BIOL 4004 UMN twin cities Cell Bio Exam 3
1/115
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
116 Terms
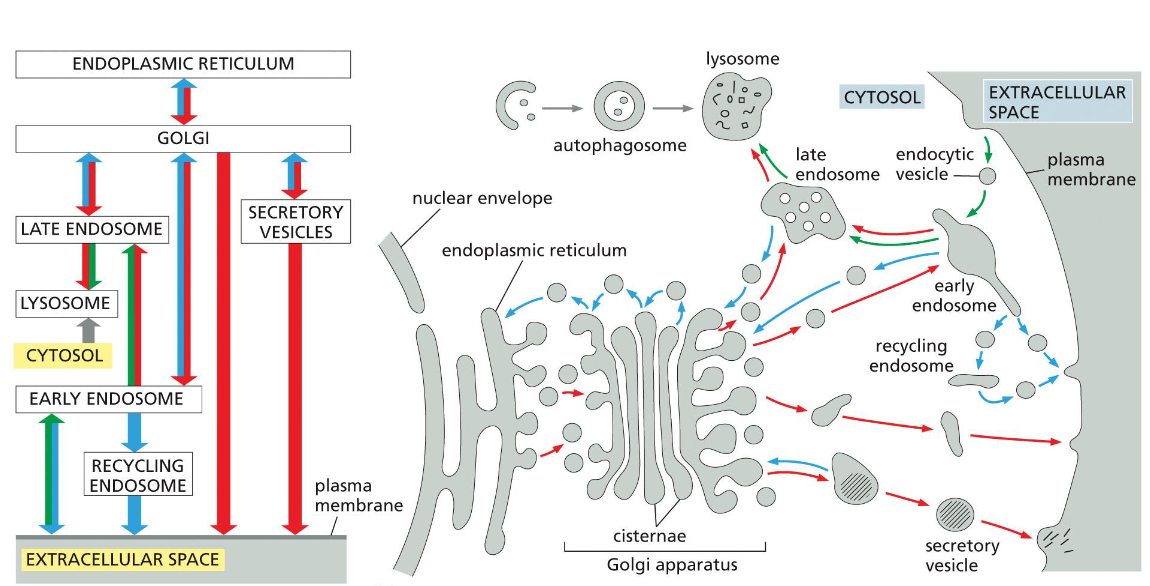
roadmap of the secretory (red), endocytic (green), retrieval (blue), recycling (green+blue), and autophagy (grey) pathways
golgi ←(secretory/retrieval)→ secretory vesicles → (secretory) → extracellular space
lysosome → autophagy → cytosol
late endosome → recycling → lysosome
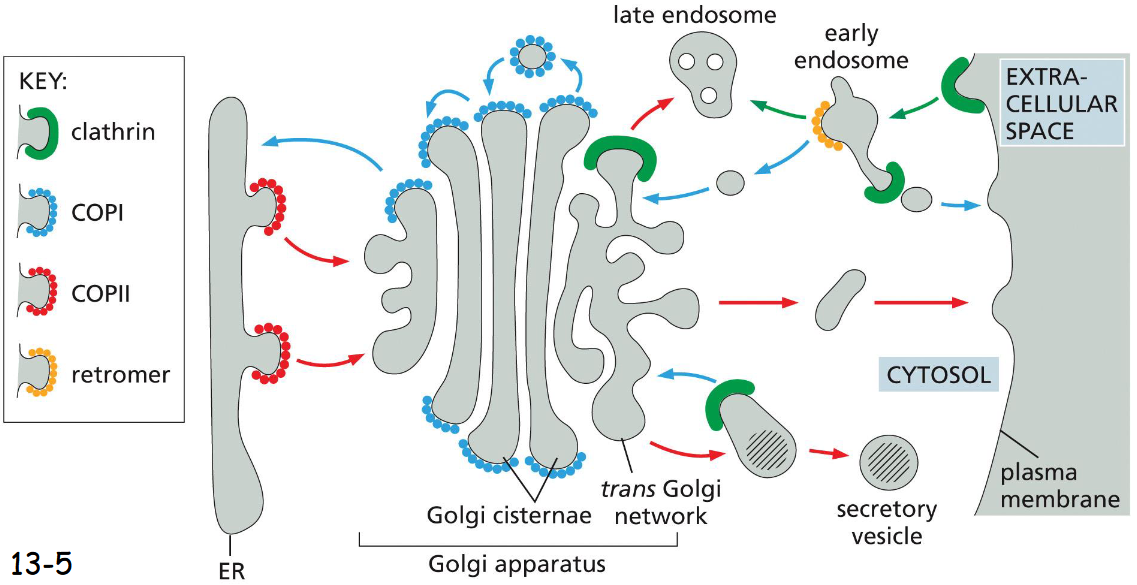
types of coated vesicles and why we need them
clathrin
COPI
COPII
retromer
helps with shaping membranes, protection + stabilization, recycling, transport specificity
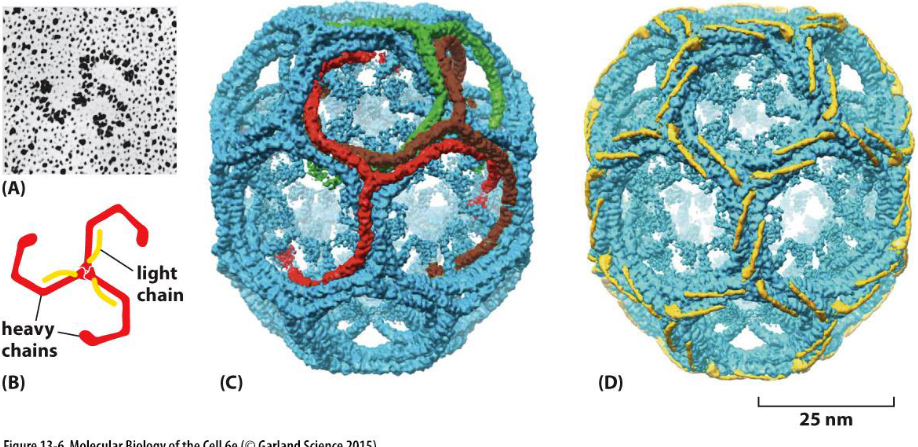
what drives vesicle formation
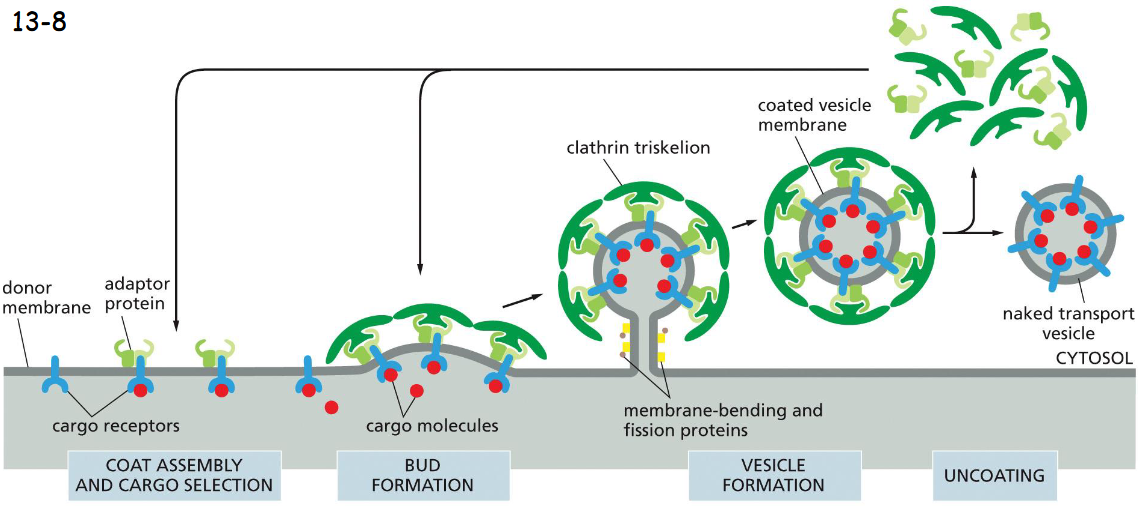
the assembly of a clathrin coat
clathrin forms the outer layer of the coat (movie 13-1)
3 large and 3 small polypeptide chains form a triskelion (A and B)
36 triskelions form a network of 12 pentagons and 6 hexagons with heavy chains (C) and light chains (D) highlighted
the light chains link to actin filaments to generate force for membrane budding
the N-terminal domains of the heavy chains protrude inward and bind to the adaptor proteins in the second layer of coated vesicles
clathrin vesicle assembly
cargo molecules bind to cargo receptors which have adaptor proteins, adaptor proteins bind clathrin triskelions
membrane bending and pinching proteins (dynamin) pinches off vesicle
the coat is rapidly lost shortly after the vesicle buds off
phosphoinositides mark:
organelles
phosphoinositol undergoes phosphorylation and de-phosphorylation at various position of their inositol sugar ring to form phosphoinositides or phosphotidylinositol phosphates (PIPs)
the head groups are recognized by adaptor proteins that discriminate between different forms, PI(3)P and PI(4,5)P2
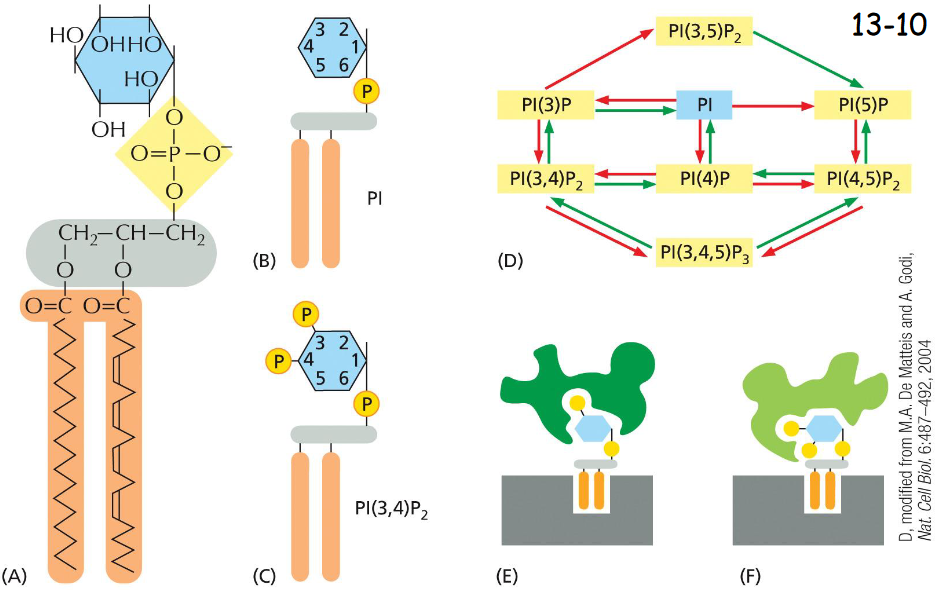
phosphoinositide changes in secretory vesicle pathway
secretory vesicles: PI(4)P
when vesicles fuse with the plasma membrane a PI(5)-kinase that is localized there converts the PI(4)P into PI(4,5)P2
the PI(4,5)P2 helps recruit adaptor proteins to initiate the formation of a clathrin-coated endocytic vesicle
once the clathrin coated vesicle buds off from the plasma membrane, a PI(5)P phosphatase hydrolyzes PI(4,5)P2 to PI(4)P
PI(4)P weakens biding to the adaptor proteins and promotes vesicle uncoating
dynamins
dynamins assemble into a spiral at the neck of each bud. The detailed events are unknown.
It has a GTPase domain that regulates the rate of pinch-off, membrane fusion and seal-off.
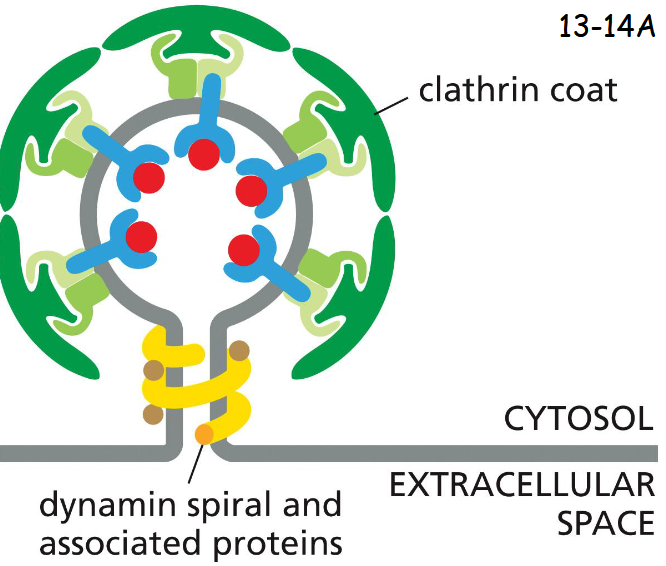
How do the vesicles rapidly lose its clathrin coat and what is the key?
A PIP phosphatase depletes PI(4,5)P2 from the vesicle membrane, changes to PI(4)P, weaken the binding of the adaptor proteins to the vesicle membrane
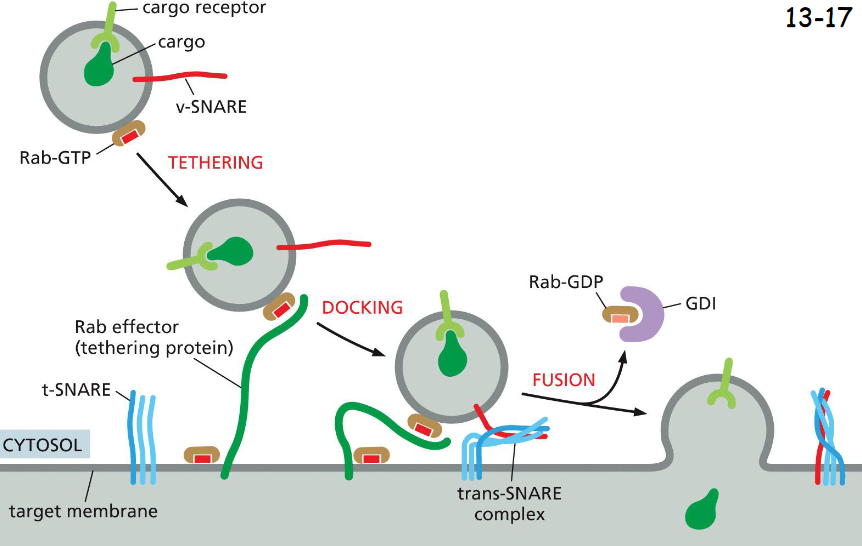
Rab proteins guide
transport vesicles to their target membrane
Rab GTPases in their GTP form associate with membrane and binds Rab effectors that tether the vesicles.
The two SNARE (synaptobrevin) proteins pair and dock the vesicle to the membrane for fusion
Rab-GDP form binds GDI (Rab-GDP disassociation factor), being released to the cytosol to allow the fusion to occur
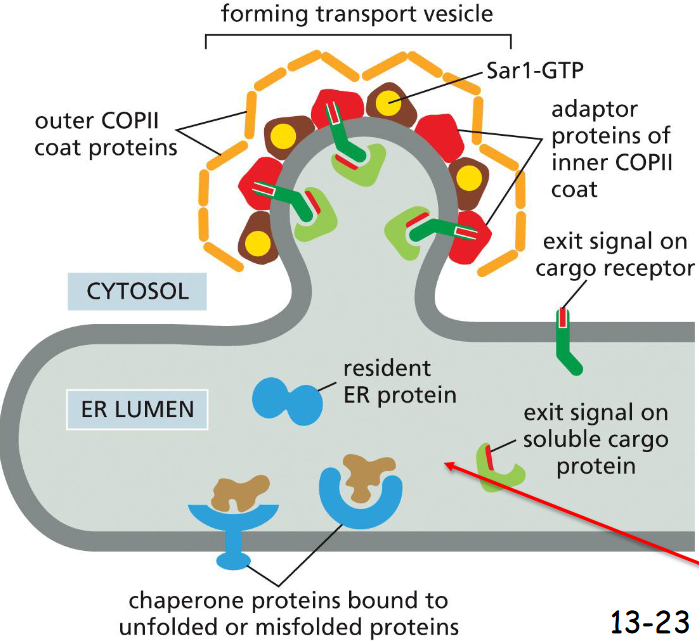
proteins leave the ER in ____-coated transport vesicles
COPII-Coated
Adaptor proteins on the second layer of COPII-coated vesicles interact with the N-termini exit signals of cargo receptor proteins
The C-termini of the cargo receptor proteins interact with the exit signals in soluble cargo proteins
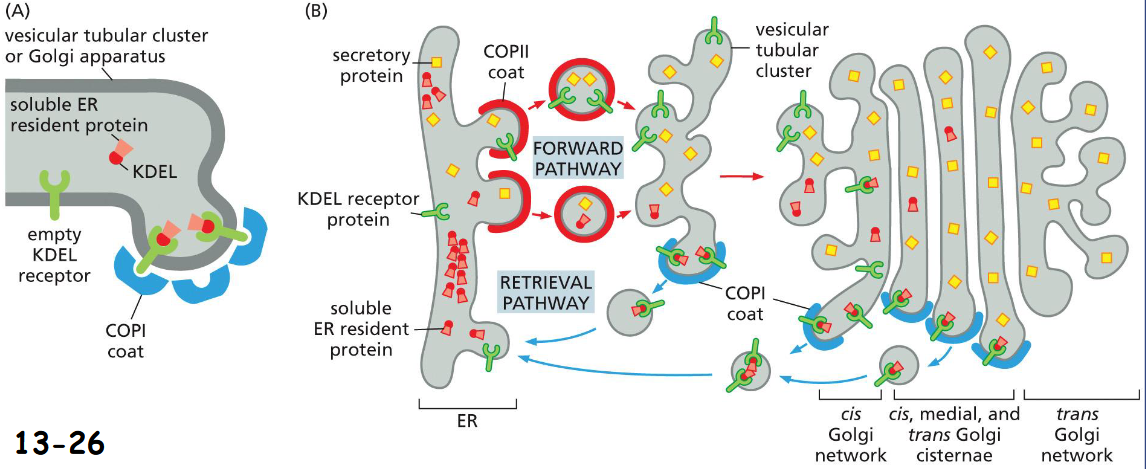
Retrieval of ER resident soluble proteins with COPI-coated vesicles:
C-terminal signal for soluble ER resident protein Lys-Asp-Glu-Leu (KDEL) binds KDEL receptor in COPI-coated vesicles back to ER
(A). The retrieval pathway begins at tubular cluster and continues until late parts of the Golgi apparatus
(B). Signals for escaped membrane ER resident proteins with a KKXX sequence in their C-terminus are also packed into COPI-coated vesicles back to ER
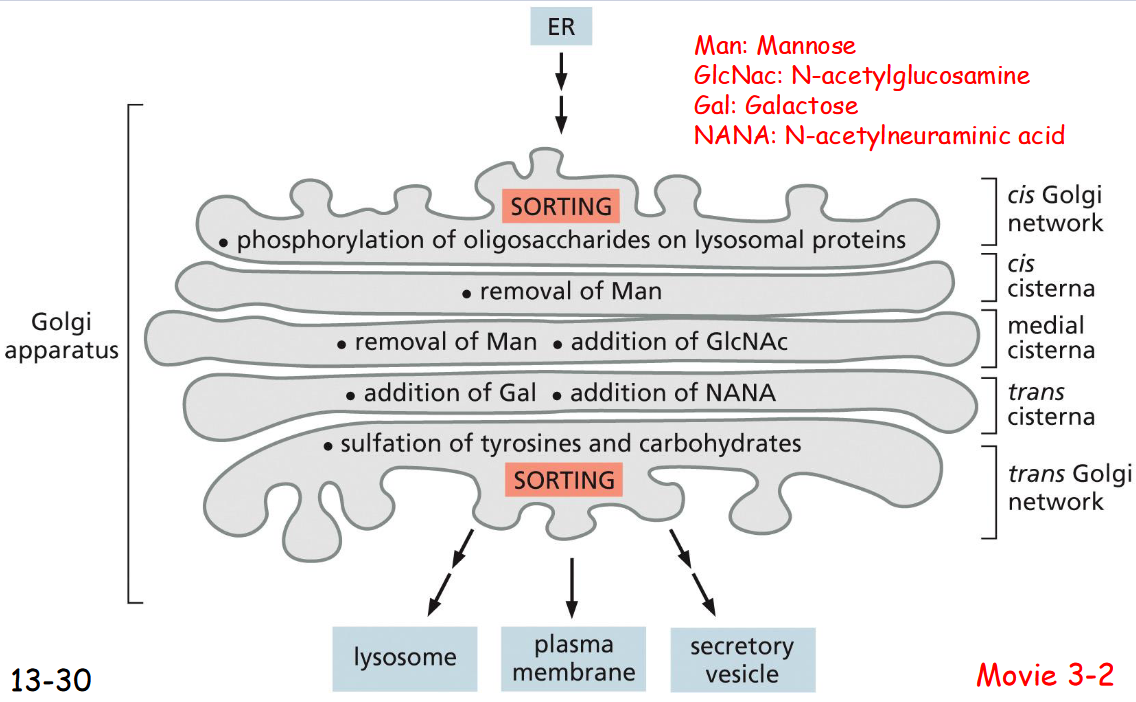
The Golgi Apparatus Consists of
an Ordered Series of Compartments
ER → Cis golgi
phosphorylation of oligosaccharides on lysosomal proteins
removal of Mannose
removal of Mannose , addition of GlcNAc
addition of Gal, addition of NANA
suflation of tyrosines and carbohydrates
trans golgi → lysosome, plasma membrane, secretory vesicle
Many Proteins and Lipids Are Carried Automatically from the Trans ____ Network to the _____
Many Proteins and Lipids Are Carried Automatically from the Trans Golgi Network to the Cell Surface
trans golgi network to cell exterior and endosomes pathway 2 and 3
elected cargos are stored in secretory vesicles in specialized cells until an extracellular signal arrives.
