of prior cold work (strain) (i.e. more cold work = smaller grains, less cold work = larger grains)
• Annealing temperature (diffusion controlled process) (i.e. higher temperature = larger grain size)
What does diffusion depend on? (i.e. how can it be controlled?)
• Temperature (faster diffusion at higher temperatures, so therefore faster recrystallization)
• Time (longer times allow more atoms to move, allowing for more complete grain formation)