Pathway 3 operates to deliver proteins and others to cell surface Constitutively in un-polarized cells. In polarized cells, a specific signal is needed to direct them to specific domains
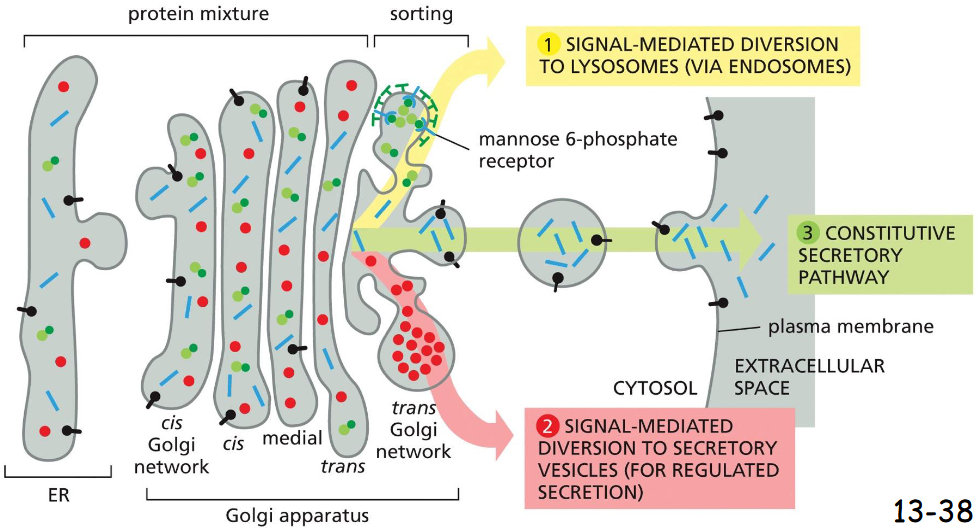
trans golgi network to cell exterior and endosomes pathway 1
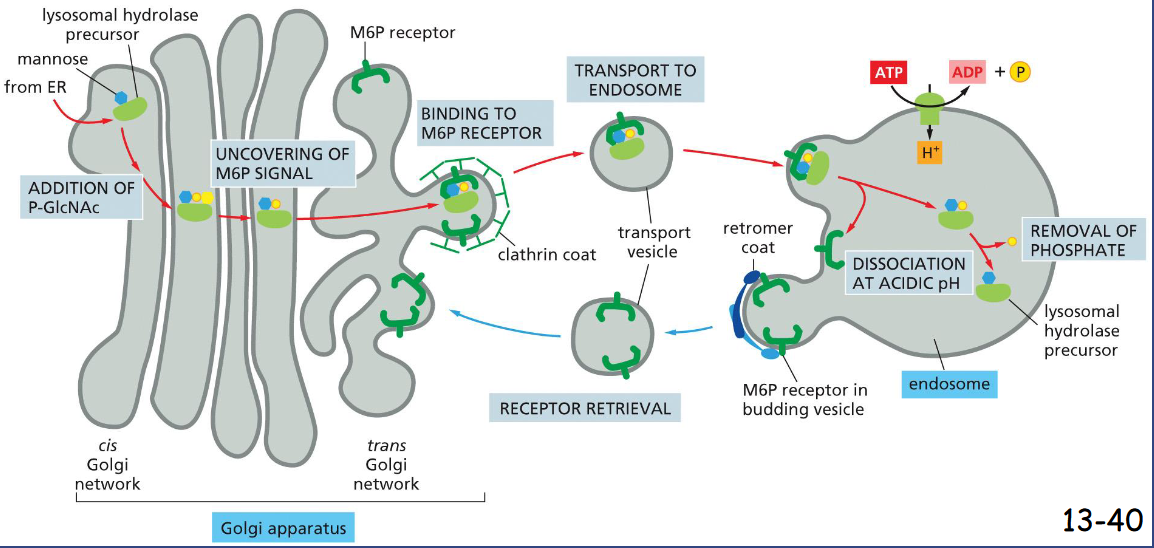
sorted by mannose-6-phosphate receptor
addition of P-GlcNAc (cis)
uncovering of M6P signal (middle)
binding to M6P receptor (trans)
budding off via clathrin coat
transport to endosome
endocytosis by endosome
M6P receptor dissociation in acidic PH of endosome
removal of phosphate
receptor leaves (w/ retromer coat), received by M6P
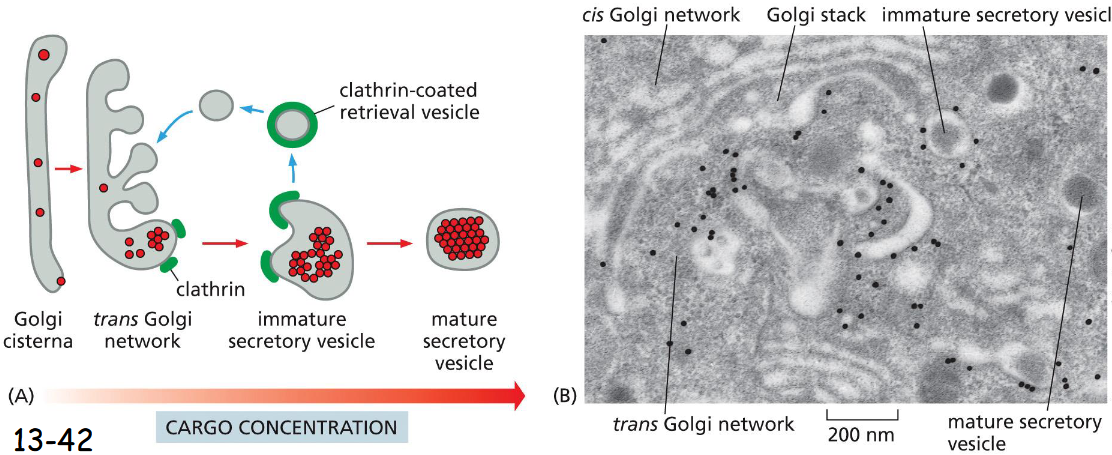
Secretory Vesicles Bud from the _____ Network
Trans Golgi
Formation of secretory vesicles:
Secretory proteins aggregate in trans Golgi network. The immature secretory vesicles contain clathrin coats.
Once the vesicle is mature the clathrin coat disassembles and sent back to trans Golgi
regulated pathway
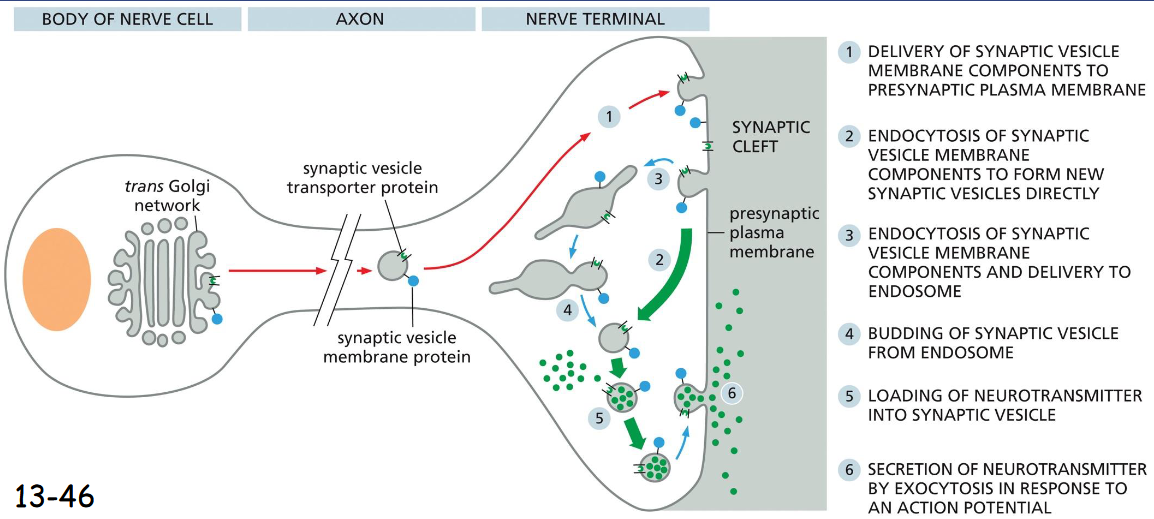
synaptic vesicle formation
can form directly from Endocytic Vesicles
delivery of synaptic membrane components to presynaptic plasma membrane
endocytosis of synaptic vesicle membrane components to form new synaptic vesicles directly
endocytosis of synaptic vesicle membrane components and delivery to endosome
budding of synaptic vesicle from endosome
loading of neurotransmitter into synaptic vesicle
secretion of neurotransmitter by exocytosis in response to an action potential
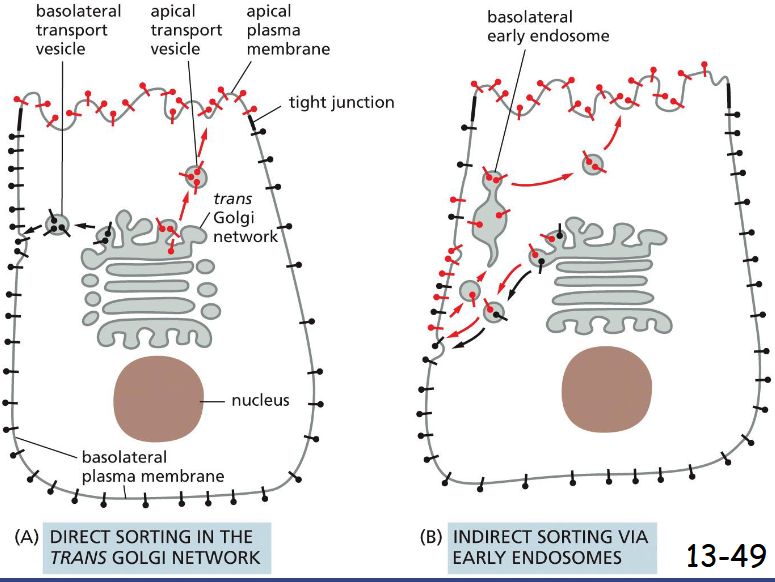
Polarized Cells Direct Proteins from the ______ to the Appropriate Domain of the_____
Polarized Cells Direct Proteins from the Trans Golgi Network to the Appropriate Domain of the Plasma Membrane
Two ways of sorting from trans golgi in a polarized epithelial cell
A) Proteins are packaged into different vesicles. The apical membrane is enriched with glycosphigolipids and some proteins are linked to the lipid bilayer through a GPI anchor.
(B) A protein is selected from the inappropriate plasma membrane domain by endocytosis and transported to the right location via the early endosomes. The indirect pathway is also called transcytosis.
A used primarily
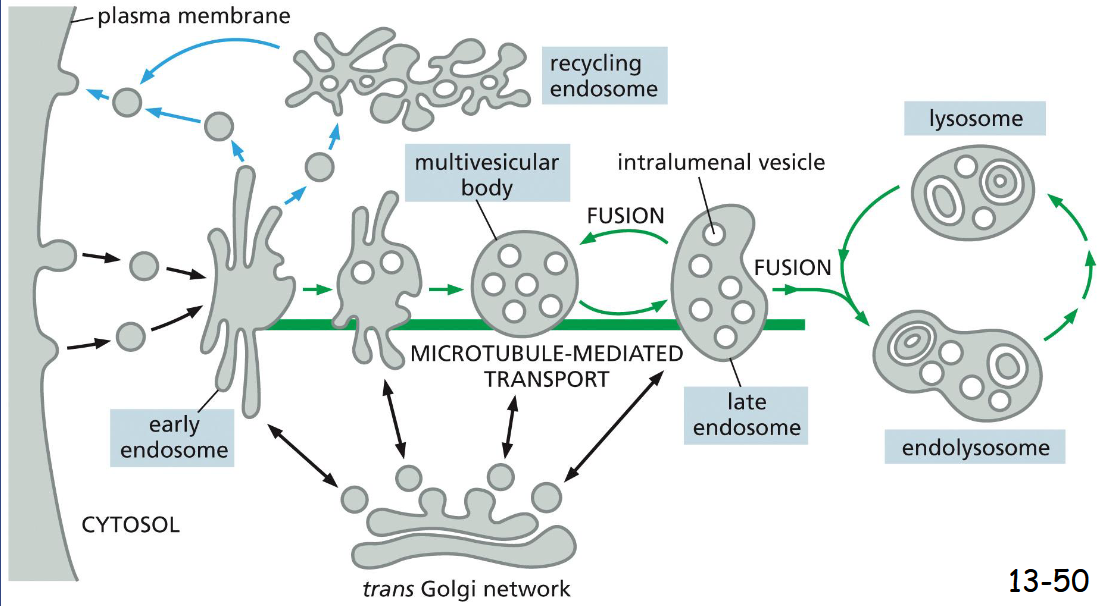
steps in transport from the plasma membrane to endosomes
plasma membrane → early endosome → late endosome → endolysosome ←→ lysosome
Vesicle formation needs _________ filaments, but vesicle traffic needs _____
formation: actin filaments
traffic: microtubules
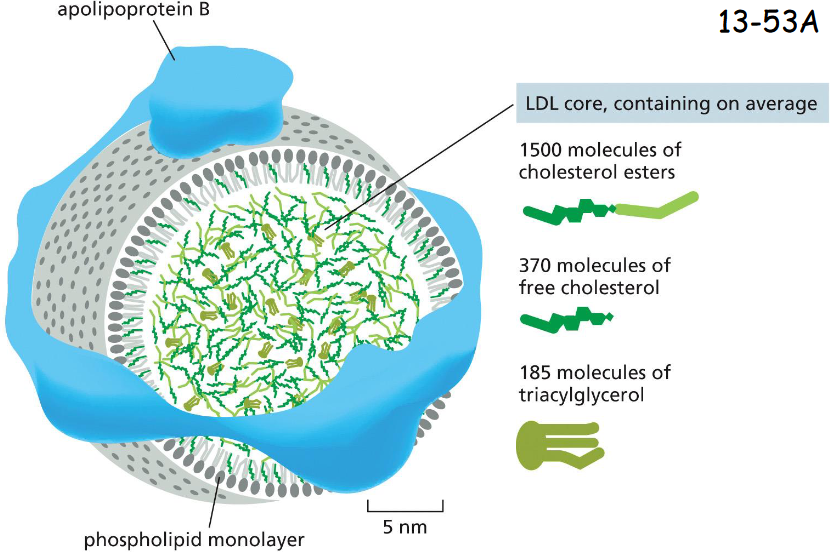
apoliprotein B receptor-mediated endocytosis
Animal cells take up cholesterol from low–density lipoprotein (LDL) particles, which are transported in the bloodstream
Apolipoprotein B organizes the lipid-protein particle and mediates the binding of LDL to cell surface LDL receptors in the plasma membranes (movie 13-3)
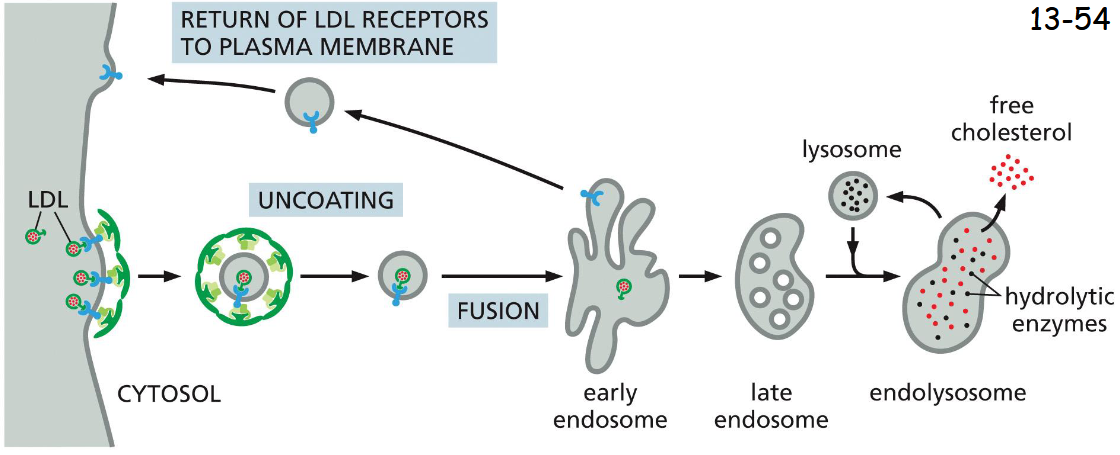
LDL receptors Are Retrieved from Early Endosomes and Returned to the _____
plasma membrane
LDL receptor binds AP2 adaptor protein which then recruits clathrin to initiate endocytosis (movie 13-3).
After shedding their coats, the vesicles deliver their contents to early endosomes. In acidic early endosome, LDL is released from its receptor and is delivered via late endosome to lysosomes.
The LDL receptors are recycled back to the plasma membrane for reuse
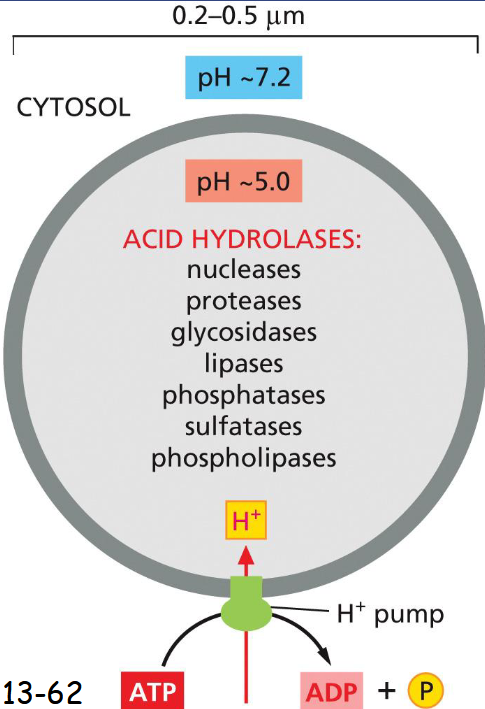
lysosome are the principal sites of
intracellular digestion
contain acid hydrolases (active under acidic conditions)
An H+ ATPase in the membrane pumps H+ into the lysosome maintaining acidic pH
multiple pathways deliver materials to lysosome
Endocytosis of macromolecules;
Macropinocytosis of fluids,
membranes and particles attached to the plasma membrane;
Phagocytosis of microorganisms or dead cells;
Autophagosome to digest cytosol and worn-out organelles
One pathway is missed for delivery to lysosomes. What is it?
secretory pathway
What is the key difference between endocytosis and macropinocytosis or phagocytosis and autophagy
endocytosis: cell internalizes specific extracellular molecules
macropinocytosis and phagocytosis capture larger volumes and particles from outside the cell
autophagy digests intracellular components
Specialized Phagocytic Cells Can Ingest Large Particles
Formation of large phagocytic vesicles by white blood cells such as macrophages and neutrophils to ingest microorganisms or dead cells
Pseudopod formation and extension are driven by actin polymerization and reorganization
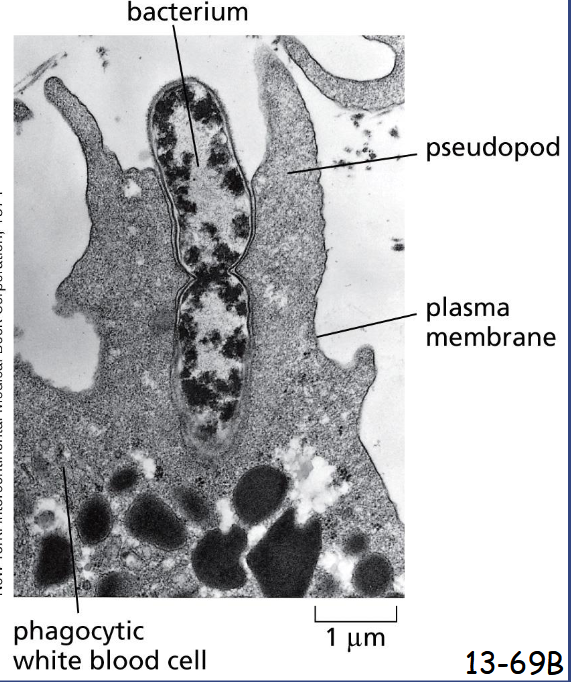
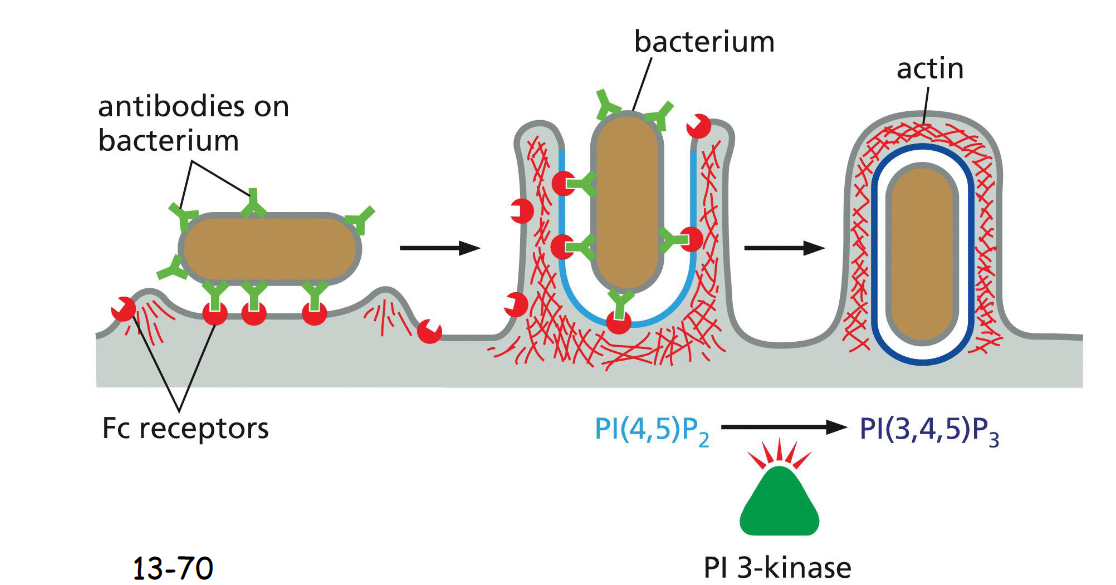
Specialized Phagocytic Cells Can Ingest Large Particles
The Fc receptor on the surface of phagocytic cells recognizes the antibody, recruiting the bacterium to the plasma membrane.
PI(4,5)P2 stimulates actin polymerization and PI(3,4,5)P3 depolymerize actins at the base, mediated by P3 kinase
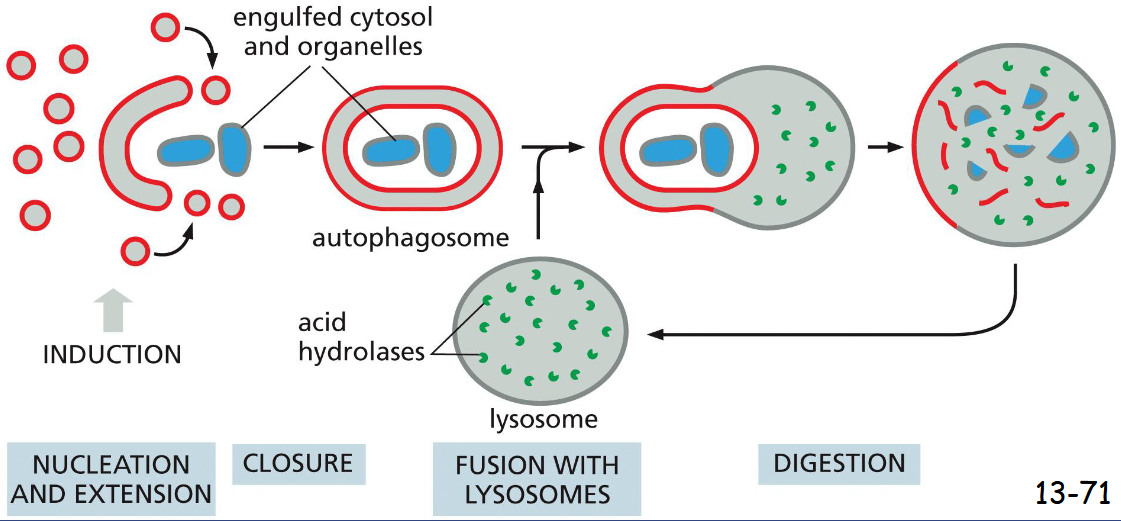
Autophagy Degrades _____
unwanted proteins and organelles
Vesicles of unknown origin fuses and grows to form a double membrane-enclosed autophagosome, which is then fused with lysosomes
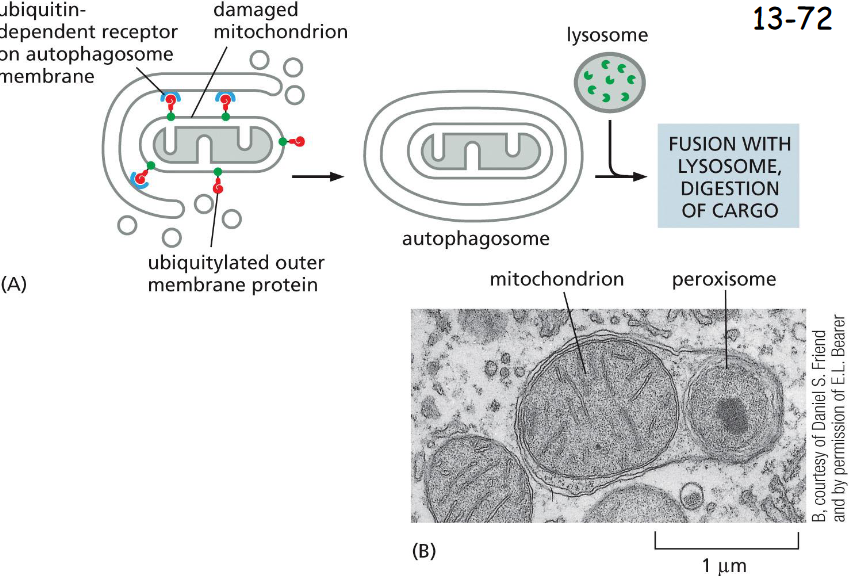
A Family of ____-Receptors Mediates Selective Autophagy
cargo-specific
(A) A cargo receptor (blue) in the autophagosome membrane that directs it to a specific cargo, in this case a damaged mitochondrion.
(B) An electron micrograph of an autophagosome containing a mitochondrion and a peroxisome
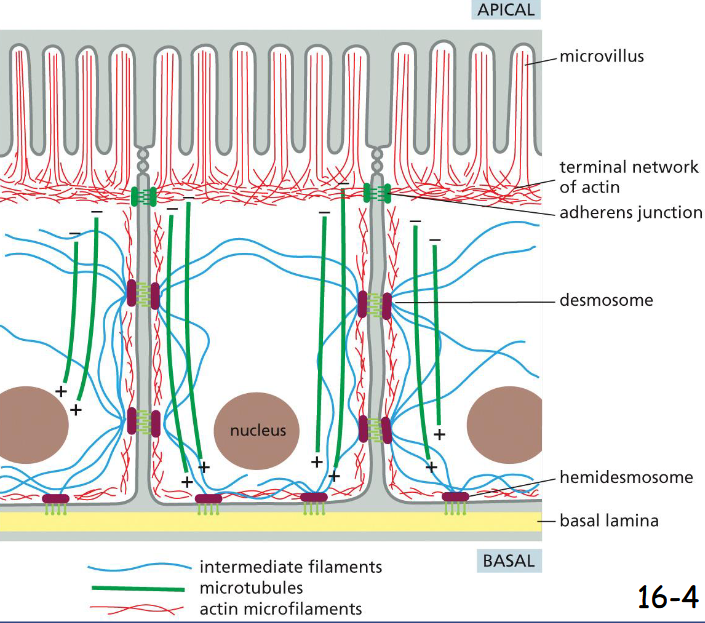
the cytoskeleton determine
cellular organization and polarity
in intestinal epithelial cells, bundled actin filaments support microvilli at the apical surface.
Below a circumferential band of actin filaments is connected to cell-cell adherens junction (anchor cell to each other and support the plasma membrane).
Microtubules run vertically from the top to the bottom and provide global coordination of organelles and vesicle transport.
Intermediate filaments are anchored to desmosomes (cell-cell connection) and hemidesmosomes (attached to extracellular matrix)
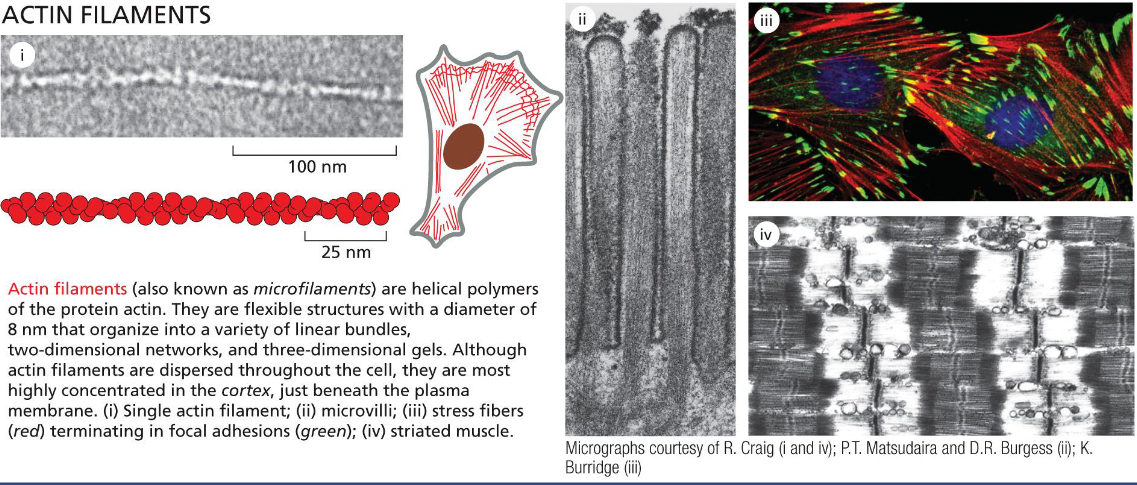
actin filaments adapt to form ___ or ___ structures
dynamic or stable structures
actin filaments determine the shape of cell surface (strength to its thin lipid bilayer and cell surface projections); are necessary for whole cell locomotion (muscle); and drive the pinching of one cell into two
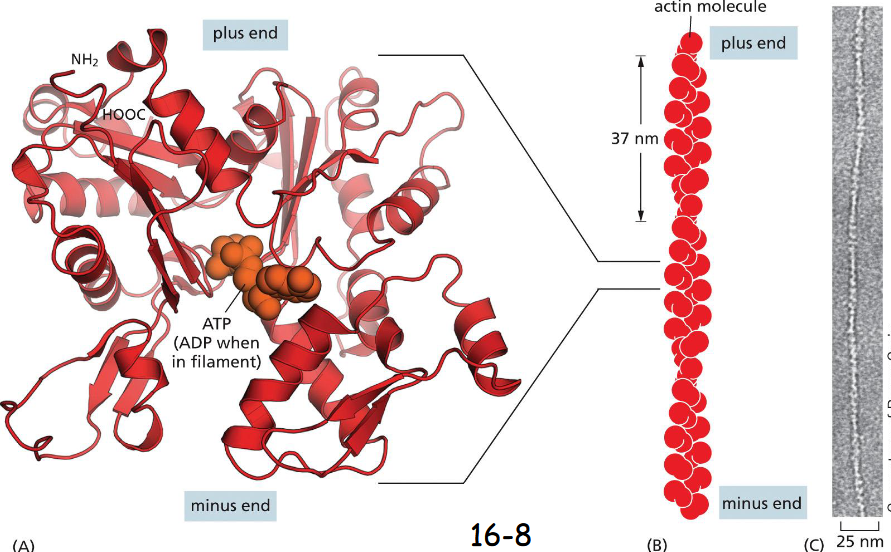
Actin Subunits assemble Head-to-Tail to Create ____ and _____ Filaments
to create flexible and polar filaments
A monomer (A) and an actin filament of two protofilaments with a twist repeat of 37 nm
(B) The filaments are assembled head-to-tail to form a right-handed helix with a faster growing plus end and a slower growing minus end
Actin-Binding Proteins Influence
Filament Dynamics and Organization
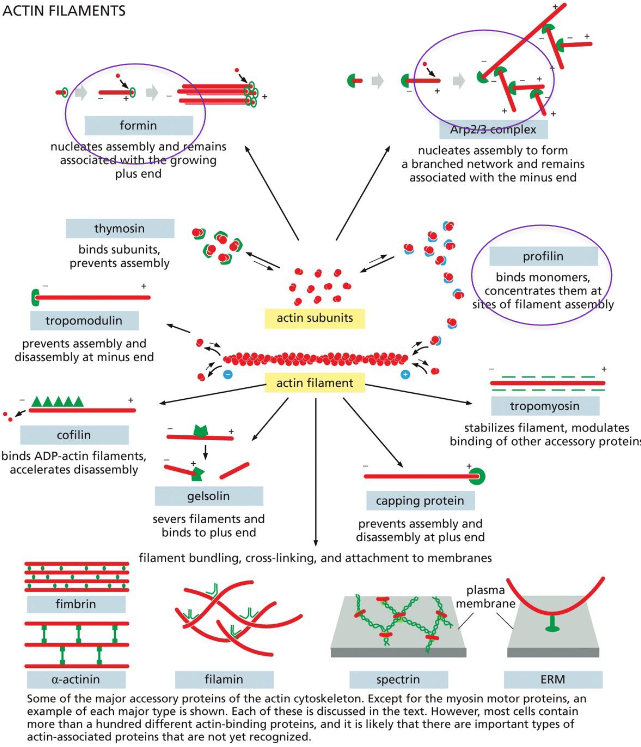
actin binding proteins: 3 to know
formin: nucleates (initiates) assembly and remains associated with the growing plus end
Arp2/3 Complex: nucleates (initiates) assembly to form a branched network and remains associated with the minus end
Profilin: binds actin monomers, concentrates them at sites of filament assembly
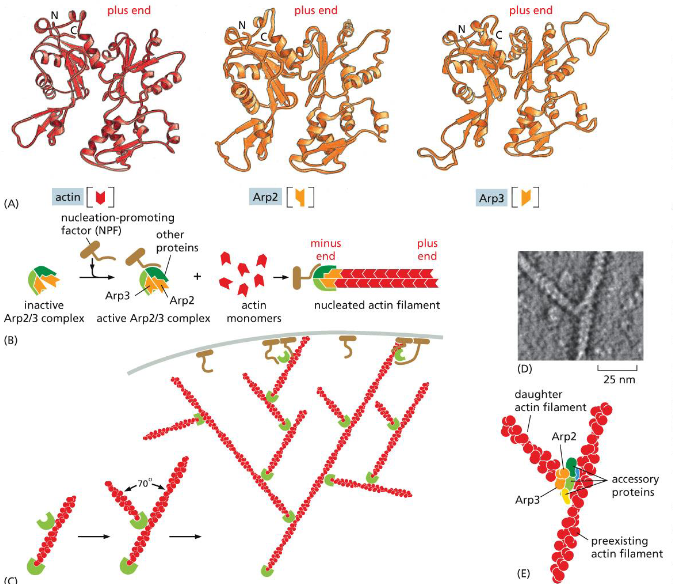
Actin-Nucleation Is Tightly Regulated and generates ___ or ___ filaments
Generates Straight or Branched Filaments
Two actin related proteins, Arp2 and Arp3 have their plus end but not the minus end similar to actin, preventing them from being part of the filaments (A).
Arp2 and Arp3 form an inactive complex, and a nucleation promoting factor then binds the complex, nucleating actin filament growth at the plus end
The Arp2/Arp3 complex acts more efficiently when it is bound to a pre-existing actin filament (C).
Branched actin filaments formed in vitro (top) and overlap of the crystal structures with the actin filament image (D).
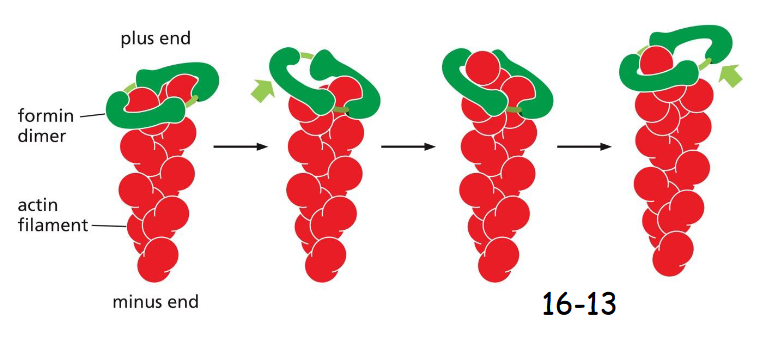
dimeric formins associate with
the plus end
facilitate the addition of a new actin monomer. Each formin monomer has a binding site for monomeric actin
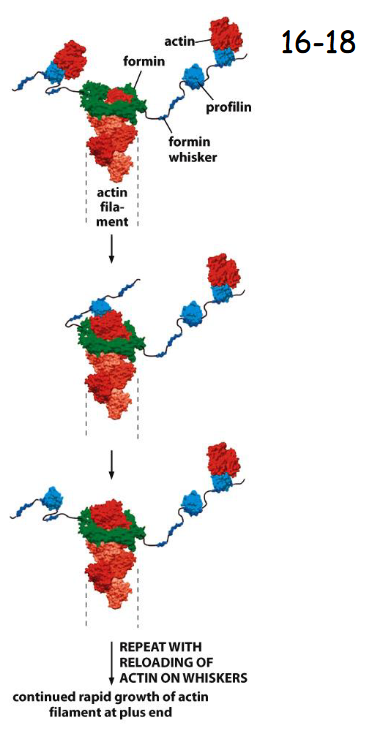
Formin and profilin
Formins facilitate the addition of actin to the growing plus end when profilin is bound. Formins actually have binding sites to recruit profilin
What are the two functions of formins?
nucleation of actin filaments (initiation)
elongation of actin filaments
Actin Filament Elongation is regulated by _____
Monomer-binding Proteins
(A) Many nucleation-promoting factors (NPFs) bind profilin, which is bound to actin monomers. NPF activation leads not only to nucleation of branched actin filaments by Arp2/3, but also to elongation of the new filaments.
(B) Some formin proteins possess whisker-like domains that contain several binding sites for profilin–actin complexes.
Like NPFs, formin proteins inside the cell are activated to promote actin filament polymerization at membrane surfaces (movie 16-1)
Actin-Based Motor Proteins Are Members of the ____ Superfamily
Myosin
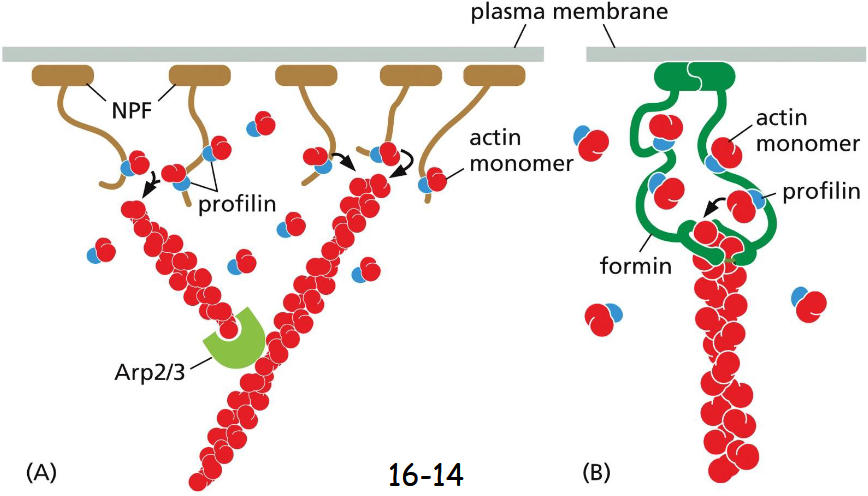
Myosin II has two heavy chains (green) and four light chains (blue).
The coiled-coil tails of the heavy chain bundle with the tails of other myosin II to form thick myosin filaments. Its globular head contains the walking or moving machinery, hydrolyzes ATP, and walk toward the plus end of an actin filament. In contrast, the actin filament moves toward its minus end.
The four light chains bind close to the head of the heavy chain and are regulatory
Schematic diagram for the myosin II molecules
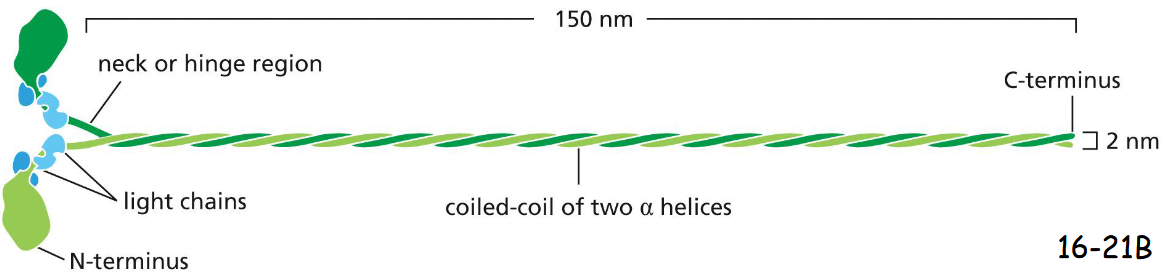
myosin II molecules aggregate by means of their tail regions (bare zone), with their heads projecting to the outside of the filament (movie 16-2)
myosin in relaxed state
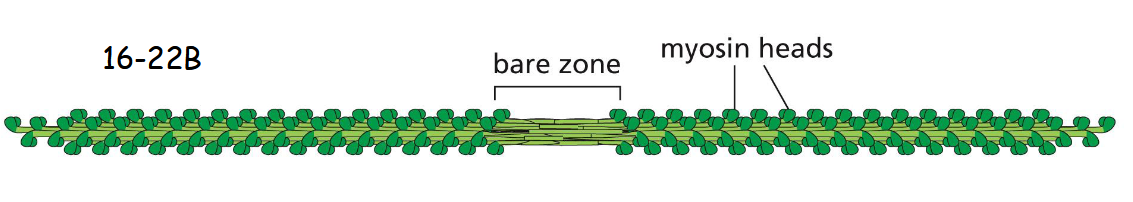
In the relaxed (noncontracting) state, the two heads of a myosin molecule are bent backward and sterically interfere with each other to switch off their activity. An individual myosin molecule in the inactive state is highlighted in green. The cytoplasmic myosin II filaments in non- muscle cells are much smaller but are organized similarly
Motor activity of the myosin head
Purified myosin heads were attached to a glass slide, and actin filaments labeled with fluorescent phalloidin were added to allow the two interact.
(A) When ATP was added, the actin filaments glide along the surface.
(B) The large red arrows indicate the directions of actin filament movement
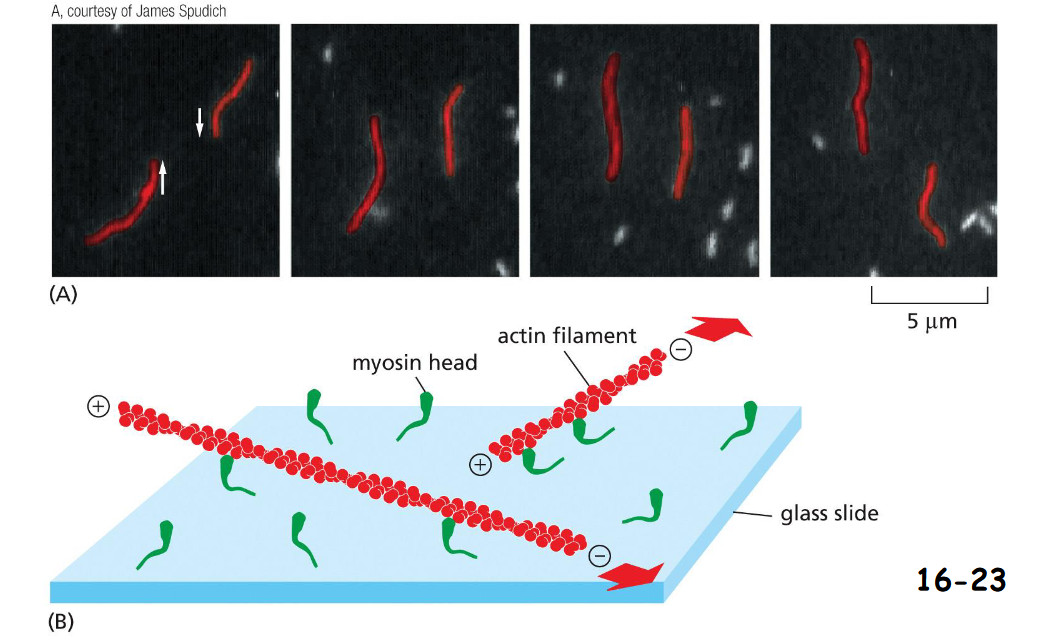
Sliding of Myosin II Along Actin Filaments Causes Muscles to:
contract
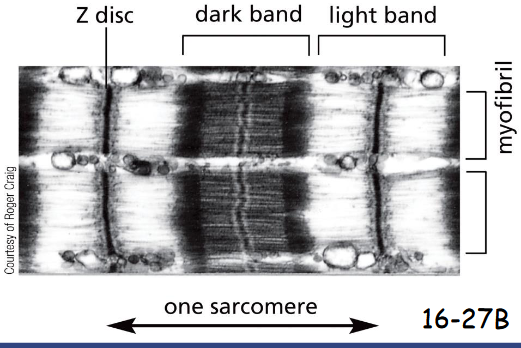
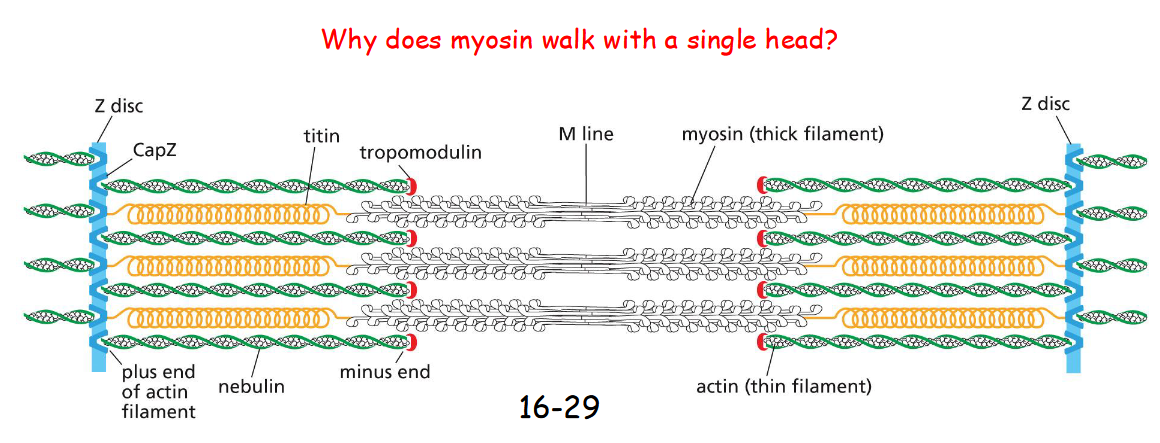
The Z-discs are the attachment sites for the plus end of actin filaments.
Skeleton muscle cells:
the huge single cells formed by the fusion of many muscle precursor cells, myoblasts, with many nuclei (syncytium)
The Z-discs are
the attachment sites for the plus end of actin filaments.
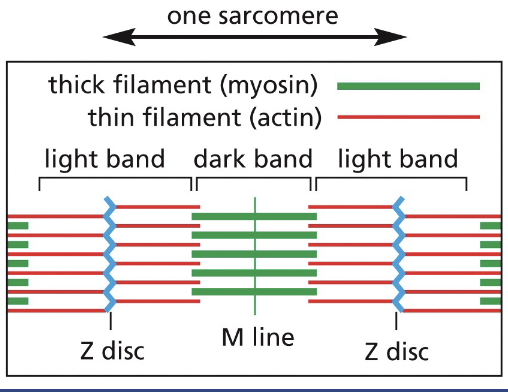
M-line sarcomere
The M-line (midline) is the location of proteins that link adjacent myosin II filaments, each myosin filament has 300 heads.
When the sarcomere contracts, the myosin filaments slide past one another toward the plus end of actin filaments (movie 16-3)
myosin accessory proteins
Tropomodulin – caps and stabilizes the minus end of actin filaments.
Nebulin – binds actin filaments to influence their length.
Titin – extend from Z disc and associate with myosin thick element as molecular spring and ruler (help the engagement of myosin with actin)
A Sudden Rise in Cytosolic Ca2+ Concentration Initiates _____ ______
Muscle Contraction
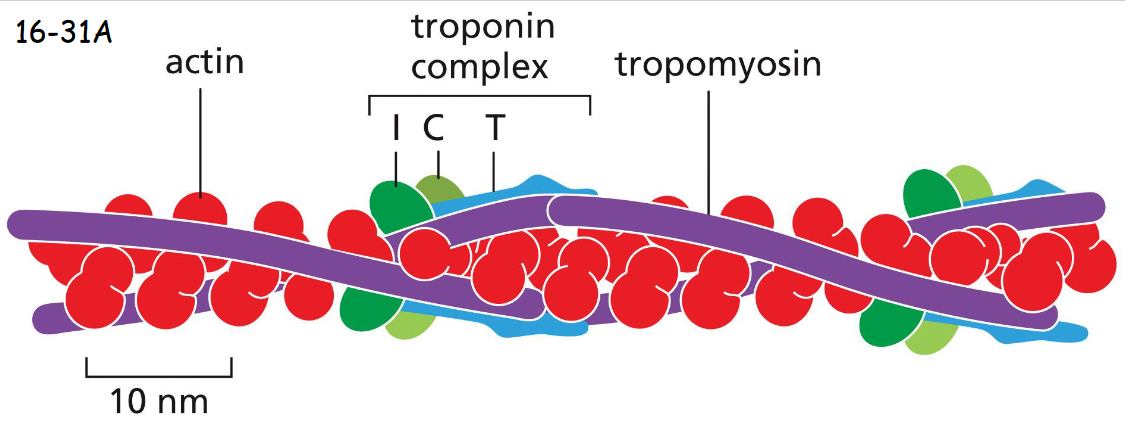
A thin filament of a skeletal muscle cell, showing the positions of tropomyosin and troponin along the actin filament.
Tropomyosin is locked on actin filament by the binding of troponin T and I (inhibitory) domains to tropomyosin in the absence of of calcium. Troponin C binds calcium and causes troponin I and tropomyosin (moves first) to release their hold on actin
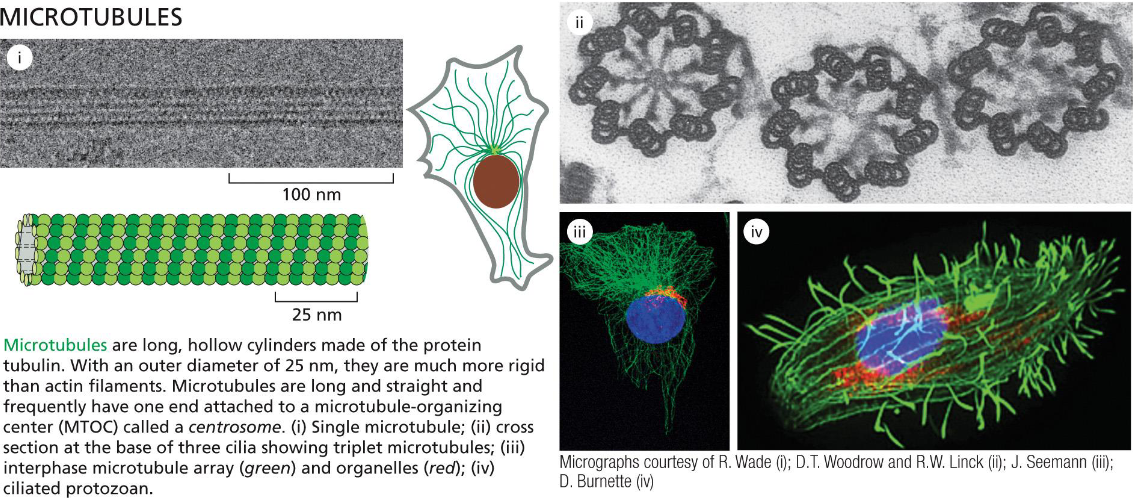
Microtubule Filaments Adapt to Form ___ or ____ Structures
Dynamic or Stable
Microtubules form the mitotic spindles and cilia that function as motile whips or sensory devices. Microtubules also determine the position of organelles and direct intracellular vesicle transport. In protozoans, microtubules form the framework upon which the entire cell is built (movie 16-4)
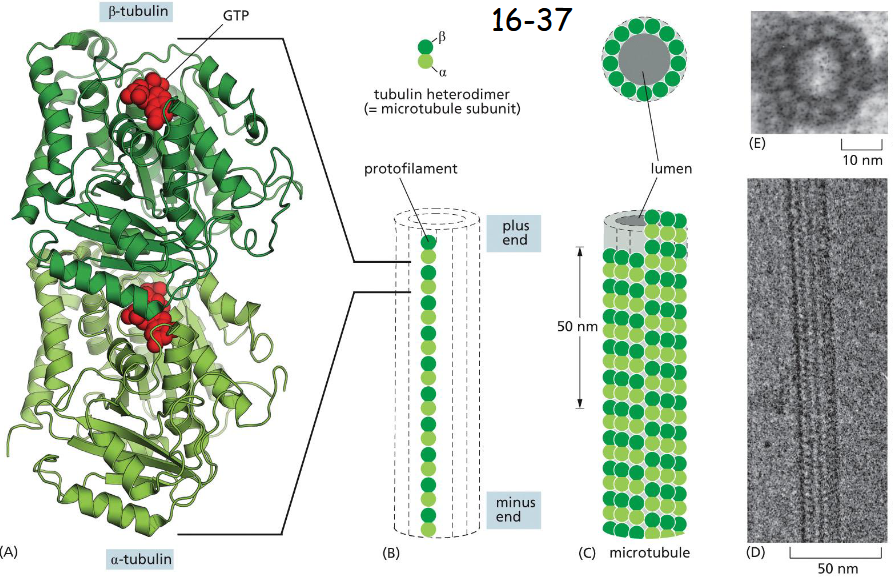
Microtubules Are Hollow Tubes Made of
13 Protofilaments
the subunit is a tubulin heterodimer
GTP with B-tubulin is embedded deep or tightly. Either GTP or GDP can be associated with B-tubulin. GTP-tubulin tends to polymerize.
B is at the plus end and alpha is at the minus end. The plus ends grow and shrink more rapidly (B).
The interface between dimer is much like the one between alpha-beta interface plus lateral alpha-alpha and beta-beta contacts (C)
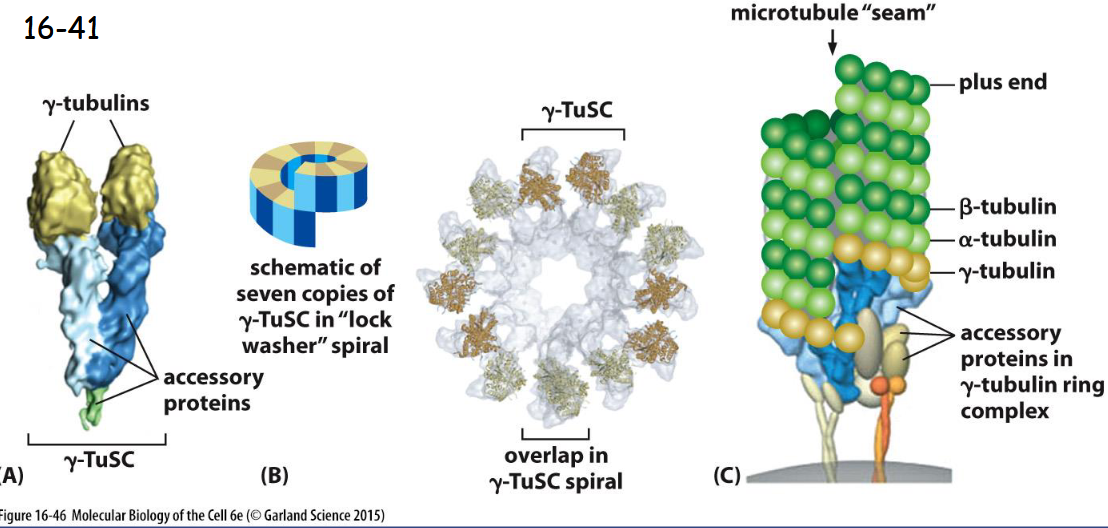
A Protein Complex Containing ______ Nucleates Microtubules
gamma-Tubulin
A) Two copies of gamma-tubulins with a pair of accessory proteins form the gamma-tubulin small complex (gamma-TuSC).
(B) Seven copies of the gamma-TuSC associate to form a spiral structure with the last gamma-tubulin beneath the first, resulting in 13 exposed gamma-tubulin subunits.
(C) In many cells, the gamma-TuSC spiral associates with additional proteins to form the gamma-tubulin ring complex (gamma-TuRC), which nucleates at the minus end of a microtubule
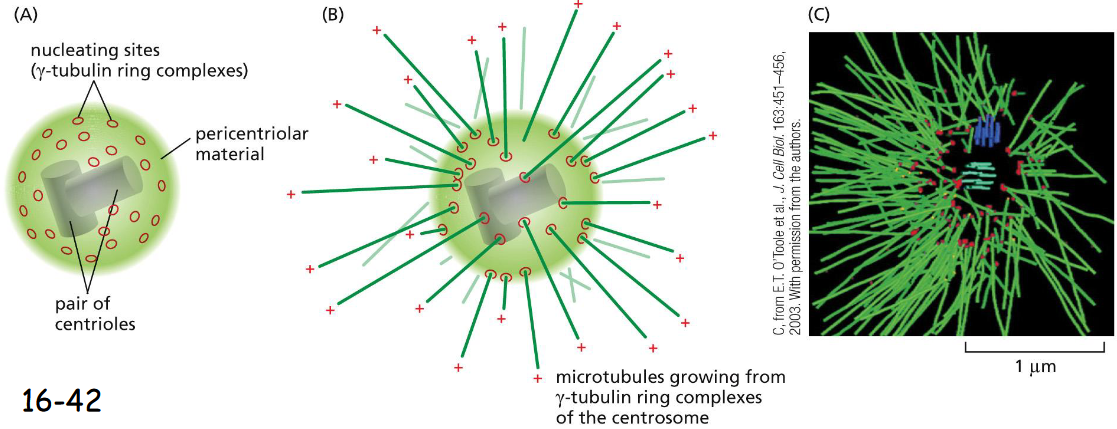
The Centrosome is a Prominent _____ Site
Microtubule Nucleation Site
(A) The centrosome is the Microtubule Organization Center (MTOC) of animal cells. Located near the nucleus, centrosome is organized by a pair of cylindrical centrioles (made of modified microtubules) arranged at right angles, and consists of a matrix of fibrous proteins.
(B) A centrosome with attached microtubules, which are nucleated at their minus ends by the gamma-tubulin ring complexes (gamma-TuRC) and the plus ends point outward.
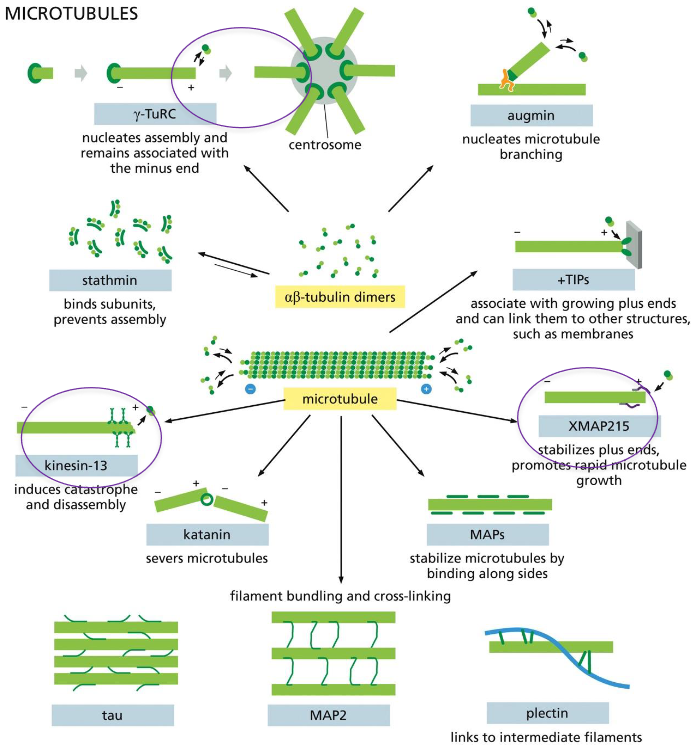
Microtubule-Binding Proteins Modulate Filament ___ and ____
Dynamics and Organization
The microtubule dynamics inside a cell are governed by a variety of proteins that bind tubulin dimers or microtubules.
They are called Microtubule-associated proteins or MAPs.
microtubule-binding proteins: 3 to know
gamma-TuRC: nucleates assembly and remains associated w/ minus end, helps with formation of centrosome
kinsein-13: induces catastrophe and disassembly at plus end
XMAP215: stabilizes plus ends, promotes rapid microtubule growth
kinesin-13 and XMAP215: end-binding proteins that modulate microtubule dynamics and attachments
A microtubule switches from a growing to a shrinking state (the frequency of catastrophes) or from a shrinking to a growing state (the frequency of rescues).
Kinesin-13 is a member of the kinesin motor protein superfamily, and binds to plus ends prying them apart.
XMAP215 binds tubulin dimers and delivers them to the microtubule plus end
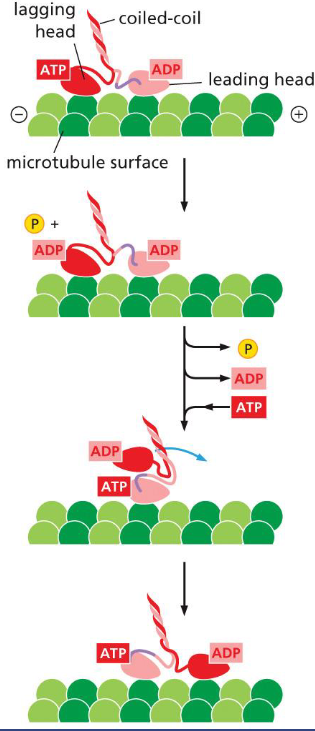
Kinesins
Kinesins carry organelles or vesicles on their long coiled-coil tails, and walk toward the plus ends with their two nucleotide binding motor heads.
The lagging head leaves it tubulin binding site, passes the leading head, and rebinds to the next tubulin binding site. ATP hydrolysis in the lagging head and binding of ATP by the leading head pull the rear head forward (movie 16-5)
Dyneins
Dyneins have minus-end directed movement, and are used for organelle and vesicle trafficking, and positioning the centrosome and nucleus during cell migration.
(A) A molecule of cytoplasmic dynein. Like myosin II and kinesin-1, cytoplasmic dynein is a two-headed molecule.
(B) The two heavy chains contain a motor head with domains for microtubule binding and ATP hydrolysis, connected by long linkers. Bound to the linker domain are multiple intermediate chains and light chains
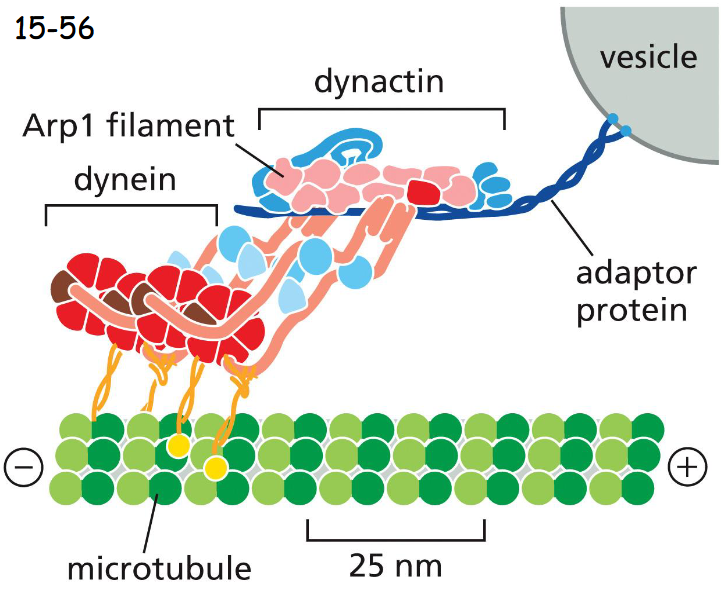
dynactin (has Arp1 filament) mediates attachment of dynein to membrane enclosed vesicle or organellee
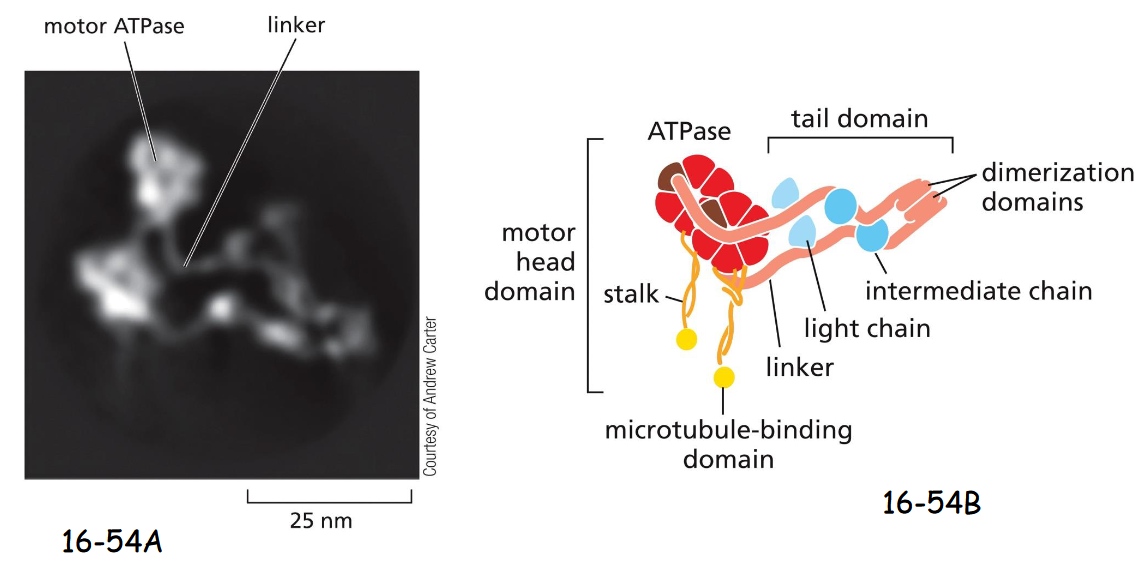
dynein and dynactin
Dynein walks toward the minus end (kinesin to plus end)
works to move organelles
Dynactin mediates the attachment of dynein to a membrane-enclosed vesicle or organelle (movie 16-6)
Dynactin is a large protein complex and includes proteins that bind to dynein, and proteins that form an actin-like filament made of the actin-related protein Arp1
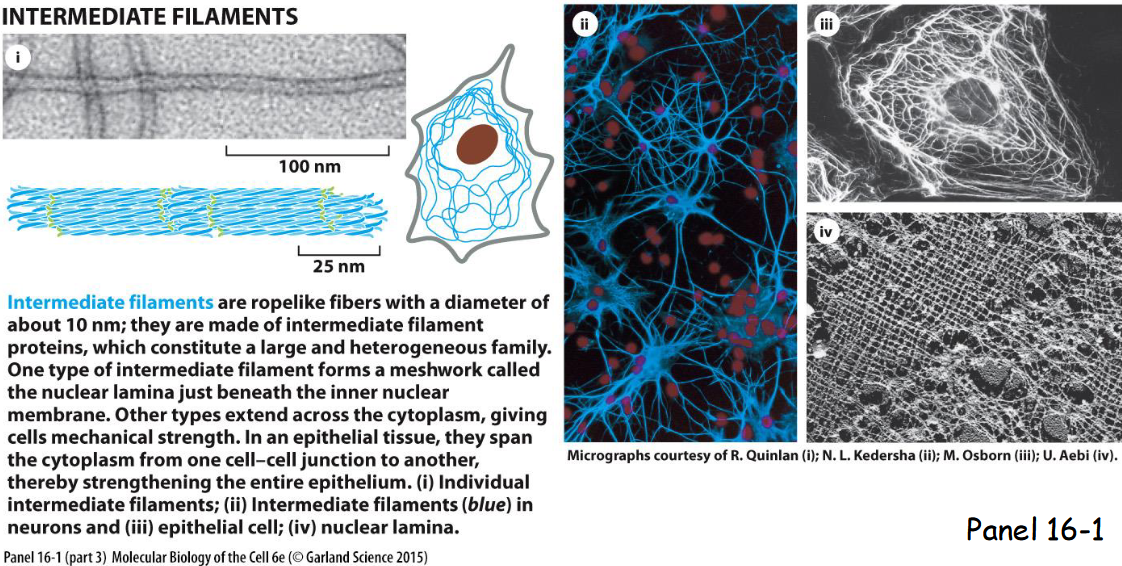
intermediate cytoskeletal filaments
Intermediate filaments extend across the cytoplasm to provide cells mechanical strength, line the inner face of nuclear envelope (nuclear lamins form a meshwork to provide anchorage sites for chromosomes and nuclear pores), and span at sites of cell-cell contact called desmosomes to hold epithelial cell sheets together or cell-matrix contact called hemidesmosomes
Intermediate Filament Structure Depends on the Lateral Bundling and
Twisting of Coiled-Coils
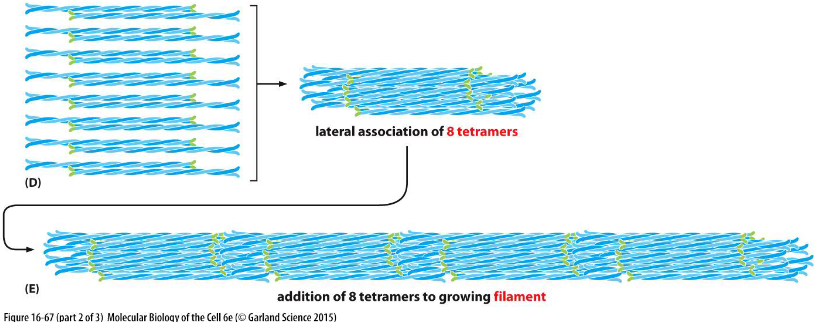
The monomer (A) pairs with another monomer to form a dimer (B) of wound coiled-coil. Two dimers then line up to form a tetramer Antiparallelly (C).
Antiparallel, no ATP binding, and no polarity
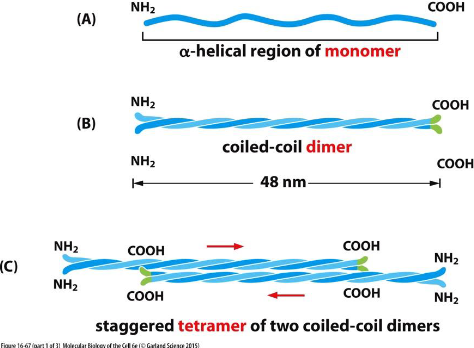
intermediate filament structure: D and E
Lateral association of 8 tetramers (D) and tetramers are packed together to form rope-like filaments (E).
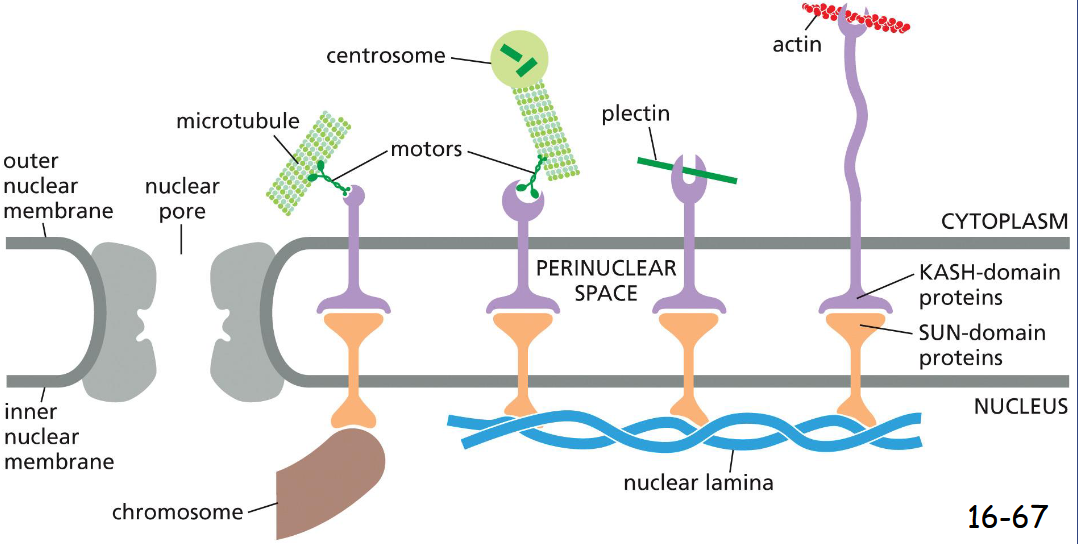
SUN and KASH proteins
Linker proteins, Connect Cytoskeletal Filaments and Bridge the Nuclear Envelope
inner nuclear envelope: SUN proteins connect to the nuclear lamina or chromosomes
outer nuclear envelope: KASH proteins connect to the cytoplasmic cytoskeleton by binding microtubule motor proteins, plectin, or actin filaments
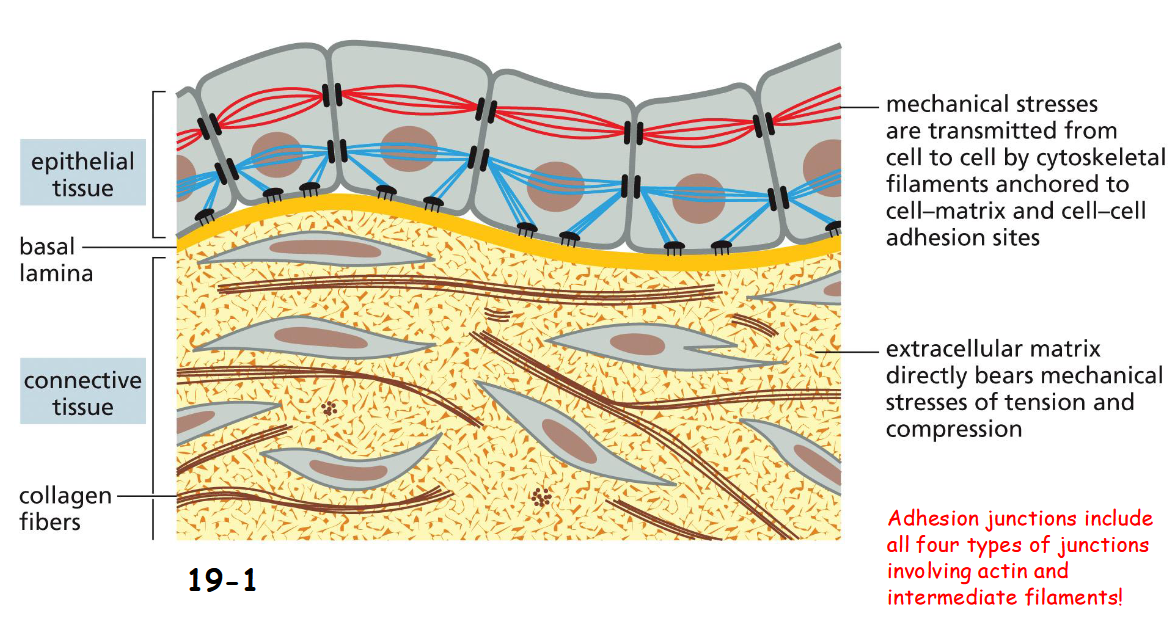
two major ways in which animal cells are bound together:
In epithelial tissue, cytoskeletons link from cell to cell through adhesion junctions.
Cell-matrix junctions attach epithelial tissue to the connective tissue
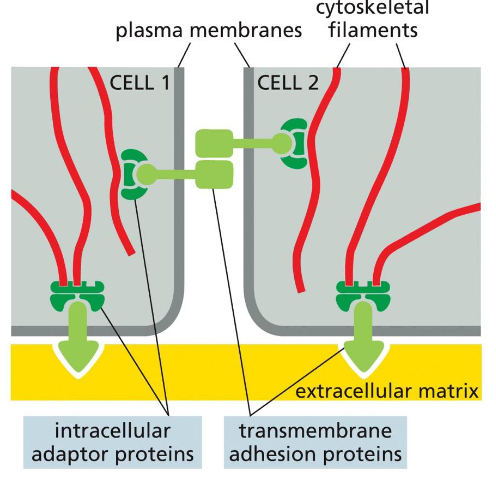
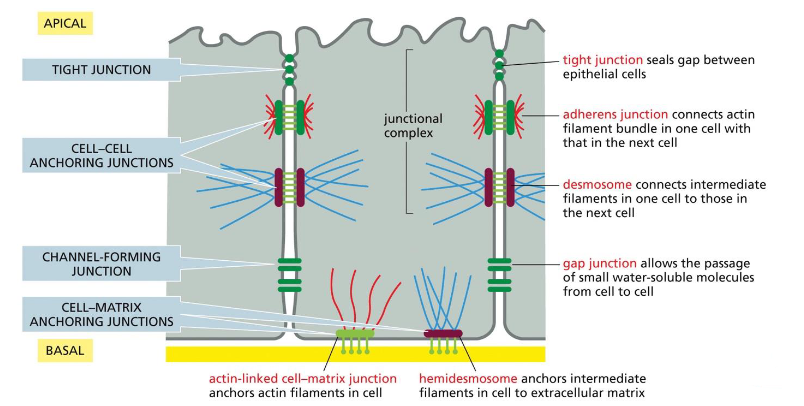
A general organization for the four cell-cell and cell-matrix connections
Transmembrane adhesion proteins link the cytoskeleton from cell to cell or to extracellular matrix with the help of adaptor proteins
various epithelial junctions found in epithelial cell
tight junction: seals gap between epithelial cells
adherens junction: connects actin filament bundle in one cell with that in the next cell
desmosome: connects intermediate filaments in one cell to the next
gap junction: allows passage of small water-soluble molecules from cell to cell
hemidesmosomes: anchors intermediate filaments in cell to extracellular matrix
actin-linked cell-matrix junction: anchors actin filaments in cell
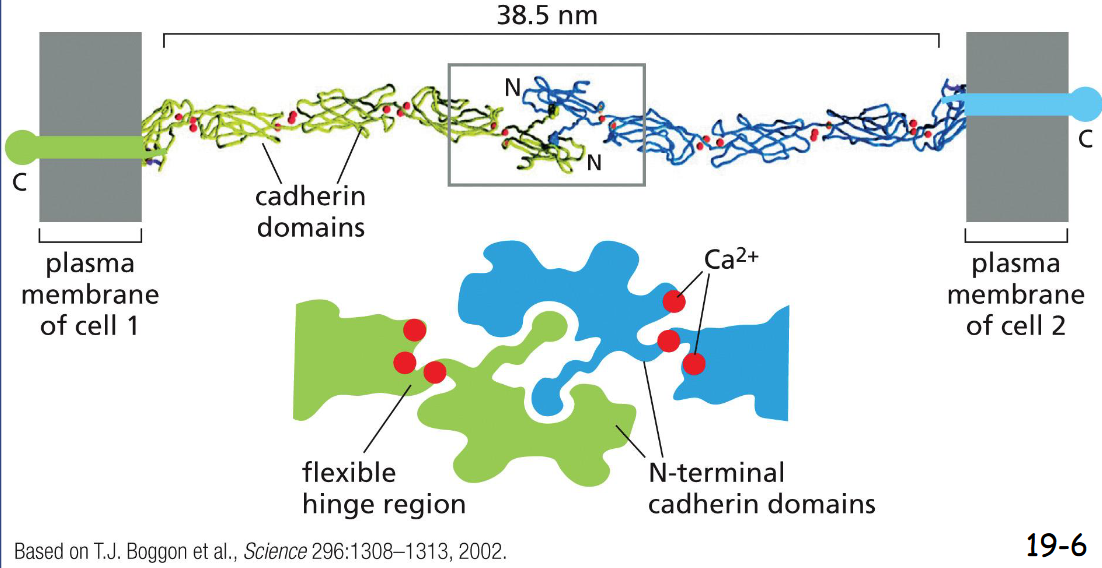
Cadherins Mediate :
Homophilic Adhesion
The N-terminal domain of a cadherin in one cell binds to the N-terminal domain of a cadherin from adjacent cell. At the edge, it is filled with Ca2+ to limit flexing
Catenins Link:
Adherens Junctions Respond to:
catenins link Classical Cadherins to the Actin Cytoskeleton and link to other catenins in adjacent cells
Three adaptor proteins, or catenins, link cadherins to the actin filaments
Adherens junctions Respond to Tension from Inside and Outside the Tissue
catenin tension vs no tension
When stretched by tension from an adjacent cell, a domain in alpha-catenin is unfolded to expose a binding site for vinculin
Vinculin in turn recruits more actin filaments.
Actin filaments are pulled downward by non-muscle myosin II
ONLY CELL TO CELL: CELL TO ECM USES TALIN
Does myosin II walk upward or downward?
upward (+ direction)
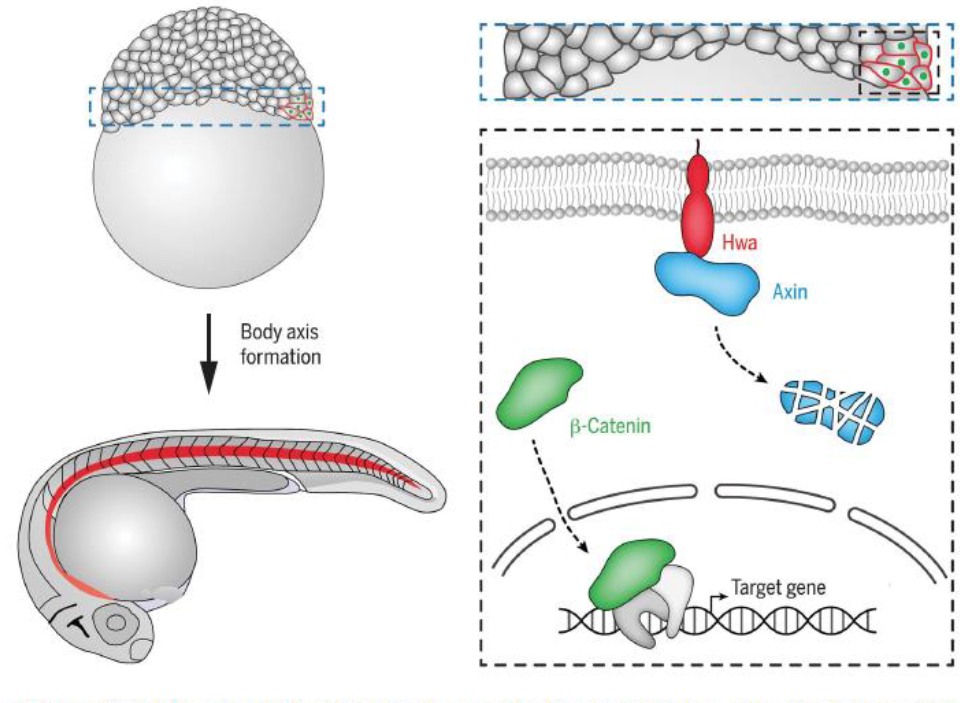
Hwa
Hwa protein is in prospective dorsal blastomeres, it degrades Axin (independent of Wnt signaling) resulting in stabliization and nuclear translocation of beta-catenin for activating organizer-specific target gene expression.
The notochord (red) is an organizer-derived tissue
tissue remodeling depends on the coordination of ___ contraction with _____ to ____ adhesion
Tissue Remodeling Depends on the Coordination of Actin-mediated Contraction with Cell–Cell Adhesion
Adhesion belt
A contractile bundle of actin filaments runs along the cytoplasmic surface of the junctional plasma membrane. The actin filament bundles are tethered by adaptor proteins to cadherins, which bind to cadherins on the adjacent cell
aids in pinching off of vesicles
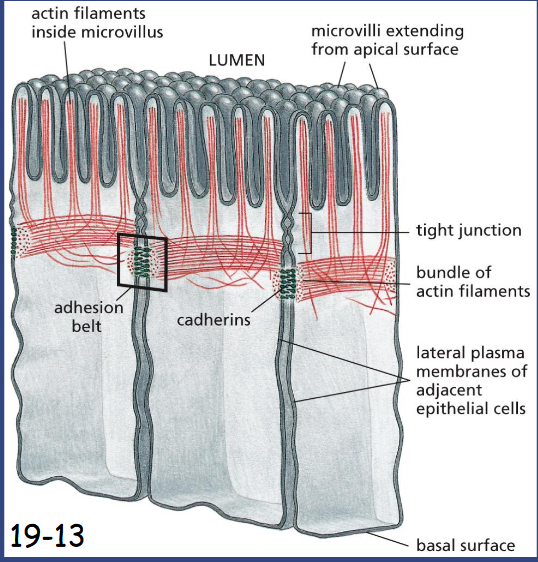
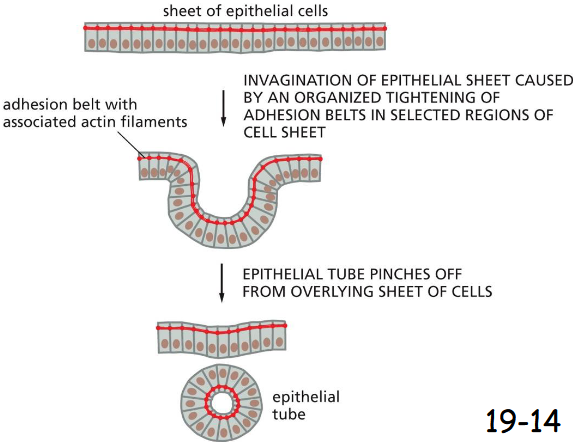
The folding of an epithelial sheet to form an epithelial tube
The oriented contraction of the bundles of actin and myosin filaments running along adhesion belts causes the epithelial cells to narrow at their apical surfaces, thereby helping the epithelial sheet to roll up into a tube in early vertebrate development
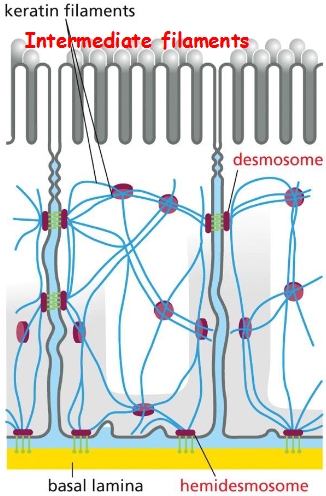
Desmosomes Give Epithelia Mechanical Strength
The epithelial cells are indirectly connected to one another through desmosomes, and to the basal lamina through hemidesmosomes
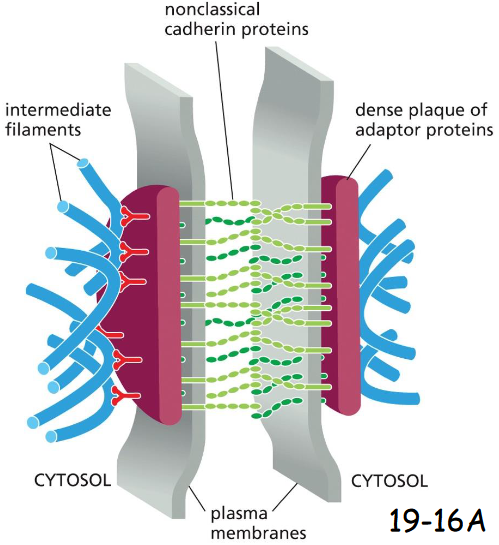
structure of desmosome
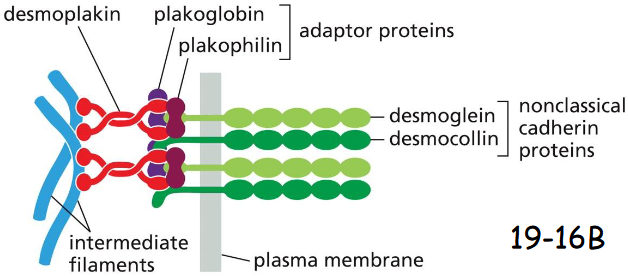
molecular components of desmosomes
Plakoglobin is gamma-catenin
Plakophilin is a distant relative of p120-catenin
Desmoplakin connects intermediate filaments to plakoglobin + plakophilin
Tight Junctions Form a Seal Between Cells and a Fence Between Plasma Membrane Domains
Tight junctions seal the adjacent cells to prevent the flow of lumen fluid into extracellular space and the backflow of glucose from the basal side into the gut lumen.
Help confine the various transport proteins to different regions or domains of the plasma membrane by acting as diffusion barriers.
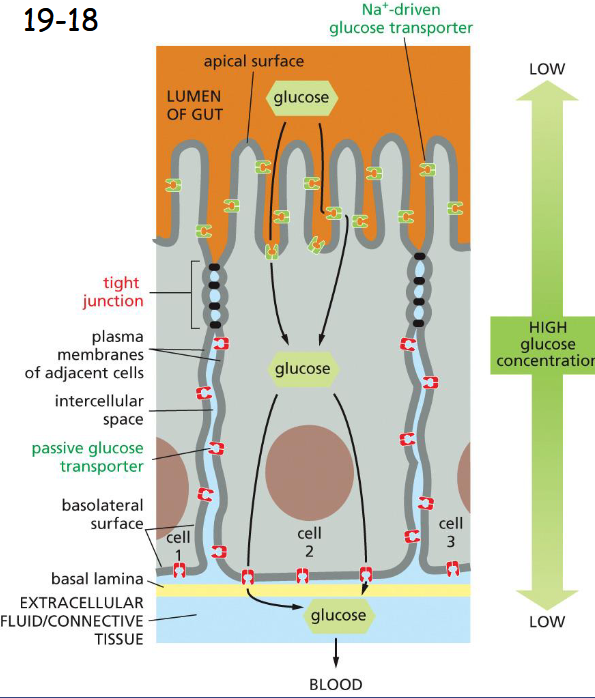
Tight Junctions Contain Strands of Transmembrane Adhesion Proteins
Claudin and occludin
interact in intercellular space
termini on cytoplasmic side bind to adaptor proteins and then actin filaments
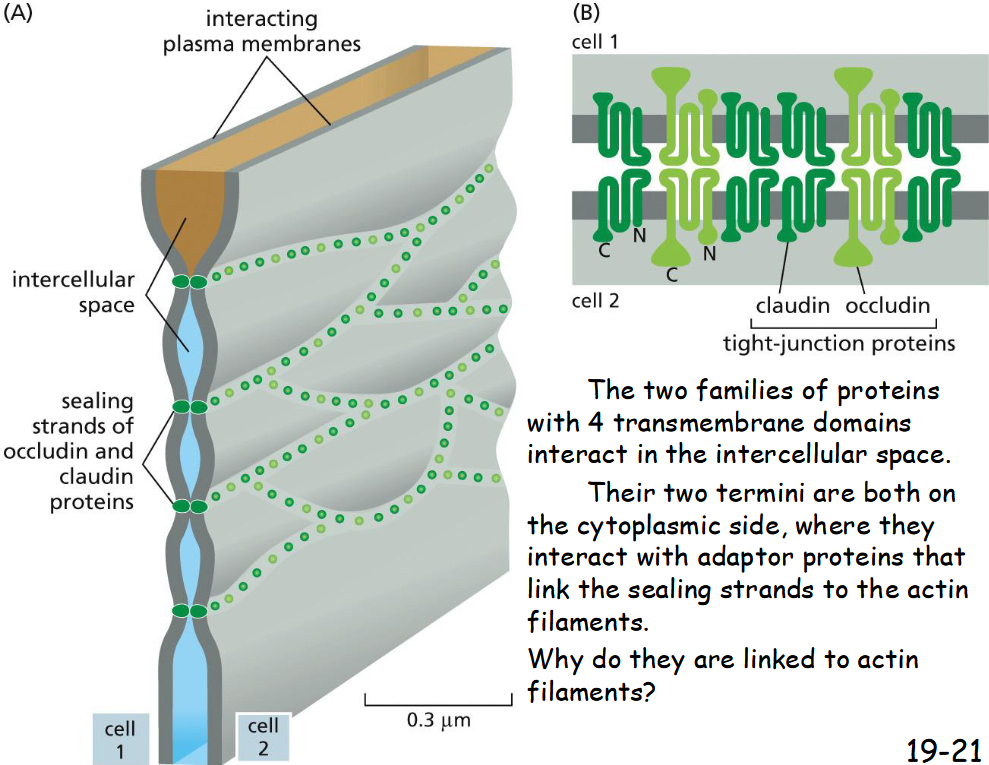
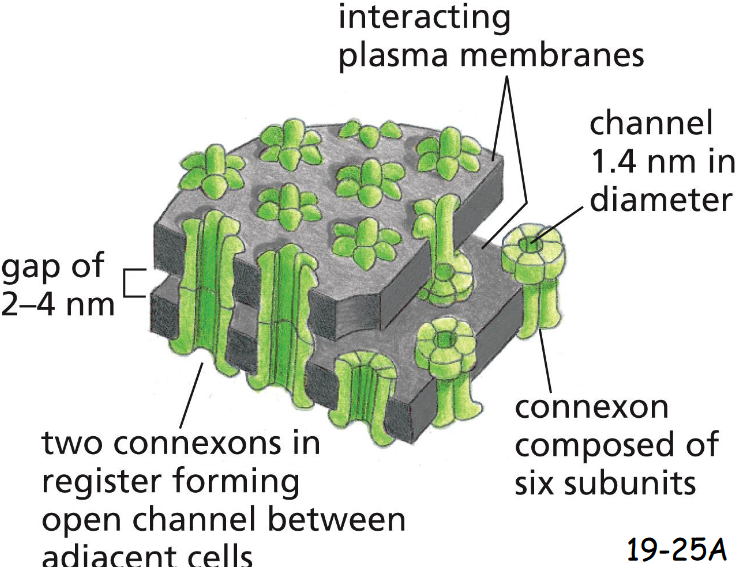
A Gap-Junction Connexon Is Made of Six ____ subunits
six Transmembrane Connexin Subunits
Gap-Junction only allows the passage of small molecules (<1000 Daltons) but not macromolecules (19-25A)
Connexons are found only in vertebrates
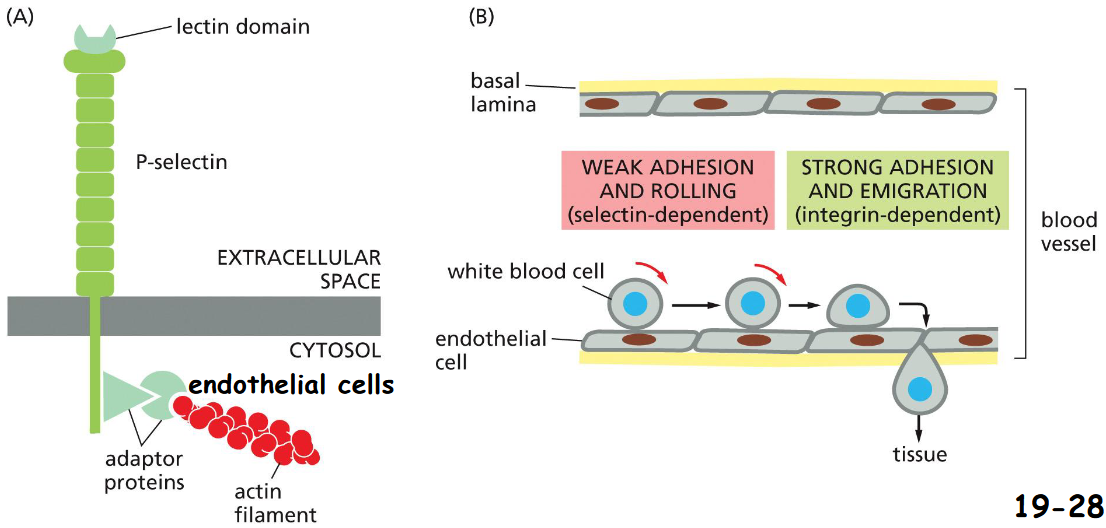
Selectins Mediate Transient Cell-Cell Adhesions in the Bloodstream
The lectin domains of selectins on endothelial cells lining blood vessels bind specific oligosaccharides of glycoproteins or glycolipids on the extracellular surface of white blood cells with a low affinity (movie 19-2)
Integrins on white blood cells bind to specific Ig-family proteins (ICAM) on the surface of endothelial cells strongly that enables white blood cells stop and leave the blood-stream
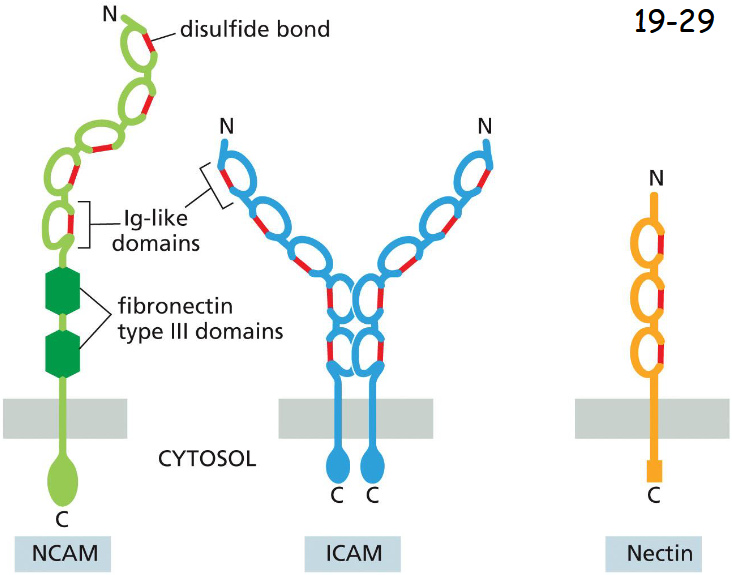
Members of the Immunoglobulin Superfamily Mediate ____- Adhesion
Ca2+-independent Cell-Cell adhesion
Intracellular cell adhesion molecule (ICAM, middle) is expressed on endothelial cells and some other cell types, and binds heterophilically to an integrin on white blood cells.
Neural cell adhesion molecule (NCAM, left) is expressed in neurons and many other cell types, and mediates homophilic binding (not major but fine-tuning compared to cadherins).
Nectin is expressed in many cell types (right) and is often found at adherens junctions, where it interacts with cadherins to help establish and strengthen specific cell– cell interactions during tissue formation
Integrins Are Transmembrane ______ That Link the
Extracellular Matrix to the _____
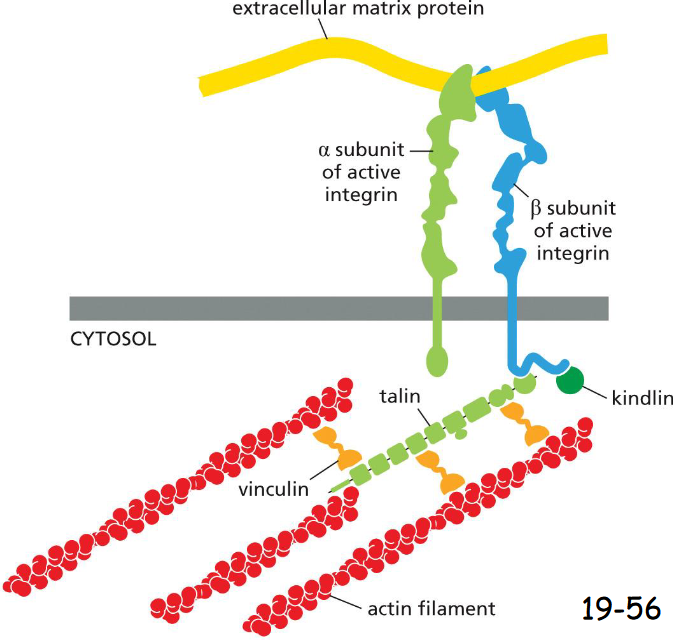
Integrins Are Transmembrane Heterodimers That Link the Extracellular Matrix to the Cytoskeleton
Integrin is a transmembrane protein. Its N-terminal extracellular domain binds Arg-Gly-Asp (RGD) sequences in fibronectin or other matrix proteins.
Beta-integrin C-terminal intracellular domain binds to adaptor proteins such as talin that interacts with actin filaments directly. Talin also brings vinculins, which attach to more actin filaments
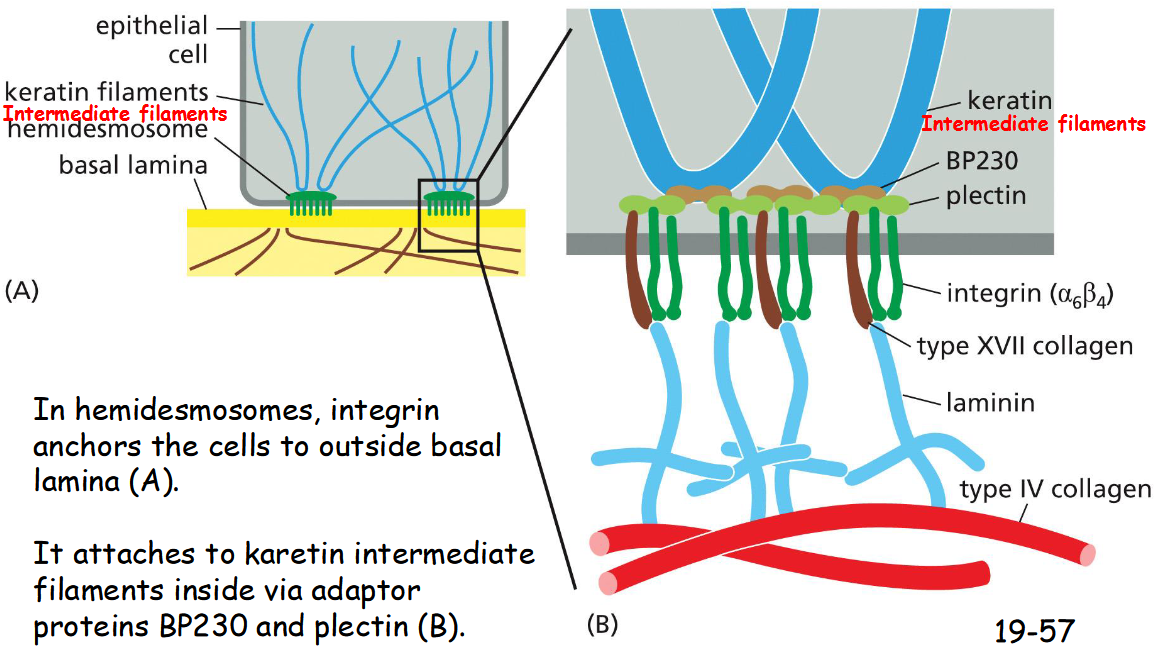
In hemidesmosomes, integrin anchors the cells to outside ___ ____
basal lamina (A)
It attaches to karetin intermediate filaments inside via adaptor proteins BP230 and plectin (B)
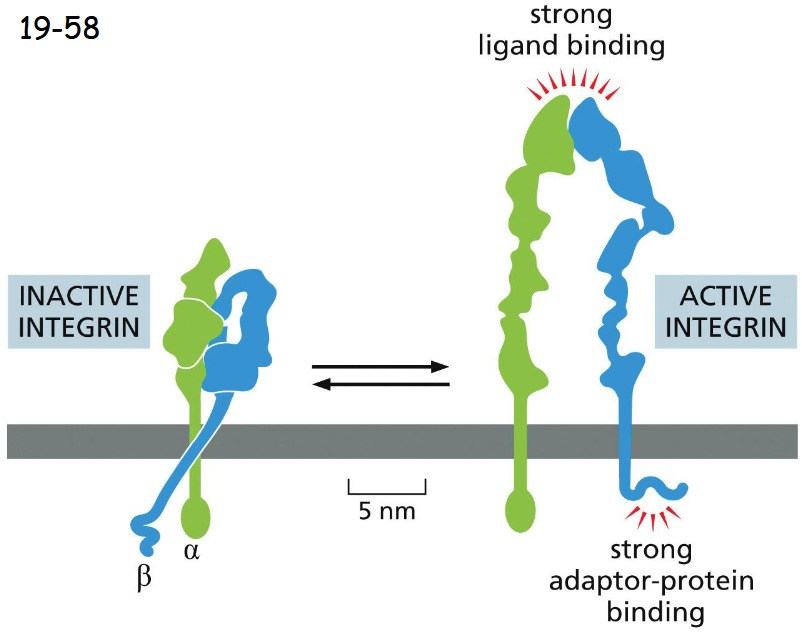
Integrins Can Switch Between an Active and an Inactive Conformation
Integrins exist in two different states:
They fold into a compact and inactive structure, but are more extended when becoming active.
When integrins are active under tension, the adaptor proteins are attached to actin filaments
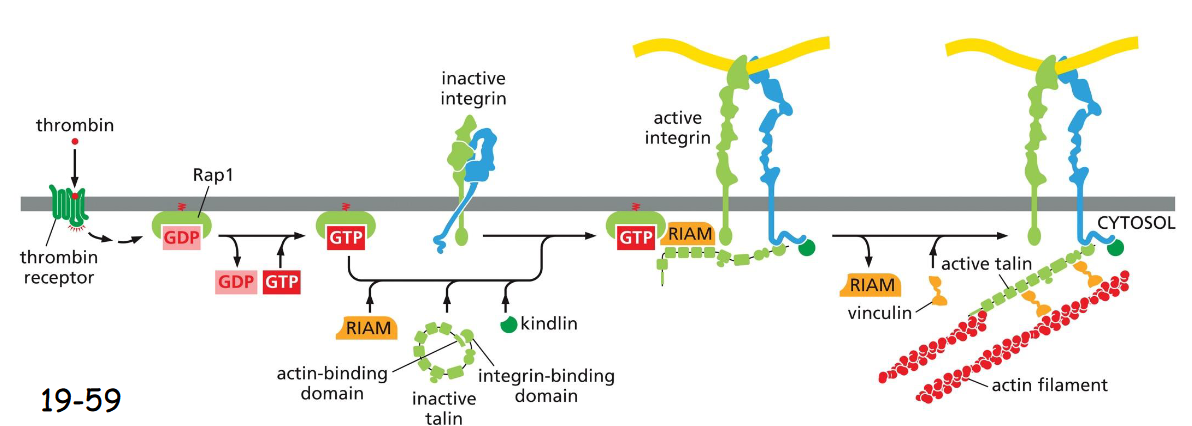
integrin activation, bleeding
occurs in platelets after wound to stop bleeding
Thrombin binds to receptor, initiates signaling cascade that activates Rap1
Rap1 interacts w/ RIAM to recruit talin and kindlin and activates integrin
leads to firm attachment of surface endothelial cells to ECM and stops bleeding
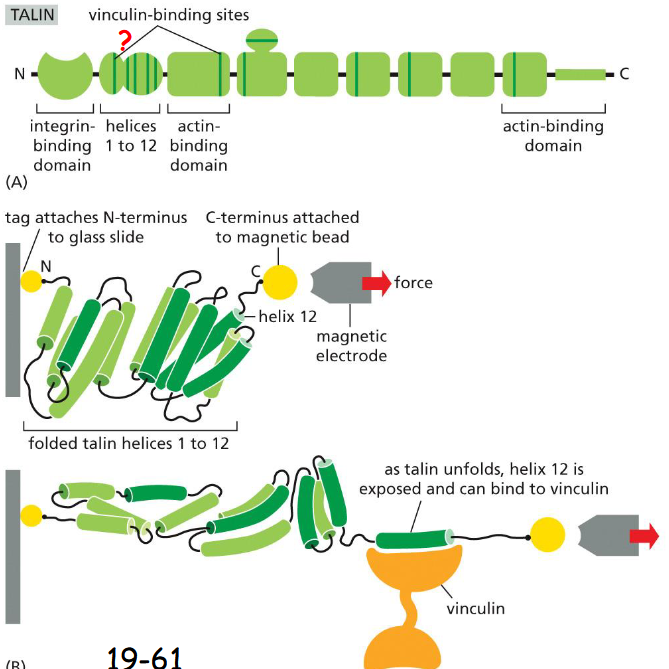
Cell–Matrix Adhesions Respond to Mechanical Forces
Tension across cell-matrix junctions stimulates the recruitment of vinculin and other proteins by talin, strengthening the attachment of the junction to cytoskeleton.
(A) The vinculin binding sites are hidden and inaccessible.
(B) Tension stretches the 12 alpha-helixes and expose the vinculin- binding sites.
Vinculins are fluorescently labeled. After the talin protein was stretched and excess vinculin solution was washed away, determine if vinculin was able to bind to the talin protein
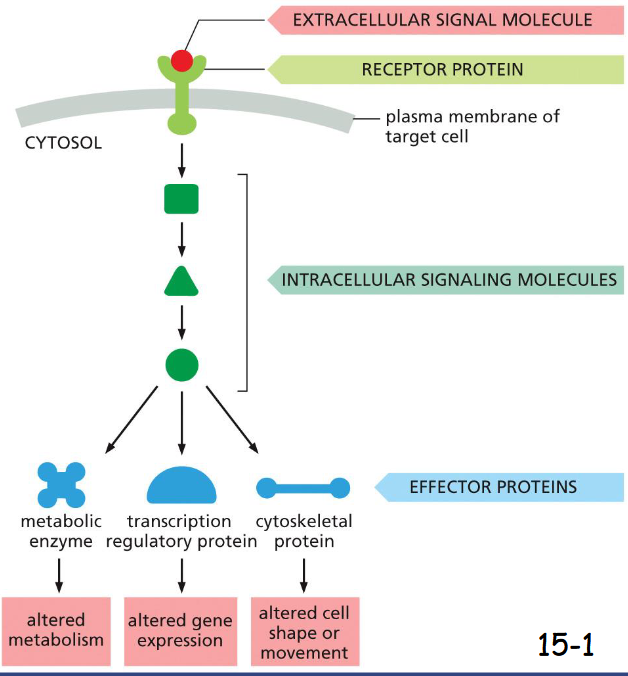
cell signaling basic steps
A signaling pathway is activated by an extracellular signal molecule.
The extracellular signal is perceived by receptor in the plasma membrane of the target cell.
The receptor activates one or more intracellular signaling steps.
The signaling proteins alter the activity of effector proteins and cause various responses
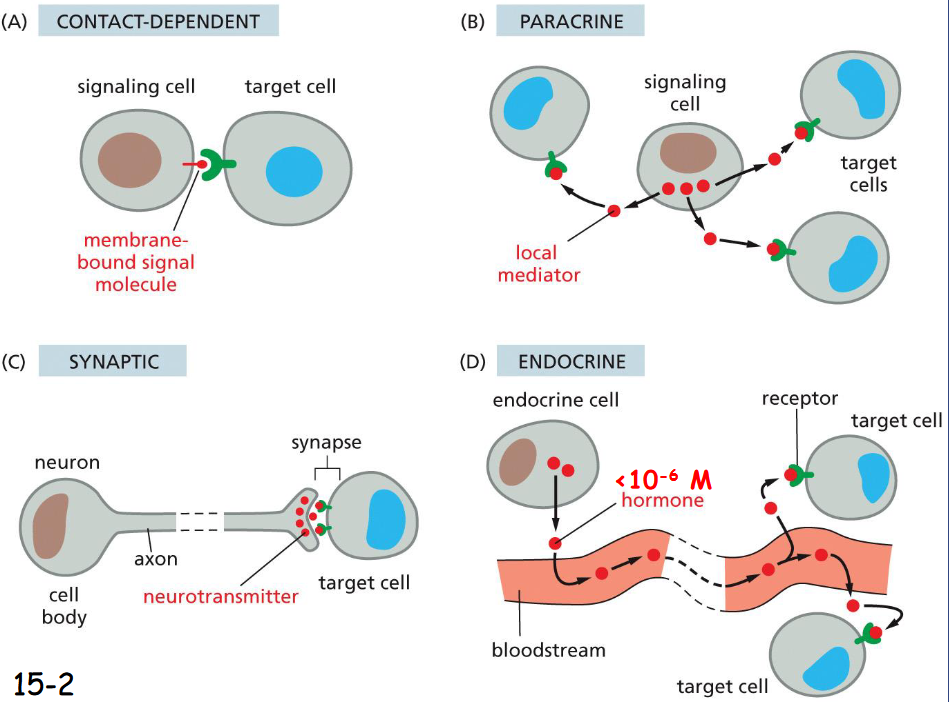
Extracellular Signals Can Act Over Short or Long Distances
shortest to longest: contact dependent, paracrine, synaptic, endocrine
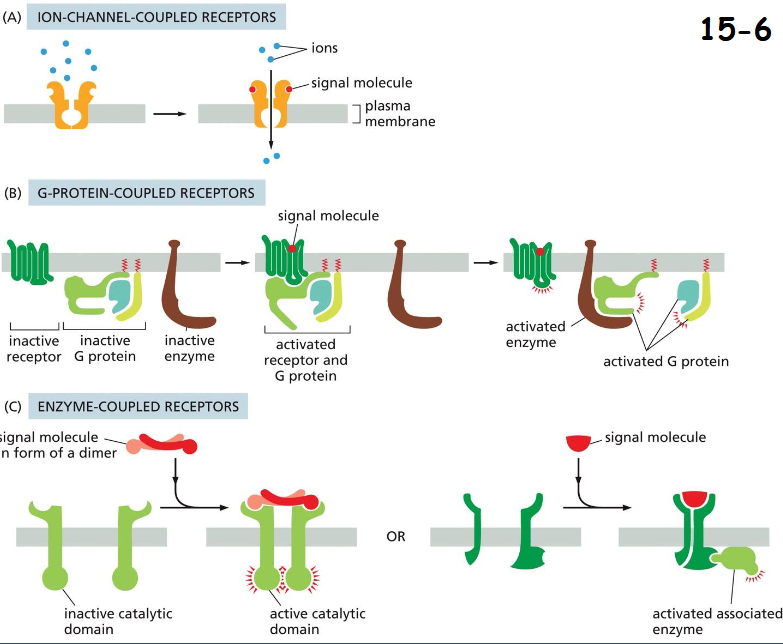
Three Major Classes of Cell-Surface Receptor Proteins
(A) ION-CHANNEL COUPLED RECEPTORS:
Neurotransmitter-gated ion channel between nerve cells and target muscle cells.
(B) GPCRs
The receptor recruits G-protein and indirectly regulates plasma membrane-bound enzyme or an ion channel.
(C) Enzyme-Coupled Receptors (RTKs)
The receptors have either intrinsic enzyme activity or act through associated enzymes.
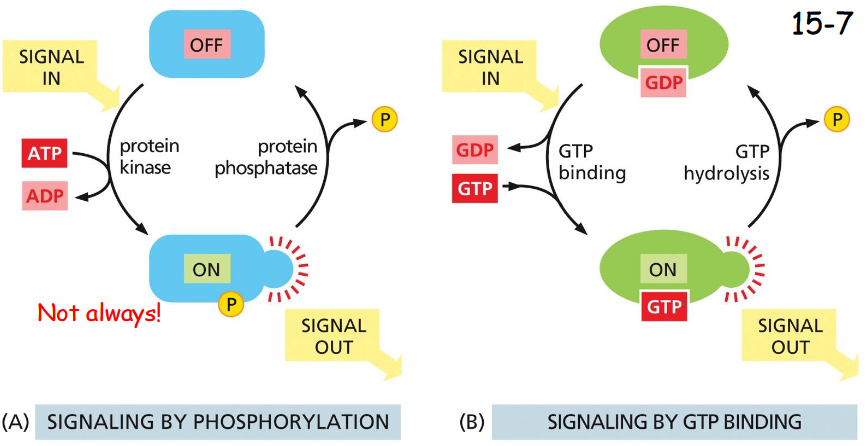
signaling by phosphorylation vs GTP-binding
Signaling by phosphorylation:
phosphorylation by protein kinase (ATP dependent) and de-phosphorylation by phosphatase (A)
Signaling by GTP-binding:
Inactive: GTP hydrolyzed to GDP by action of GTPase-Activating-Protein GAP
Activation: GDP dissociated by action of GEF (guanine nucleotide exchange factor) and GTP binds (10x higher concentration), activating signal
In addition, other small molecules such as cyclic AMP and Ca2+ act as second messengers, and diacylglycerol diffusing in the membrane also acts as second messengers (movie 15-1)
Cells Can Adjust Their Sensitivity to a Signal
The mechanism often involves phosphorylation and ubiquitination of the receptor proteins or signaling proteins
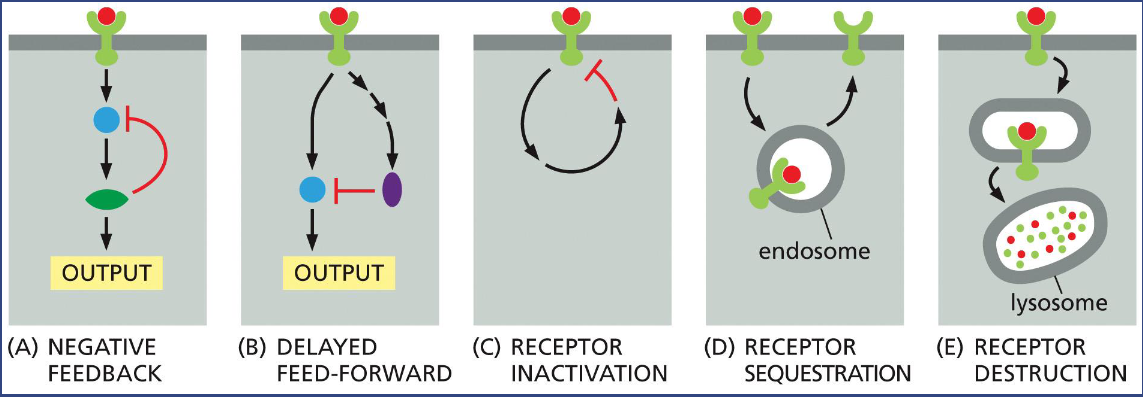
Can you suggest a common feature for the five ways of signaling termination?
Why?
Common feature: removal or inactivation of signaling molecule or receptor
Why> ensures that signaling remains tightly regulated
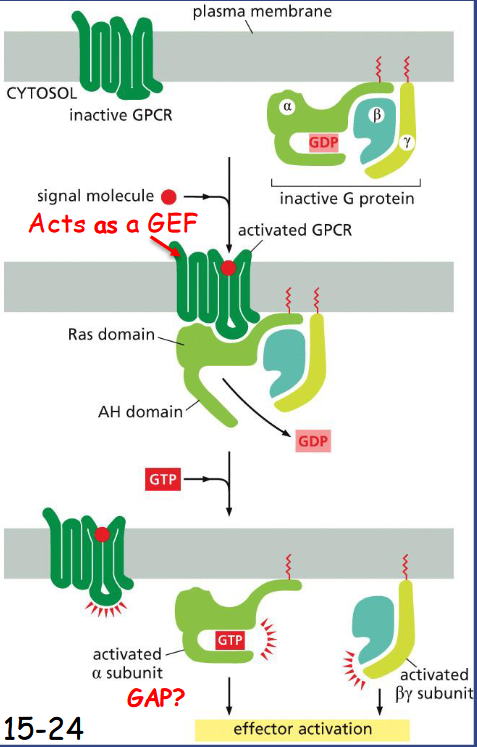
Trimeric G Proteins Relay Signals From ____
GPRCs
G-protein coupled receptor (GPCR) situates near a trimeric G protein. Both G-alpha and G-gamma are anchored to the membrane (movie 15-2).
Once bound by a signaling molecule, GPCR acts like a GEF (Guanine nucleotide exchange factor) to induce alpha subunit to release GDP and bind GTP.
The receptor is then separated from the G-alpha and G-beta-gamma is released.
Regulator of G-protein signaling (RGS) interacts with G-alpha and acts as a GTPase activating protein (GAP) to shut off G- protein-mediated responses
Some G Proteins Regulate the Production of ____
Cyclic AMP
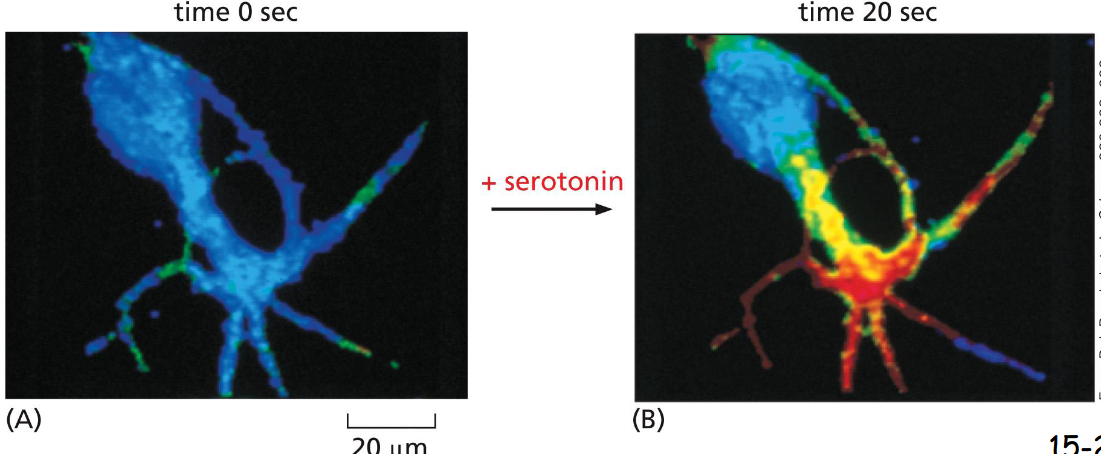
A nerve cell in culture was loaded with a fluorescent protein that changes fluorescence when it binds cAMP at a high level (red). Serotonin is a neurotransmitter and a ligand for a GPCR. When fed with serotonin, the cell shows a twenty-fold increase in cyclic AMP production in 20 sec
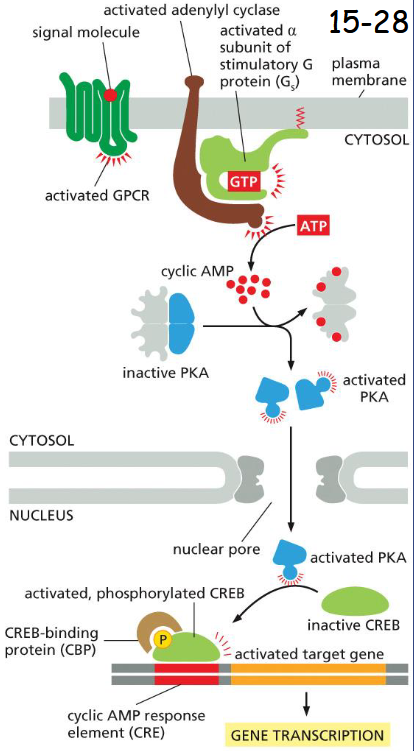
Cyclic-AMP-Dependent ______ Mediates Most of the Effects of Cyclic AMP
Protein Kinase (PKA)
The alpha-subunit of trimeric G protein (Gs) activates adenylyl cyclase (movie 15-3).
The rise in cAMP then activates protein kinase A (PKA). cAMP binds the two regulatory subunits of PKA and causes the release of its catalytic subunits.
In endocrine cells, activated PKA enters the nucleus and phosphorylates transcription factor CREB which further recruits a transcription co-activator CBP, activates transcription of target gene
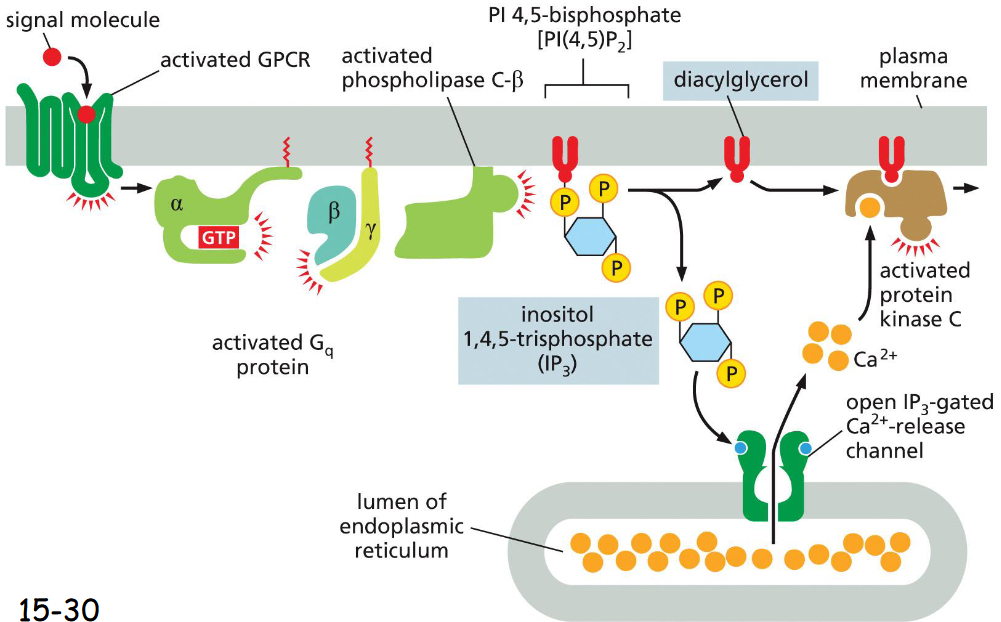
GPCR IP3 diacylglycerol phospholipid signaling
Activation of GPCR → activation of G protein → G protein dissociates into alpha and gamma+beta, activates phospholipase C-beta which splits IP3 and diacylglycerol (PI45P2)
IP3 (inositol 1,4,5-triphosphate) increases cytosolic Ca2+ by opening of IP3 gated Ca2+ channel (from ER)
diacylglycerol stays in the membrane and recruits PKC to the cytosolic face
Ca2+ then activates protein kinase C, PKC phosphorylates target proteins
__ ____ Gas Can Mediate Signaling Between Cells
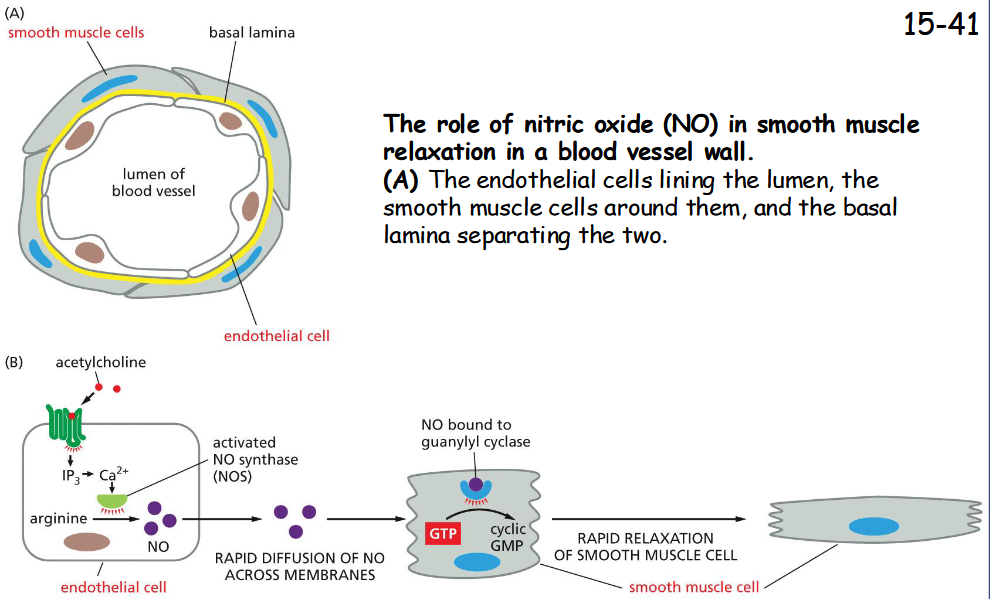
nitric oxide gas
(B) Acetylcholine stimulates blood vessel dilation by activating a GPCR on the surface of endothelial cells.
This receptor activates a G protein and stimulates IP3 synthesis and Ca2+ release from the ER.
Increased Ca2+ activates nitric oxide synthase, causing the production of NO from arginine.
NO diffuses out of the endothelial cells and into the neighboring smooth muscle cells, where it activates guanylyl cyclase to produce cyclic GMP, which triggers the smooth muscle cells to relax, increasing blood flow through the vessel